Abstract
The antimicrobial agent linezolid is approved for the treatment of severe infections caused by, e.g., methicillin-resistant Staphylococcus strains. In order to evaluate the penetration of linezolid into the interstitial space fluid (ISF) of subcutaneous adipose tissue and skeletal muscle of the target population, a microdialysis study was performed with 12 patients with sepsis or septic shock after multiple intravenous infusions. Unbound linezolid concentrations were determined for plasma and microdialysates by use of a validated high-performance liquid chromatography method. Individual compartmental pharmacokinetic (PK) analysis was performed using WinNonlin. In vivo microdialysis was found to be feasible for the determination of unbound linezolid concentrations at steady state in the ISF of critically ill patients. On average, linezolid showed good distribution into ISF but with high interindividual variability. A two-compartment model was fitted to unbound concentrations in plasma with a geometric mean distribution volume of 62.9 liters and a mean clearance of 9.18 liters/h at steady state. However, disposition characteristics changed intraindividually within the time course. In addition, an integrated model for simultaneous prediction of concentrations in all matrices was developed and revealed similar results. Based on the model-predicted unbound concentrations in ISF, a scheme of more-frequent daily dosing of linezolid for some critically ill patients might be taken into consideration to avoid subinhibitory unbound concentrations in the infected tissue. The developed integrated model will be a valuable basis for further PK data analysis to explore refined dosing guidelines that achieve effective antimicrobial therapy in all patients by use of the population PK approach.
The oxazolidinone linezolid (Zyvoxid; Pharmacia, Erlangen, Germany), an antimicrobial drug, was approved for the treatment of severe skin and skin structure infections and community-acquired and hospital-acquired pneumonia (39) caused by multiresistant gram-positive pathogens, e.g., methicillin-resistant Staphylococcus aureus or vancomycin-resistant Enterococcus sp. strains.
For effective treatment of infectious diseases, it is extremely important to reach pharmacologically active drug concentrations at the site of action (16, 19). It is generally accepted that the interstitial space fluid (ISF) of tissue represents the site of action for the vast majority of bacterial infections (45, 46), and since only unbound molecules account for the drug effect, it would be desirable to directly measure this drug fraction. These specifications are met by in vivo microdialysis (14, 35, 41).
Currently available pharmacokinetic (PK) data about the tissue distribution of linezolid were assessed by use of healthy volunteers (10, 11, 22) and by use of patients (4, 29, 31, 43). In these studies, various methods to investigate total or unbound tissue concentrations were used. However, none of them suitably reflected the unbound concentration-time course in the interstitia of peripheral tissues of critically ill patients. Critically ill patients are well known to exhibit substantial alterations in drug pharmacokinetics and pharmacodynamics—especially those for antibiotics—due to their complex pathophysiological situations (36).
In the present study, we investigated the pharmacokinetics of unbound linezolid in plasma of patients with sepsis and septic shock after multiple dosing as well as the distribution of linezolid into ISF of subcutaneous (s.c.) adipose tissue and intramuscular (i.m.) tissue as assessed by microdialysis. The objective was to characterize the pharmacokinetics of linezolid in the tissue interstitium of critically ill patients. An integrated PK model incorporating the concentration-time profiles of all three matrices simultaneously was developed. In addition, pharmacodynamic (PD) parameters were used to evaluate the current dosing guidelines.
MATERIALS AND METHODS
The clinical study was conducted at the Department of Clinical Pharmacology, Medical University of Vienna, Vienna, Austria. The study protocol was approved by the local ethics committee and was performed in accordance with the declaration of Helsinki (1964) in the revised version of 2000 (Edinburgh) (54), the Guidelines of the International Conference on Harmonization (25), the Good Clinical Practice Guidelines (15), and Austrian drug law.
Subjects.
Twelve patients (nine males and three females) were included in the study. Sepsis was diagnosed according to the following criteria of the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference Committee (3): systolic blood pressure of <90 mm Hg; tachycardia of >90 beats/min; respiratory rate of >20 breaths/min or a partial CO2 pressure of <32 mm Hg; a temperature of >38.0 or <36.0°C; and a leukocytosis count of >12,000/μl or a leukopenia count of <4,000/μl or >10% immature (band) forms. A patient could be enrolled in the study if at least two of these criteria were met. Septic shock was defined as sepsis-induced hypotension along with the presence of organ dysfunction and hypoperfusion abnormalities despite bolus fluid resuscitation of 500 ml. Patients who received inotropic or vasopressive agents did not need to be hypotensive at the times that perfusion abnormalities were measured. The indication for linezolid therapy was made by an independent physician, and no linezolid therapy within the past 72 h was allowed. Conventional therapies were not changed.
Study protocol.
After admittance to the intensive care unit, patients were treated with linezolid. Blood and microdialysis samples were taken after single (study visit 1) and multiple (study visit 2) dosing. All patients received 600 mg of linezolid as a short-term infusion over ∼30 min every 12 h. Aberrant to this, one patient received an infusion over 1.25 h at study visit 2. Another patient received only three doses at intervals of 21 and 24 h.
Study visit 1 was performed according to the schedule described for study visit 2 with minor changes in the microdialysis probe calibration procedure (see below). For data assessment under steady-state conditions, study visit 2 was carried out at least 3 days after repeated linezolid application twice a day according to the following procedure. Two intravenous catheters were applied to administer linezolid or to draw blood samples at predefined time points. For in vivo microdialysis investigations, commercially available microdialysis probes (CMA60; CMA Microdialysis AB, Solna, Sweden) with a molecular mass cutoff of 20 kDa, an outer diameter of 0.6 mm, and a membrane length of 30 mm were used. Perfusion fluid was delivered by use of a precision pump (CMA102; CMA Microdialysis AB, Solna, Sweden). The microdialysis method has been described in more detail previously (34, 41). Two microdialysis probes were placed in s.c. and i.m. tissue of the lower extremities. Subsequently, the probes were perfused with Ringer's solution at a flow rate of 1.5 μl/min, and a baseline sample was collected for 30 min prior to drug infusion. Blood and microdialysate samples were collected every 20 min (0 to 3 h after the start of infusion) and at intervals of 30 min (3 to 8 h after the start of infusion). In total, the complete sampling schedule comprised 20 time points per matrix and per patient.
In vivo calibration of the microdialysis probes.
Calibration of the microdialysis probes in vivo was performed by use of the retrodialysis method (48). For study visit 1, the microdialysis probes were calibrated prior to the linezolid application by use of Ringer's solution containing 10 mg/liter of linezolid. The calibration procedure was followed by a washing step of 30 min to carefully switch the perfusion fluid to pure Ringer's solution. After completion of the microdialysis sampling period on study visit 2, the probes were perfused with Ringer's solution containing 150 mg/liter linezolid. Two dialysate fractions were collected at intervals of 15 min. The linezolid concentrations (C) were determined in the dialysate and in the perfusate. Relative recovery (RR; %) was calculated as (1 − Cdialysate/Cperfusate) · 100%. For a reliable calibration of microdialysis probes, the concentration of linezolid in the perfusate should substantially exceed expected ISF concentrations as described by Tegeder et al. (50). This setup ensured that drug present in ISF would not significantly affect its diffusion out of the probe during retrodialysis.
In principle, the mean RR per probe was used for the calculation of unbound linezolid concentration in the ISF of each patient. If calibration samples of a probe were missing (4 of 20 probes), the median value of all other probes from the same tissue of the patients was used for the calculation of RR. Unbound linezolid concentration in the ISF of s.c. or i.m. tissue was calculated according to the equation
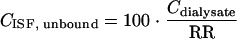 |
where CISF, unbound and Cdialysate are expressed in μg/ml and RR is expressed as a percentage.
Sampling and sample storage.
At predefined time points, blood and microdialysate samples were taken and the actual clock time was documented. Blood was centrifuged for 5 min at 2,550 × g immediately after sampling. The plasma supernatant was collected. Plasma and microdialysate were stored at approximately −70°C. Sample transport was carried out using dry ice and adhering to a cold chain.
Measurement of linezolid in biological matrices.
Linezolid was quantified in plasma, ultrafiltrate, and microdialysate samples by use of a previously described high-performance liquid chromatography method with an RP-18 stationary phase and UV detection at 251 nm (8). The method was validated according to an international FDA guideline (18) with lower limits of quantification of 0.2 mg/liter and 0.8 mg/liter for plasma and ultrafiltrate/microdialysate samples, respectively. In total, it showed an interday variability of ≤6.1% coefficient of variation (CV) and a relative error of <±3.4% across the entire concentration range.
Determination of unbound linezolid.
To evaluate the individual plasma protein binding to a commercially available ultrafiltration membrane, a Centrifree ultrafiltration device from Millipore (Eschborn, Germany) with a regenerated cellulose membrane (molecular mass cutoff, 30 kDa) was used. Two hundred microliters of a plasma sample was transferred into the ultrafiltration device and centrifuged for 10 min at 1,064 × g (Megafuge; Heraeus, Hanau, Germany) at ambient temperature. The ultrafiltrate was then subjected to high-performance liquid chromatography analysis for quantification of linezolid.
Prior to analysis of the human samples, the adsorption characteristics of linezolid to the ultrafiltration device were investigated in vitro. It was demonstrated that linezolid did not adsorb to the ultrafiltration membrane and that the unbound fraction (fu) was independent of the concentration (11). These findings are a prerequisite for determining the unbound plasma linezolid concentration in human samples.
Individual noncompartmental pharmacokinetic analysis.
For the determination of tissue penetration parameters of linezolid in ISF after systemic application, a noncompartmental analysis was performed using WinNonlin (version 4.0; Pharsight Corp., Mountain View, Calif.). The area under the concentration-time curve (AUC) from the start of infusion at t0 to the last measured value at tz was calculated by application of the linear trapezoidal rule. For the determination of the unbound AUC0-12 (fAUC0-12), the concentration-time curve was extrapolated from the last measured unbound concentration (Cz) to the concentration at 12 h (C12) by use of the equation 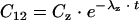 , where t denotes the time interval between tz and 12 h and λz denotes the slope of the terminal phase of the concentration-time curve. The fAUC0-12 was doubled to yield fAUC0-24.
, where t denotes the time interval between tz and 12 h and λz denotes the slope of the terminal phase of the concentration-time curve. The fAUC0-12 was doubled to yield fAUC0-24.
To allow the simultaneous fitting of unbound plasma and ISF data, specific penetration factors (factors of tissue penetration [FT]) were calculated from the ratio of the 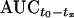 (from the start of infusion until the last measured value) for the unbound linezolid concentration in plasma to those for the unbound linezolid concentration in ISF of s.c. or i.m. tissue (12, 20, 38) according to the following equation:
(from the start of infusion until the last measured value) for the unbound linezolid concentration in plasma to those for the unbound linezolid concentration in ISF of s.c. or i.m. tissue (12, 20, 38) according to the following equation:
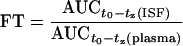 |
Compartmental pharmacokinetic data analysis.
In order to compare only the clinically relevant linezolid concentrations, the compartmental data analysis was based on unbound concentrations for all matrices. For the description of individual unbound plasma concentration-time profiles, open one- and two-compartment models were investigated using models from the WinNonlin model library. The input of the drug was assumed to follow zero-order kinetics, and elimination from the central compartment occurred with first-order kinetics.
After the incorporation of unbound ISF concentrations into the data set, user-built pharmacokinetic models had to be developed. In these models, unbound plasma concentrations were always assigned to the central compartment. During model development, open three- and four-compartment models of various structures were investigated. The models differed in terms of the number and alignment of the compartments. In addition, clearance was varied by changing the compartmental location as well as by using different numbers of clearance processes in different models. The kinetics of invasion and elimination of linezolid remained unchanged. The data were weighted according to the equation 1/Ci2, where Ci denotes the predicted concentration. All iterations were conducted using the Gauss-Newton algorithm (40).
Evaluation of the goodness of fit.
In addition to the evaluation of the weighted sum of squares, the evaluation of the goodness of fit and the estimated parameters was based on the Akaike information criterion (5), the variability (CV) of the parameter estimates, the random distribution of weighted residuals between measured and predicted concentrations with respect to time, and the absence of a significant correlation between independent model parameters (<0.95) (21).
Calculation of pharmacokinetic parameters.
From the estimated pharmacokinetic parameters of the model, other parameters were calculated. The unbound plasma concentrations at the end of an intravenous (i.v.) infusion (Cmax) as well as the minimal concentrations (Cmin) were calculated from the corresponding model equations at the respective time points. The AUC during steady state within the dosing interval τ (AUCss) equaled the AUC0-∞ and characterized the extent of drug exposition. If a model included more than one compartment with an elimination process, the single-clearance values were added to calculate the total clearance (CLtot). The volume of distribution during steady state (Vss) was obtained by adding the volumes of the different compartments (e.g., Vss = V1 + V2 + V3 + V4).
Calculation of pharmacodynamic parameters.
In order to evaluate current dosing guidelines, the PD indices fT>MIC, reflecting the cumulative percentage of time over a period of 24 h that concentration exceeds MIC (33), and fAUC/MIC, the ratio of the fAUC0-24 and MIC, were examined in all three matrices at steady state. A MIC of 4 mg/liter was chosen because that concentration is considered as a breakpoint value for the susceptibility of bacteria against linezolid (17, 30). For the determination of fT>MIC, the individual model-predicted concentration-time profiles were evaluated.
Statistics.
The statistical analysis was conducted using SPSS for Windows (version 11.5; SPSS Inc.). Prior to the analysis, the distribution of data (Shapiro-Wilk test) and the homogeneity of variances (Levene's test) were evaluated. For comparison of means, Student's t test was used for normally distributed data with homogeneous variances. For data not normally distributed, the Mann-Whitney test was applied. In cases where paired samples were not normally distributed, the comparison was performed using the Wilcoxon test. A P value of <0.05 was considered statistically significant. Where not stated otherwise, data are presented as mean CV (%).
RESULTS
Subjects.
Critically ill patients with a median age of 62 years (range, 51 to 74 years) were included into the study. The patients' body weights ranged from 55 to 133 kg (median, 81 kg). Eleven patients (91.7%) developed septic shock, whereas 1 patient (8.3%) suffered from severe sepsis. All patients were sedated and mechanically ventilated. Overall, two patients (16.7%) suffered from serious adverse events. In both cases, the patients died. For one of the remaining patients, sampling on study visit 2 had to be discontinued 2 hours after administration of the linezolid dose. Therefore, complete data sets for graphical analysis were obtained from nine patients. However, PK evaluation for study visits 1 and 2 could be performed for 12 and 10 patients, respectively.
In vivo relative recovery in patients.
s.c. ISF probes displayed a mean RR of 53.1% (31.0%; n = 8) and i.m. ISF probes a mean RR of 59.1% (17.0%; n = 8) on study visit 2. There was no statistically significant difference between the RR values with regard to the matrix (P = 0.10).
Determination of unbound linezolid.
Two plasma samples per patient and study visit were selected to determine the individual protein binding (1 − fu). fu was calculated as the ratio between unbound and total plasma concentrations. The median of both fu values per study visit and patient was calculated. The resulting individual unbound fractions ranged from 73.0 to 95.9% with a mean of 86.6% (CV = 7.9%; n = 22).
Pharmacokinetics of linezolid in critically ill patients.
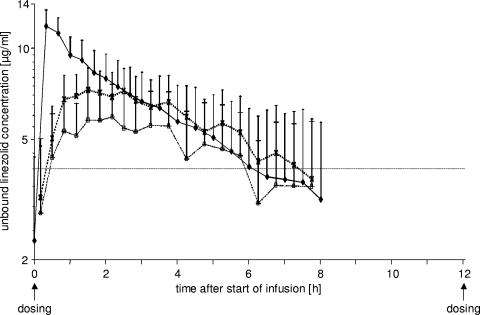
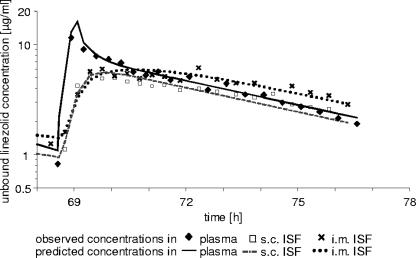
For each matrix (plasma and s.c. and i.m. ISF), the geometric mean of the unbound linezolid concentrations versus time of at least six observed single values is depicted in Fig. 1. For all matrices, the mean minimal concentrations were below 4 mg/liter prior to the administration of linezolid at steady state. For plasma, mean unbound concentrations above 4 mg/liter were observed until 7 h after the start of the linezolid infusion. A biphasic concentration decline could be ascertained.
FIG. 1.
Geometric means of observed unbound linezolid concentrations in plasma (filled diamonds, solid line), s.c. ISF (open squares, dashed line), and i.m. ISF (crosses, dotted line) of septic patients (n = 6 to 10) after multiple i.v. dosing. Error bars represent the geometric standard deviations.
The extent of penetration of unbound linezolid into ISF was quantified by determining the 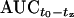 ratios at steady state. The median penetration of linezolid into tissue interstitium in intensive care patients was found to be 89.6% and 99.9% in s.c. and i.m. ISF (n = 10 each), respectively, ranging substantially from 20.2% to 118% and from 24.1% to 144%. While most of the patients displayed a drug exposition in ISF of more than 80%, three subjects (25%) exhibited significantly lower ISF s.c. and/or i.m. AUC ratios. For one patient, AUC ratios were below 25% in both ISF matrices.
ratios at steady state. The median penetration of linezolid into tissue interstitium in intensive care patients was found to be 89.6% and 99.9% in s.c. and i.m. ISF (n = 10 each), respectively, ranging substantially from 20.2% to 118% and from 24.1% to 144%. While most of the patients displayed a drug exposition in ISF of more than 80%, three subjects (25%) exhibited significantly lower ISF s.c. and/or i.m. AUC ratios. For one patient, AUC ratios were below 25% in both ISF matrices.
Compartmental pharmacokinetic data analysis in ultrafiltrated plasma.
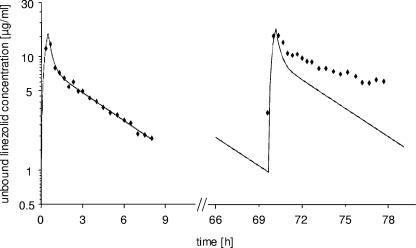
All unbound plasma data were best described by a two-compartment model. Nevertheless, it was not possible to describe the entirely measured concentrations after single and multiple dosing by using the same individual PK parameter sets with the underlying assumption of unchanged PK disposition characteristics. To demonstrate the change in PK parameters within each individual patient from single to multiple dosing, individual concentration-time profiles were simulated for the entire investigation (generally up to ∼60 to 84 h) by use of the parameters estimated by the two-compartment model after single dosing. The model-predicted time course and the observed concentrations are illustrated in the semilogarithmic concentration-time profile of a representative patient (Fig. 2). If the PK remained unchanged from the first dose to the steady state, one would expect the model-predicted concentration curve with the observed concentrations randomly spread around this curve in the right part. However, the observed concentrations at steady state were much higher than the expected concentrations due to intraindividual changes in PK. Considering all individual profiles, simulated steady-state concentrations differed from measured concentrations to different extents. Shallower slopes were observed for four patients (44%). For five patients (56%), no changes in slope were visible.
FIG. 2.
Intraindividual changes in PK of unbound linezolid in plasma from single dosing (left) to steady state (right). Model-predicted concentrations based only on single-dose data are represented by the solid line, observed concentrations by filled diamonds. See text for details.
In order to improve the insufficient joint model fit, unbound plasma data investigated at steady state were analyzed separately. An open two-compartment model with different parameter estimates was successfully applied to data for all 10 patients. The geometric mean maximum concentrations predicted by the model at the end of the infusion were almost unchanged from the first administration to the steady-state administration. At the beginning of the linezolid treatment, the model-predicted Cmax was on average 16.1 mg/liter (25.4%; n = 12). After multiple dosing, concentrations reached a Cmax of 16.4 mg/liter on average (30.4%; n = 9). Prior to the linezolid infusion at steady state, minimal concentrations were estimated as 1.83 mg/liter (188%; n = 9) with a very broad range between 0.109 and 9.52 mg/liter. The PK parameters estimated by this model are summarized in Table 1. Except for Vss, pronounced variability was observed in the PK parameters clearance and intercompartmental clearance (CLD2) and in the derived parameters AUC and half-life.
TABLE 1.
PK parameters of unbound linezolid in plasma of critically ill patients after multiple i.v. dosinga
| Vss (liter) | CL (liter/h) | CLD2 (liter/h) | AUC (mg · h/liter) | t1/2 (h)b |
|---|---|---|---|---|
| 62.9 (19.2) | 9.18 (57.9) | 62.1 (87.9) | 65.3 (57.9) | 5.0 (45.5) |
Values in parentheses are cv (%) geometric means (n = 10).
t1/2, terminal elimination half-life.
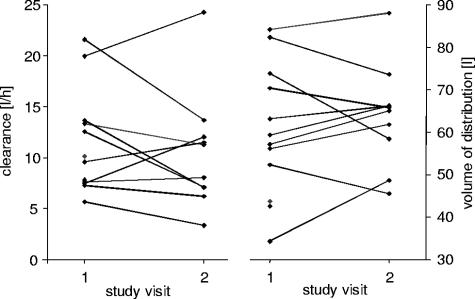
The comparison of mean, i.e., not individual, PK parameters of all patients between single and multiple dosing, however, revealed no significant differences in the volumes of distribution (P = 0.878), clearances (P = 0.285), or AUCs (P = 0.169). In Fig. 3, the changes in individual values for the PK parameters CL and Vss, which are contrary to the overall results, are depicted. If the ratio of CL and Vss had remained constant, the terminal elimination half-life would have been unaffected. However, in the observed patient population, the two parameters changed to higher and lower values for all patients, but to different extents. Thus, the terminal elimination half-life changed in each individual case to a different degree.
FIG. 3.
Individual changes in clearance and volume of distribution of unbound linezolid in plasma after multiple i.v. dosing of 600 mg twice a day (each symbol represents the estimated parameter value for one patient).
Compartmental pharmacokinetic analysis of unbound plasma and ISF data.
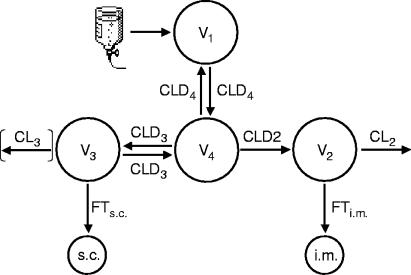
The structure of the integrated model incorporating all unbound plasma and both ISF data sets after i.v. multiple dosing is depicted in Fig. 4. For reasons of model stability, no combined analysis of data after single and multiple dosing was conducted. The specific factors of tissue penetration served for modeling purposes as a constant proportionality factor. For four patients, the parameter CL3 (elimination from compartment 3) was included into the individual models. In these cases, the respective intercompartmental clearances (CLD3) were incorporated as monodirectional clearances. The peripheral volumes of distribution V2 and V3 were fixed to 1 liter to increase the model stability.
FIG. 4.
Structure of the integrated pharmacokinetic models for simultaneous modeling of unbound plasma and s.c. and i.m. ISF data after multiple dosing of linezolid (see text for details).
The mean pharmacokinetic parameters of unbound linezolid after single and multiple i.v. dosing estimated by the integrated model are displayed in Table 2. The volume of distribution after the first i.v. infusion was similar to the distribution volume calculated by the unbound plasma model after a single dose and was consistent in terms of variability characteristics. The mean clearance corresponded to the result of the unbound plasma model in size and variability. A semilogarithmic unbound concentration-time profile in plasma and ISF after multiple i.v. dosing of a representative patient is depicted in Fig. 5.
TABLE 2.
PK parameters of unbound linezolid estimated by the integrated model after single and multiple linezolid dosinga
| i.v. dosing type | Vss (liter) | CLtot (liter/h) |
|---|---|---|
| Single (n = 12) | 61.4 (29.0) | 9.91 (45.0) |
| Multiple (n = 9) | 79.8 (23.6) | 8.44 (60.1) |
For Vss and CLtot, values in parentheses are cv (%) geometric means.
FIG. 5.
Model-predicted concentration-time profile of a representative patient after multiple linezolid dosing.
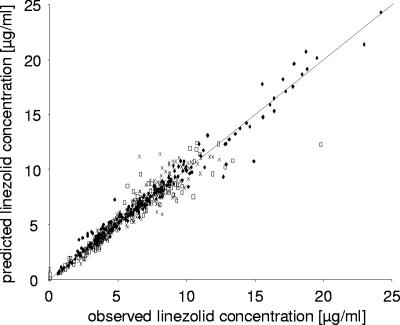
A goodness-of-fit plot is depicted in Fig. 6. It shows the overall performance of the integrated model to predict the unbound plasma and s.c. and i.m. ISF data. Since the data of the model-predicted and observed concentrations of all three matrices randomly spread around the line of identity, there was no bias or misspecification in the structural model. All data were close to the line of identity, indicating a good precision of the predictions.
FIG. 6.
Goodness of fit of the integrated PK model after multiple i.v. dosing showing the unbound model-predicted data versus observed data from the three matrices plasma (filled diamonds), s.c. ISF (open squares), and i.m. ISF (crosses) (n = 9). The solid line represents the line of identity.
Although linezolid showed an ISF penetration with a median value of at least 89.6%, one cannot conclude that effective concentrations will be achieved at the target site for a sufficient time period in all individual patients. Therefore, the PD indices fT>MIC and fAUC/MIC, based on unbound concentrations, were calculated, and the results are given in Table 3. At steady state, three of nine patients (33%) showed fT>MIC values in plasma of less than 40%. In s.c. and i.m. ISF, fT>MIC remained below 40% in four of nine and two of nine individuals (44% and 22%), respectively. fAUC/MICs exceeded the value of 51 only in two cases: for one patient in plasma and for another in i.m. ISF. Additionally, the large range of the calculated data was remarkable. While some patients achieved sufficient unbound linezolid concentrations in plasma and ISF during the whole dosing interval, concentrations in ISF of other subjects remained below the MIC for a large proportion or even the entirety of the dosing interval.
TABLE 3.
fAUC/MIC and fT>MIC values for linezolid in ultrafiltrated plasma and s.c. and i.m. ISF of critically ill patients after multiple dosinga
| Parameter |
fAUC/MIC in:
|
fT>MIC (%) in:
|
||||
|---|---|---|---|---|---|---|
| UF | ISF
|
UF | ISF
|
|||
| s.c. | i.m. | s.c. | i.m. | |||
 |
29.9 | 29.1 | 33.0 | 59 | 54 | 90 |
| Minimum | 12.0 | 10.1 | 14.1 | 16 | 0 | 24 |
| Maximum | 88.2 | 48.7 | 53.0 | 100 | 100 | 100 |
MIC = 4 mg/liter. For s.c. and i.m. ISF, n = 9. For ultrafiltrated plasma (UF), n = 10.
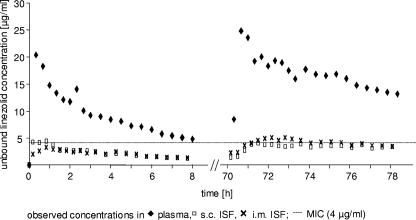
As described above, the distributions of linezolid into ISF varied among the patients. In 1/12 patients (8.3%), the extent of ISF distribution differed considerably from that for the remaining patient population. The concentration-time profile of this patient is presented in Fig. 7. Although unbound plasma concentrations during the time of linezolid therapy were at the high end compared to those for other patients, ISF concentrations showed a very deviant pattern. After single dosing as well as after multiple dosing, the MIC was exceeded only for a very short time period.
FIG. 7.
Observed concentration-time plot of a patient with impaired drug distribution into tissue interstitium after single and multiple dosing of linezolid.
DISCUSSION
In the present study, the unbound concentrations of linezolid in plasma and in s.c. and i.m. ISF of septic patients were quantified. The microdialysis method was successfully applied after the administration of multiple intravenous infusions in this important but also difficult-to-investigate patient population. For the first time, the direct measurement of unbound ISF linezolid concentrations was performed under steady-state conditions by applying the microdialysis technique to critically ill patients.
Distribution of linezolid into tissue interstitium was rapid in most patients. Other investigations considering the ISF distribution of the anti-infective agent in patients are not directly comparable, since in these investigations, total concentration (29, 31, 43) or the concentration in epithelial lining fluid (ELF) (4) was measured. Lovering et al. (31) and Rana et al. (43) used the biopsy sampling technique and in addition collected drainage fluid. The first group found linezolid penetrations of 26.5 and 93.0% for adipose and muscle tissue, respectively (31). In particular, the penetration values for adipose tissue were smaller than those in our study. One reason might be the difference in the techniques used. Microdialysis measures unbound concentrations in the interstitium of the tissue, while biopsy sampling does not differentiate between intra- and extracellular concentrations and includes various types of tissue. Only the unbound concentration in the interstitium, however, is considered to contribute to the efficacy of anti-infectives. When performing a comparison with the results obtained from drainage fluid (31), one should keep in mind that this is a pathophysiological fluid caused by reactions after surgery and is not representative of interstitial fluids. The measurement of linezolid concentrations in the epithelial lining fluid of mechanically ventilated patients at two certain time points suggested an adequate distribution into lung tissue (4). Considering the concentration in ELF, Boselli et al. (4) observed a maximum concentration twofold higher than that we found in ISF of skeletal muscle. Obviously, the results gained in the ELF study do not reflect the situation for other peripheral tissues, due to the special pathophysiological conditions described below. As the study presented here included the continuous measurement of ISF concentrations, the area under the concentration-time curve was ascertainable for ISF data. This allowed a more comprehensive evaluation of drug exposition and penetration in the tissue interstitium and might therefore explain the differences between these and other published results.
The concentration-time curves of unbound linezolid in plasma and s.c. and i.m. ISF were simultaneously modeled by a stepwise approach. After the unbound concentrations in plasma alone were analyzed, an integrated pharmacokinetic model based on plasma and ISF concentrations was developed. The use of a two-compartment model for the description of unbound linezolid pharmacokinetics in plasma is in concordance with the results of other investigations (32, 53). While never before reported for linezolid, comodeling of unbound plasma and ISF concentrations with an individual compartmental PK analysis approach (12, 20, 38) or by using population pharmacokinetic analysis techniques (6, 50, 51) for other drugs has previously been described, but application is still rare. Here, an integrated model for linezolid with minor interindividual variations in the model structure was developed.
Compared to previously published data for healthy volunteers, the average values we found for volume of distribution and clearance were higher (9, 11, 47, 49). Because the bioavailability of linezolid is reported to be 100% (37), the parameter values of Vss and CL reported after oral administration can be directly compared without a correction. Nevertheless, other pharmacokinetic studies with critically ill patients (4, 32) yielded results similar to ours. In general, the model-predicted maximum concentrations at the end of the infusion did not substantially increase from single to multiple dosing. Thus, persistent accumulation of linezolid during the twice-daily treatment could not be observed. This can be explained by the relatively small half-life/dosing interval ratio resulting in high drug fluctuation. Although the elimination rate changed individually in the course of therapy, there was also no accumulation in those patients where the clearance decreased over time. In these individuals, the increased half-life was still considerably smaller than the dosing interval. In addition, other PK parameters, e.g., Vss, changed as well. The increase in the volume of distribution in critically ill patients might be caused by fluid retention due to an insufficiency of elimination pathways and/or high fluid input resulting in significant “third spacing.” In accordance with the results obtained by Meagher et al., we did not observe any significant increase in minimum and maximum concentrations over time in critically ill patients (32), while Whitehouse et al. (53) described a distinctive linezolid accumulation in this patient population. Compared to healthy volunteers, our study patients had a significantly decreased drug exposition expressed as AUCss after multiple dosing (P = 0.017) (data not shown) due to substantially increased clearance values. These results corroborate the findings of Meagher et al. (32). Considering the metabolism of linezolid as a pathway of nonenzymatic oxidation as previously suggested (47), the more rapid elimination may be caused by increased oxidative stress in the septic patients compared to non-critically ill patients (32). In a recent short notice, Egle et al. reported decreased linezolid plasma concentrations due to an interaction with rifampin (13).
The pharmacokinetic changes observed when intraindividually comparing single and multiple dosing data could not be statistically proven for the average population values. Closer examination of the data revealed that individual changes in parameters occurred heterogeneously due to the high variability across the patient population. Meagher et al. also observed changes of clearance within subjects (32).
For critically ill patients, linezolid showed a generally good but interindividually variable penetration into the tissue interstitium. The results demonstrated that for the majority, unbound concentration kinetics in ISF mostly mimicked the unbound concentration kinetics in plasma. However, 1/12 patients (8.3%) showed a dramatically lower distribution into the ISF. As this pattern was found in both skeletal muscle and s.c. adipose tissue during the whole study period, i.e., with four different microdialysis probes, experimental problems can be excluded. Other investigators also found impaired drug distribution in the critically ill (7, 26-28, 50). Besides the physicochemical properties of a compound, distribution into tissue and the drug concentration achieved at the target site are mainly the results of tissue perfusion, the existence of diffusion barriers, and the volume of the interstitial space fluid. For septic patients, the overwhelming inflammatory state of sepsis leads to capillary leakage and a distinctive peripheral vasodilatation followed by a displacement of fluid, solutes, and proteins into the interstitial space (42). The administration of large amounts of fluid to achieve hemodynamic stability for septic patients is a basic therapeutic principle (24) and contributes to the increase of fluid volume in the body. Subsequently, the extracellular volume and hence the compartment of drugs being distributed into it will be enlarged as well. On the other hand, the sustained compromised microcirculation in peripheral tissues, e.g., skeletal muscle, may be an additional reason for impaired drug distribution into the tissue. Even after complete restoration of the macrohemodynamics, e.g., the intravascular volume, a significant proportion of malperfused vessels was observed with septic patients (52). The alteration of the composition of the interstitial space fluid due to the capillary leakage, especially the shift of proteins, may alter the concentration of unbound drug (42). Due to the relatively low protein binding of linezolid, this effect may not be responsible for the very low ISF concentrations observed with the particular patient. Dosing recommendation based solely on (unbound) plasma concentration for this particular patient would have led to an overestimation of ISF concentrations, resulting in turn in a continuation of the twice-daily dosing scheme, with the consequence of subinhibitory concentrations in infected tissue interstitium and a possible treatment failure.
In general, concentrations measured in microdialysate lead to the conclusion that linezolid distributed into ISF with a high maximum but for various durations. The PD indices fT>MIC and fAUC/MIC were identified as important determinants of linezolid efficacy in vitro and in vivo (1, 2, 23, 44). In several animal infection models, an fT>MIC of >40% significantly enhanced bacterial killing of pneumococci (1, 23), and fAUC/MICs between 48 and 147 were necessary for the bacteriostatic effect of linezolid (2, 23). Taking these fT>MIC values into account, 7/10 patients had effective unbound linezolid concentrations in plasma. For critically ill patients, Rayner et al. found breakpoint values for fT>MIC of >82% and fAUC/MIC values of >51 for the probability of bacterial eradication with respect to the infection site (44). In our study, fT>MIC in plasma and ISF was >82% only in four patients (40%), and nearly all patients showed fAUC/MICs in plasma and ISF of less than 51. Assuming that a bacterial strain with a MIC of 4 mg/liter caused the infection, a more frequent linezolid dosing scheme, e.g., 600 mg three times a day, might be considered to avoid subinhibitory concentrations in the infected tissue interstitium and thus to circumvent an ineffective antimicrobial therapy and the development of resistance. Finally, it should be kept in mind that PD target values were established aiming at unbound plasma concentrations (2, 23). To our best knowledge, it has not yet been demonstrated that these values are also applicable to concentrations in tissue interstitium.
In conclusion, the study presented here underlines the need for additional pharmacokinetic investigations with critically ill patients. Due to their special pathophysiological conditions, results from studies with healthy volunteers or patients with mild diseases may not be applied to this patient population. Microdialysis investigations determining unbound drug concentrations in the critically ill are feasible. Further sophisticated PK modeling is needed to systematically evaluate the described differences in PK parameters. Modeling the data by using a population pharmacokinetic approach may help to assess the different types of variability (inter- and intraindividual; predictive and random components) and to predict ISF concentrations based on unbound plasma data. Relating the pharmacokinetic data to results of further pharmacodynamic investigations using unbound concentrations in tissue interstitium will be a valuable basis for optimizing sampling schedules for septic patients and possibly for individualizing the antimicrobial therapy as well.
Acknowledgments
We acknowledge Judith Mueller, Sabrina Albrecht, and Petra Zeleny for their valuable contribution to the realization of this study.
REFERENCES
- 1.Andes, D., M. L. van Ogtrop, and W. A. Craig. 1998. Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-9.
- 2.Andes, D., M. L. van Ogtrop, J. Peng, and W. A. Craig. 2002. In vivo pharmacodynamics of a new oxazolidinone (linezolid). Antimicrob. Agents Chemother. 46:3484-3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bone, R. C., W. J. Sibbald, and C. L. Sprung. 1992. The ACCP-SCCM consensus conference on sepsis and organ failure. Chest 101:1481-1483. [DOI] [PubMed] [Google Scholar]
- 4.Boselli, E., D. Breilh, T. Rimmele, S. Djabarouti, J. Toutain, D. Chassard, M. C. Saux, and B. Allaouchiche. 2005. Pharmacokinetics and intrapulmonary concentrations of linezolid administered to critically ill patients with ventilator-associated pneumonia. Crit. Care Med. 33:1529-1533. [DOI] [PubMed] [Google Scholar]
- 5.Bourne, D. 1995. Mathematical modeling of pharmacokinetic data, vol. 1, p. 109. Technomic Publishing AG, Lancaster, Pa. [Google Scholar]
- 6.Bouw, M. R., M. Gardmark, and M. Hammarlund-Udenaes. 2000. Pharmacokinetic-pharmacodynamic modelling of morphine transport across the blood-brain barrier as a cause of the antinociceptive effect delay in rats—a microdialysis study. Pharm. Res. 17:1220-1227. [DOI] [PubMed] [Google Scholar]
- 7.Brunner, M., T. Pernerstorfer, B. X. Mayer, H. G. Eichler, and M. Muller. 2000. Surgery and intensive care procedures affect the target site distribution of piperacillin. Crit. Care Med. 28:1754-1759. [DOI] [PubMed] [Google Scholar]
- 8.Buerger, C., C. Joukhadar, M. Muller, and C. Kloft. 2003. Development of a liquid chromatography method for the determination of linezolid and its application to in vitro and human microdialysis samples. J. Chromatogr. B 796:155-164. [DOI] [PubMed] [Google Scholar]
- 9.Burkhardt, O., K. Borner, N. Von Der Hoh, P. Koppe, M. W. Pletz, C. E. Nord, and H. Lode. 2002. Single- and multiple-dose pharmacokinetics of linezolid and co-amoxiclav in healthy human volunteers. J. Antimicrob. Chemother. 50:707-712. [DOI] [PubMed] [Google Scholar]
- 10.Conte, J. E., Jr., J. A. Golden, J. Kipps, and E. Zurlinden. 2002. Intrapulmonary pharmacokinetics of linezolid. Antimicrob. Agents Chemother. 46:1475-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dehghanyar, P., C. Burger, M. Zeitlinger, F. Islinger, F. Kovar, M. Muller, C. Kloft, and C. Joukhadar. 2005. Penetration of linezolid into soft tissues of healthy volunteers after single and multiple doses. Antimicrob. Agents Chemother. 49:2367-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De La Pena, A., T. Dalla Costa, J. D. Talton, E. Rehak, J. Gross, U. Thyroff-Friesinger, A. I. Webb, M. Muller, and H. Derendorf. 2001. Penetration of cefaclor into the interstitial space fluid of skeletal muscle and lung tissue in rats. Pharm. Res. 18:1310-1314. [DOI] [PubMed] [Google Scholar]
- 13.Egle, H., R. Trittler, and K. Kuemmerer. 2005. Linezolid and rifampin: drug interaction contrary to expectations? Clin. Pharmacol. Ther. 77:451-454. [DOI] [PubMed] [Google Scholar]
- 14.Elmquist, W. F., and R. J. Sawchuk. 1997. Application of microdialysis in pharmacokinetic studies. Pharm. Res. 14:267-288. [DOI] [PubMed] [Google Scholar]
- 15.European Agency for the Evaluation of Medicinal Products. 1996. Guideline for good clinical practice. [Online.] http://www.emea.eu.int/pdfs/human/ich/013595en.pdf.
- 16.European Agency for the Evaluation of Medicinal Products. 2000. Points to consider on pharmacokinetics and pharmacodynamics in the development of antibacterial medicinal products. [Online.] www.emea.eu.int/pdfs/human/ewp/265599en.pdf.
- 17.European Committee on Antimicrobial Susceptibility Testing. 2001. Linezolid breakpoints. Clin. Microbiol. Infect. 7:283-284.11422259 [Google Scholar]
- 18.Food and Drug Administration. 2001. Guidance for industry. Bioanalytical method validation. [Online.] http://www.fda.gov/cder/guidance/4252fnl.pdf.
- 19.Food and Drug Administration. 1998. Guidance for industry. Developing antimicrobial drugs—general considerations for clinical trials. Draft guidance. [Online.] http://www.fda.gov/cder/guidance/2580dft.pdf.
- 20.Freddo, R. J., and T. Dalla Costa. 2002. Determination of norfloxacin free interstitial levels in skeletal muscle by microdialysis. J. Pharm. Sci. 91:2433-2440. [DOI] [PubMed] [Google Scholar]
- 21.Gabrielsson, J., and D. Weiner. 2000. Pharmacokinetic and pharmacodynamic data analysis: concepts and applications, vol. 3, p. 311. Apotekarsocieteten (Swedish Pharmaceutical Society), Stockholm, Sweden. [Google Scholar]
- 22.Gee, T., R. Ellis, G. Marshall, J. Andrews, J. Ashby, and R. Wise. 2001. Pharmacokinetics and tissue penetration of linezolid following multiple oral doses. Antimicrob. Agents Chemother. 45:1843-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gentry-Nielsen, M. J., K. M. Olsen, and L. C. Preheim. 2002. Pharmacodynamic activity and efficacy of linezolid in a rat model of pneumococcal pneumonia. Antimicrob. Agents Chemother. 46:1345-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hollenberg, S. M., T. S. Ahrens, D. Annane, M. E. Astiz, D. B. Chalfin, J. F. Dasta, S. O. Heard, C. Martin, L. M. Napolitano, G. M. Susla, R. Totaro, J. L. Vincent, and S. Zanotti-Cavazzoni. 2004. Practice parameters for hemodynamic support of sepsis in adult patients: 2004 update. Crit. Care Med. 32:1928-1948. [DOI] [PubMed] [Google Scholar]
- 25.International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. 2000. Guidelines. [Online.] http://www.ich.org/cache/compo/276-254-1.html. [DOI] [PMC free article] [PubMed]
- 26.Joukhadar, C., M. Frossard, B. X. Mayer, M. Brunner, N. Klein, P. Siostrzonek, H. G. Eichler, and M. Muller. 2001. Impaired target site penetration of beta-lactams may account for therapeutic failure in patients with septic shock. Crit. Care Med. 29:385-391. [DOI] [PubMed] [Google Scholar]
- 27.Joukhadar, C., N. Klein, M. Frossard, E. Minar, H. Stass, E. Lackner, M. Herrmann, E. Riedmuller, and M. Muller. 2001. Angioplasty increases target site concentrations of ciprofloxacin in patients with peripheral arterial occlusive disease. Clin. Pharmacol. Ther. 70:532-539. [DOI] [PubMed] [Google Scholar]
- 28.Joukhadar, C., N. Klein, B. X. Mayer, N. Kreischitz, G. Delle-Karth, P. Palkovits, G. Heinz, and M. Muller. 2002. Plasma and tissue pharmacokinetics of cefpirome in patients with sepsis. Crit. Care Med. 30:1478-1482. [DOI] [PubMed] [Google Scholar]
- 29.Kutscha-Lissberg, F., U. Hebler, G. Muhr, and M. Koller. 2003. Linezolid penetration into bone and joint tissues infected with methicillin-resistant staphylococci. Antimicrob. Agents Chemother. 47:3964-3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Livermore, D. M., S. Mushtaq, and M. Warner. 2001. Susceptibility testing with linezolid by different methods, in relation to published “general breakpoints.” J. Antimicrob. Chemother. 48:452-454. [DOI] [PubMed] [Google Scholar]
- 31.Lovering, A. M., J. Zhang, G. C. Bannister, B. J. Lankester, J. H. Brown, G. Narendra, and A. P. MacGowan. 2002. Penetration of linezolid into bone, fat, muscle and haematoma of patients undergoing routine hip replacement. J. Antimicrob. Chemother. 50:73-77. [DOI] [PubMed] [Google Scholar]
- 32.Meagher, A. K., A. Forrest, C. R. Rayner, M. C. Birmingham, and J. J. Schentag. 2003. Population pharmacokinetics of linezolid in patients treated in a compassionate-use program. Antimicrob. Agents Chemother. 47:548-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mouton, J. W., M. N. Dudley, O. Cars, H. Derendorf, and G. L. Drusano. 2005. Standardization of pharmacokinetic/pharmacodynamic (PK/PD) terminology for anti-infective drugs: an update. J. Antimicrob. Chemother. 55:601-607. [DOI] [PubMed] [Google Scholar]
- 34.Muller, M., O. Haag, T. Burgdorff, A. Georgopoulos, W. Weninger, B. Jansen, G. Stanek, H. Pehamberger, E. Agneter, and H. G. Eichler. 1996. Characterization of peripheral-compartment kinetics of antibiotics by in vivo microdialysis in humans. Antimicrob. Agents Chemother. 40:2703-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muller, M., R. Schmid, A. Georgopoulos, A. Buxbaum, C. Wasicek, and H. G. Eichler. 1995. Application of microdialysis to clinical pharmacokinetics in humans. Clin. Pharmacol. Ther. 57:371-380. [DOI] [PubMed] [Google Scholar]
- 36.Pea, F., P. Viale, and M. Furlanut. 2005. Antimicrobial therapy in critically ill patients: a review of pathophysiological conditions responsible for altered disposition and pharmacokinetic variability. Clin. Pharmacokinet. 44:1009-1034. [DOI] [PubMed] [Google Scholar]
- 37.Perry, C. M., and B. Jarvis. 2001. Linezolid: a review of its use in the management of serious gram-positive infections. Drugs 61:525-551. [DOI] [PubMed] [Google Scholar]
- 38.Persky, A. M., M. Muller, H. Derendorf, M. Grant, G. A. Brazeau, and G. Hochhaus. 2003. Single- and multiple-dose pharmacokinetics of oral creatine. J. Clin. Pharmacol. 43:29-37. [DOI] [PubMed] [Google Scholar]
- 39.Pharmacia. 2002. Zyvoxid Fachinformation. Pharmacia, Erlangen, Germany.
- 40.Pharsight. 1999. WinNonlin reference guide. Pharsight, Mountain View, Calif.
- 41.Plock, N., and C. Kloft. 2005. Microdialysis—theoretical background and recent implementation in applied life-sciences. Eur. J. Pharm. Sci. 25:1-24. [DOI] [PubMed] [Google Scholar]
- 42.Power, B. M., A. M. Forbes, P. V. van Heerden, and K. F. Ilett. 1998. Pharmacokinetics of drugs used in critically ill adults. Clin. Pharmacokinet. 34:25-56. [DOI] [PubMed] [Google Scholar]
- 43.Rana, B., I. Butcher, P. Grigoris, C. Murnaghan, R. A. Seaton, and C. M. Tobin. 2002. Linezolid penetration into osteo-articular tissues. J. Antimicrob. Chemother. 50:747-750. [DOI] [PubMed] [Google Scholar]
- 44.Rayner, C. R., A. Forrest, A. K. Meagher, M. C. Birmingham, and J. J. Schentag. 2003. Clinical pharmacodynamics of linezolid in seriously ill patients treated in a compassionate use programme. Clin. Pharmacokinet. 42:1411-1423. [DOI] [PubMed] [Google Scholar]
- 45.Ryan, D. M. 1993. Pharmacokinetics of antibiotics in natural and experimental superficial compartments in animals and humans. J. Antimicrob. Chemother. 31(Suppl. D):1-16. [DOI] [PubMed] [Google Scholar]
- 46.Ryan, D. M., O. Cars, and B. Hoffstedt. 1986. The use of antibiotic serum levels to predict concentrations in tissues. Scand. J. Infect. Dis. 18:381-388. [DOI] [PubMed] [Google Scholar]
- 47.Slatter, J. G., D. J. Stalker, K. L. Feenstra, I. R. Welshman, J. B. Bruss, J. P. Sams, M. G. Johnson, P. E. Sanders, M. J. Hauer, P. E. Fagerness, R. P. Stryd, G. W. Peng, and E. M. Shobe. 2001. Pharmacokinetics, metabolism, and excretion of linezolid following an oral dose of [14C]linezolid to healthy human subjects. Drug Metab. Dispos. 29:1136-1145. [PubMed] [Google Scholar]
- 48.Stahle, L., P. Arner, and U. Ungerstedt. 1991. Drug distribution studies with microdialysis. III: extracellular concentration of caffeine in adipose tissue in man. Life Sci. 49:1853-1858. [DOI] [PubMed] [Google Scholar]
- 49.Stalker, D. J., G. L. Jungbluth, N. K. Hopkins, and D. H. Batts. 2003. Pharmacokinetics and tolerance of single- and multiple-dose oral or intravenous linezolid, an oxazolidinone antibiotic, in healthy volunteers. J. Antimicrob. Chemother. 51:1239-1246. [DOI] [PubMed] [Google Scholar]
- 50.Tegeder, I., A. Schmidtko, L. Brautigam, A. Kirschbaum, G. Geisslinger, and J. Lotsch. 2002. Tissue distribution of imipenem in critically ill patients. Clin. Pharmacol. Ther. 71:325-333. [DOI] [PubMed] [Google Scholar]
- 51.Tunblad, K., M. Hammarlund-Udenaes, and E. N. Jonsson. 2004. An integrated model for the analysis of pharmacokinetic data from microdialysis experiments. Pharm. Res. 21:1698-1707. [DOI] [PubMed] [Google Scholar]
- 52.Verdant, C., and D. De Backer. 2005. How monitoring of the microcirculation may help us at the bedside. Curr. Opin. Crit. Care 11:240-244. [DOI] [PubMed] [Google Scholar]
- 53.Whitehouse, T., J. A. Cepeda, R. Shulman, L. Aarons, R. Nalda-Molina, C. Tobin, A. MacGowan, S. Shaw, C. Kibbler, M. Singer, and A. P. R. Wilson. 2005. Pharmacokinetic studies of linezolid and teicoplanin in the critically ill. J. Antimicrob. Chemother. 55:333-340. [DOI] [PubMed] [Google Scholar]
- 54.World Medical Association. 1996. Declaration of Helsinki: ethical principles for medical research involving human subjects. [Online.] http://www.wma.net/e/policy/pdf/17c.pdf. [PubMed]