Abstract
Thrombin-induced platelet microbial protein 1 (tPMP-1), a cationic antimicrobial polypeptide released from thrombin-stimulated rabbit platelets, targets the Staphylococcus aureus cytoplasmic membrane to initiate its microbicidal effects. In vitro resistance to tPMP-1 correlates with survival advantages in vivo. In S. aureus, the plasmid-carried qacA gene encodes a multidrug transporter, conferring resistance to organic cations (e.g., ethidium [Et]) via proton motive force (PMF)-energized export. We previously showed that qacA also confers a tPMP-1-resistant (tPMP-1r) phenotype in vitro. The current study evaluated whether (i) transporters encoded by the qacB and qacC multidrug resistance genes also confer tPMP-1r and (ii) tPMP-1r mediated by qacA is dependent on efflux pump activity. In contrast to tPMP-1r qacA-bearing strains, the parental strain and its isogenic qacB- and qacC-containing strains were tPMP-1 susceptible (tPMP-1s). Efflux pump inhibition by cyanide m-chlorophenylhydrazone abrogated Etr, but not tPMP-1r, in the qacA-bearing strain. In synergy assays, exposure of the qacA-bearing strain to tPMP-1 did not affect the susceptibility of Et (ruling out Et-tPMP-1 cotransport). The following cytoplasmic membrane parameters did not differ significantly between the qacA-bearing and parental strains: contents of the major phospholipids; asymmetric distributions of the positively charged species, lysyl-phosphotidylglycerol; fatty acid composition; and relative surface charge. Of note, the qacA-bearing strain exhibited greater membrane fluidity than that of the parental, qacB-, or qacC-bearing strain. In conclusion, among these families of efflux pumps, only the multidrug transporter encoded by qacA conferred a tPMP-1r phenotype. These data suggest that qacA-encoded tPMP-1r results from the impact of a specific transporter upon membrane structure or function unrelated to PMF-dependent peptide efflux.
Mammalian platelets appear to play a critical role in the innate host defense against the induction and progression of endovascular infections (6, 7, 22, 39, 43). This host defense property relates to the capacity of platelets to release a group of cationic antimicrobial peptides (CAMPs) collectively known as platelet microbicidal proteins (PMPs) at sites of endovascular damage or infection (39, 43). Both rabbit and human platelets release functionally and structurally similar PMPs (37, 45) with microbicidal activities in vitro against pathogens that commonly invade the bloodstream, including Staphylococcus aureus (11, 12, 39). To date, the best-characterized PMPs are those released from platelets following stimulation by the procoagulant molecule thrombin (i.e., thrombin-induced PMPs [tPMPs]) (11, 19, 20, 40, 45, 46). In humans and animals with catheter-induced S. aureus bacteremia, strains which are intrinsically susceptible to tPMPs (tPMPs) in vitro are less likely to cause complicated endovascular infections, such as endocarditis (11, 12).
Previous studies indicated that tPMPs target and permeabilize the S. aureus cytoplasmic membrane (CM) to initiate their microbicidal pathway (20, 44). Intracellular targets affecting macromolecular synthesis and autolytic enzyme pathways have also been implicated in the staphylocidal mechanisms of tPMPs (40, 42). However, the precise adaptations by which S. aureus strains resist the microbicidal activities of tPMPs remain incompletely defined but appear to include enhanced membrane fluidity (3); reduced transmembrane potential (Δψ) (17), and mutations of genes involved in generating the proton motive force (PMF) (2). In addition, we recently demonstrated that carriage of plasmids bearing genes encoding the QacA PMF-dependent transmembrane efflux pump specific for cationic compounds was associated with low-level tPMP resistance (tPMPr) in vitro (21).
The current study was designed to further characterize the mechanism(s) of tPMPr among strains bearing distinct multidrug efflux pumps, especially to delineate whether (i) the active export of the cationic tPMP was responsible for this phenotype and (ii) other transmembrane efflux pumps (i.e., qacB and qacC) also conferred tPMPr in vitro.
(This study was presented in part at the 40th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Ontario, Canada, 17 to 20 September 2000 [L. I. Kupferwasser, R. A. Skurray, N. Firth, M. H. Brown, M. R. Yeaman, R. Prasad, M. Smriti, and A. S. Bayer, abstr. 2288].)
MATERIALS AND METHODS
Bacterial strains.
Table 1 lists the bacterial strains and plasmids used in this investigation. The parental staphylococcal strain SK982 is plasmid-free and tPMP-1s in vitro (21). Plasmids carrying either the qacA, qacB, or qacC multidrug resistance determinants were transferred into SK982 by mixed-culture transfer as previously described (21). The multidrug transporter-bearing plasmids studied are members of different staphylococcal plasmid families, including pSK1 from the pSK1 family; pSK89, a rolling-circle member; pSK108 from the pSK639 family; and pSK21, pSK23, pSK84, and pSK156, which are β-lactamase heavy-metal resistance plasmids (23, 24, 30). pSK5754 is a derivative of pSK1 in which the qacA gene was replaced with a chloramphenicol resistance (cat) determinant (14). The Escherichia coli-S. aureus shuttle vector pSK5640 is a derivative of pSK5630 (13) in which the pUC19 lacZα-MCS fragment has been inserted into the AatII and PvuII sites. The constructs pSK5672 and pSK5691 are pSK5640-based plasmids containing a 3.5-kb qacR-qacA BamHI-HindIII fragment and a 1.7-kb qacA BamHI fragment from pSK1, respectively. All strains were kept frozen at −70°C until thawed and subcultured prior to use.
TABLE 1.
Bacterial strains and plasmids
| Strain | Background | Plasmid | Transporter | Reference(s) |
|---|---|---|---|---|
| SK982 | SK982 | 21 | ||
| SK2355 | SK982 | pSK1 | QacA | 21 |
| SK2416 | SK982 | pSK108 | QacC | 23 |
| SK2721 | SK982 | pSK89 | QacC | 24 |
| SK2725 | SK982 | pSK21 | QacB | 25 |
| SK2727 | SK982 | pSK23 | QacB | 30 |
| SK2718 | SK982 | pSK84 | QacB | 25 |
| SK2719 | SK982 | pSK156 | QacB | 25, 30 |
| SK5791 | SK982 | pSK5754 | 14 | |
| SK5871 | SK982 | pSK5640 | This study | |
| SK5872 | SK982 | pSK5672 | QacA | This study |
| SK5873 | SK982 | pSK5691 | QacA | This study |
Preparation of tPMP-1.
We focused these studies on tPMP-1 since it is the best studied and the most abundant of the tPMPs in rabbit platelets. Supernatants from thrombin-stimulated rabbit platelets were prepared and tPMP-1 was subsequently isolated as previously described (45). The bioactivity of tPMP-1 preparations was validated as previously published using a microbiologic assay with Bacillus subtilis ATCC 6633 as the test organism (37, 45). Bioactive tPMP-1 was kept frozen at −70°C until thawed for use.
tPMP-1 susceptibility assay.
Susceptibility profiles of the study constructs to tPMP-1 were determined by a time-kill assay as previously described (39). Overnight cultures of the S. aureus isolates were washed twice and suspended in phosphate-buffered saline. Dilutions of tPMP-1 and S. aureus isolates were added to polypropylene microculture tubes containing Eagle's minimal essential medium (MEM; Irvine Scientific, Santa Ana, CA) to achieve a final tPMP-1 concentration range of 2 to 12 μg/ml and a final bacterial inoculum of 103 CFU/ml (i.e., the standard inoculum used for tests of this peptide against S. aureus [39]). For determination of the percentages of survival, one tube contained only bacteria and MEM and acted as a positive growth control. After 2 h of incubation at 37°C, suspensions were mixed by vortexing, and 15-μl aliquots were removed from each tube and quantitatively cultured onto blood agar plates. The proportion of S. aureus cells of each construct that survived a 2-h exposure to tPMP-1 was expressed as a percentage of the number of CFU of the positive growth control. All assays were performed in triplicate on two separate days, and the mean percentage of survival ± the standard deviation (SD) was determined. A tPMP-1r phenotype was defined as ≥50% survival of the initial inoculum after a 2-h exposure to tPMP-1 at 37°C. This arbitrary in vitro breakpoint correlates with an enhanced capacity of such strains to induce and propagate human or experimental endovascular infections in vivo (11, 22, 39). Data represent the means (±SD) of three independent experiments.
Effect of QacA efflux pump inhibition on S. aureus tPMP-1 resistance.
The qacA-bearing strain SK2355 was exposed either to phosphate-buffered saline (control) or to 50 μM cyanide m-chlorophenylhydrazone (CCCP) for 20 min at 37°C. An additional cell population was exposed to CCCP as described above, in combination with 20 mM glucose for 10 min to counteract the CCCP effects on the PMF. After these exposures, the MICs for Et under these various conditions were determined at a final bacterial inoculum of 106 CFU and a final range of Et concentrations from 6.25 to 150 μg/ml in a microtiter assay. The MIC was defined as the lowest concentration of Et at which no visible growth was observed following a 24-h incubation period at 37°C. Data represent the means (±SD) of three independent experiments. In a parallel study, SK2355 cells (103 CFU final inoculum) were similarly prepared in the presence or absence of 50 μM CCCP with or without 20 mM glucose and then exposed to 2 μg/ml tPMP-1 for 2 h in MEM at 37°C. After tPMP-1 exposures, the percentages of survival were determined under the different exposure conditions. Since relative tPMP-1r can be induced by the PMF-lowering actions of CCCP against selected tPMP-1s strains (2), studies were conducted to distinguish this potential effect from that of CCCP-induced efflux inhibition in strain SK2355. Thus, we evaluated the tPMP-1 susceptibility profile of parental strain SK982 in the presence or absence of CCCP (12.5 to 100 mM) using the same assay as outlined above.
Impact of QacA substrate-tPMP-1 coincubations on antistaphylococcal activity.
Fractional inhibitory concentration (FIC) analysis was carried out in microtiter plates using 103 CFU of SK2355 cells with combinations of two QacA substrates, Et and chlorhexidine (Chlx), or tPMP-1. Increasing increments of each compound (3.125 μg/ml to 250 μg/ml for Et or Chlx and 1 μg/ml to 12 μg/ml for tPMP-1) were combined using the checkerboard procedure (30). Microtiter plates were incubated for 48 h at 37°C. The Et, Chlx, and tPMP-1 MICs were determined as described above. The FIC index was calculated as x/MICx + y/MICy, where x and y represent the lowest concentration of each compound (in combination) at which there is no growth and MICx and MICy represent the individual MIC of each compound. FIC indices have been defined as being indicative of the following: ≤0.50, synergy; 0.51 to 2.00, additivity; 2.01 to 4.00, indifference; and >4, antagonism (10, 27, 32). Data represent means (±SD) from three independent experiments.
FA analyses.
Fatty acids (FAs) from the total amount of lipids extracted from the S. aureus constructs were analyzed using gas liquid chromatography after methyl esterification (courtesy of Microbial ID, Inc., Newark, DE). In brief, 40 mg of bacterial cells was collected and saponified in boiling water with 1 ml of 50% alkaline methanol (15% NaOH) for 30 min. The tubes were cooled, and 2 ml of 45% methanol containing 3 N HCl was added for methylation. The FA-methyl esters were extracted using a 1:1 mixture of hexane and methyl tert-butyl ether. The aqueous (lower) phase was discarded, and the organic phase (containing FAs) was washed with 0.3 N NaOH.
The FA-methyl ester mixtures were separated by ionization analyses (Sherlock microbial identification system; Microbial ID, Newark, DE). A 25-mm by 0.2-mm phenyl methyl silicone-fused-silica capillary column with a flame ionization detector and autosampler was used for detection. Each FA-methyl ester composition was compared to database standards (straight-chained saturated fatty acids from 9 to 20 carbons in length and five hydroxy acids) using Sherlock pattern recognition software (Microbial ID, Newark, DE). The unsaturation profiles as well as the relative compositions of iso- versus anteiso-branched-chain fatty acids were calculated. All assays were performed a minimum of two times on separate days.
PL analyses. (i) Extraction, identification, and quantification of PLs.
Phospholipids (PLs) were extracted from S. aureus by standard methods (8). Briefly, cells were grown for 18 h in a brain heart infusion broth, harvested by centrifugation (3,000 × g, 4°C, 15 min), and washed in buffer A (100 mM potassium phosphate, 5 mM EDTA, pH 7.2) and then in buffer B (100 mM potassium phosphate, 600 mM potassium chloride, pH 8.2). Cells (0.6 g [wet weight], ∼1 × 108 cells/ml) were suspended in 3 ml of buffer B and transferred to a 25-ml glass conical flask and cooled to 4°C with gentle swirling. The suspension was centrifuged and the pellet washed with buffer C (200 mM potassium acetate, 600 mM potassium chloride, pH 4.5) at 4°C.
The cell pellet was then extracted using 2:1 (vol/vol) chloroform-methanol, followed by washing with 0.9% NaCl to remove nonlipid contaminants (21). The extracted organic layer was evaporated to dryness under nitrogen and stored at −20°C until analysis. The major PL species, phosphotidylglycerol (PG), cardiolipin (CL), and lysyl-phosphotidylglycerol (LPG), were separated by two-dimensional thin-layer chromatography (2D-TLC) using Silica Gel 60 F254 high-performance TLC plates (Merck, Darmstadt, Germany) and subsequently developed with chloroform-methanol-25% ammonium hydroxide (65:25:6, by volume) in the vertical orientation and chloroform-acetone-acetic acid-methanol-water (45:16:9:8:4, by volume) in the horizontal orientation, as detailed previously (9, 15). LPG (positively charged) was identified by ninhydrin staining (33). PG, CL, and other minor PLs were visualized by exposure of the TLC plate to iodine vapor. All PLs were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL) and used as internal standards to determine the positions of their spots on 2D-TLC plates.
For quantitative analysis, isolated PLs were individually recovered from TLC plates and digested at 180°C for 3 h with 0.3 ml of 70% perchloric acid. The digested samples were incubated with a colorimetric reagent (10% ascorbic acid, 2.5% ammonium molybdate, 5% perchloric acid [1:1:8, vol/vol/vol]) for 2 h at 37°C and quantified spectrophotometrically at an optical density at 660 nm (OD660). The content of each PL species was expressed as a percentage of the total PL content. All assays were performed a minimum of five times on separate days.
(ii) LPG distribution.
Fluorescamine labeling was carried out by a modification of the 2D-TLC protocol described above. After they were harvested and washed with buffers A and B as described above, cells (0.6 g [wet weight]) were suspended in 3 ml of buffer B (∼1 × 108 cells/ml), transferred to a 25-ml glass conical flask, and cooled to 4°C with gentle swirling. To the cell pellet, 90 μl of fluorescamine solution (0.52 M) in dehydrated dimethyl sulfoxide was added dropwise, with constant swirling for 30 s. The reaction was stopped by the addition of 3 ml of 1 M ammonia in 600 mM potassium chloride. The suspension was centrifuged (3,000 × g), the pellet was washed four times at 4°C with buffer C until the supernatant was free of color, and then it was subjected to 2D-TLC as described above. Fluorescamine labeling of outer-leaflet LPG was detected by using a UV detector (365-nm excitation). Of note, once bound to LPG, fluorescamine alters its mobility characteristics and attenuates its ability to be detected by ninhydrin staining (18). After detection of the fluorescamine-labeled LPG, its relative content (normalized with respect to total PL and to total LPG) was quantified by the colorimetric assay described above. All assays were performed a minimum of five times on separate days.
CM fluidity measurement.
CM fluidity was determined by fluorescence polarization using the fluorescent probe 1,6-diphenyl-1,3,5-hexatriene (DPH) as described before (3). The protocol for DPH incorporation into target CMs, measurement of fluorescence polarization, and calculation of the degree of fluorescence polarization index (p.i.) (Biotek model SFM 25 spectrofluorometer; excitation and emission wavelengths of 360 and 426 nm, respectively) is described in detail elsewhere (3). The lower the p.i. value, the higher the degree of membrane fluidity (3). Data represent the means (±SD) from three independent experiments.
Cytochrome c binding.
We performed the cytochrome c binding assay according to a previously described method (34). In brief, S. aureus cells were grown overnight and then washed twice with 20 mM MOPS buffer (morpholinepropanesulfonic acid, pH 7). The cells were suspended in the same buffer to a final OD578 of 7. The suspension was incubated with 0.5 mg/ml cytochrome c (95% purity; Sigma Chemicals, St. Louis, MO) for 10 min in the same buffer and then centrifuged. The amount of cytochrome c in the supernatant was determined spectrophotometrically at an OD530 and subtracted from the original cytochrome c content in solution before bacterial exposure to calculate the percentage of cytochrome c bound to the cell pellet for each strain. The lower the percentage of cytochrome c bound to the S. aureus pellet, the more positively charged the S. aureus cell envelope (34). Data represent the means (±SD) from three independent experiments.
Statistics.
Continuous data were analyzed by Kruskal-Wallis analysis of variance, with corrections for multiple comparisons where appropriate. A P value of ≤0.05 was considered significant.
RESULTS
tPMP-1 susceptibility phenotypes.
Table 2 depicts the susceptibility profiles of the study strains to tPMP-1 across a concentration range of 2 to 12 μg/ml. Based on the arbitrary breakpoint of 50% survival at 2-h exposures, the qacA-bearing strain SK2355 exhibited low-level resistance over the 2- to 4-μg/ml range of tPMP-1. In contrast, the qacB- and qacC-bearing strains were tPMP-1s across the entire concentration range, paralleling data for the parental strain, SK982 (Table 2). These results, irrespective of the plasmid type carrying the multidrug efflux genes, implied that the differences observed in tPMP resistance were due solely to the efflux protein. Thus, one representative strain for each transporter type was used in subsequent detailed studies (SK2355 qacA, SK2725 qacB, and SK2416 qacC).
TABLE 2.
tPMP-1 susceptibility phenotypes of the study strains
| Concn of tPMP-1 (μg/ml) | % Survival of inoculum (mean ± SD)a
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SK982 (parent) | SK2355 (pSK1) (qacA) | SK2416 (pSK108) (qacC) | SK2721 (pSK89) (qacC) | SK2725 (pSK21) (qacB) | SK2727 (pSK23) (qacB) | SK2718 (pSK84) (qacB) | SK2719 (pSK156) (qacB) | SK5791(pSK5754) (pSK1, qacA negative and qacR+) | SK5871(pSK5640) (pSK5630, E. coli-S. aureus shuttle vector) | SK5872 (pSK5672) (pSK5640, qacA+qacR) | SK5873 (pSK5691) (pSK5640, qacA only) | |
| 12 | 4 ± 1 | 19 ± 2 | 7 ± 2 | 7 ± 2 | 7 ± 3 | 6 ± 2 | 9 ± 1 | 5 ± 2 | ND | ND | ND | ND |
| 6 | 12 ± 3 | 25 ± 4 | 14 ± 2 | 14 ± 2 | 11 ± 2 | 15 ± 3 | 10 ± 3 | 16 ± 5 | ND | ND | ND | ND |
| 4 | 17 ± 2 | 56 ± 3 | 17 ± 3 | 18 ± 4 | 16 ± 3 | 14 ± 2 | 16 ± 1 | 21 ± 2 | ND | ND | ND | ND |
| 3 | 18 ± 2 | 61 ± 4 | 24 ± 2 | 22 ± 2 | 17 ± 2 | 21 ± 4 | 22 ± 3 | 25 ± 4 | ND | ND | ND | ND |
| 2 | 25 ± 4 | 84 ± 4 | 22 ± 1 | 28 ± 3 | 28 ± 5 | 28 ± 3 | 30 ± 5 | 25 ± 4 | 20 ± 0.16 | 27 ± 7 | 47 ± 0.7 | 49 ± 7 |
ND, not done.
Previous studies using pSK1 and deletion derivatives established that the presence of qacA was sufficient to confer a tPMP-1r phenotype (3, 21). However, qacR, which encodes the multidrug-binding transcriptional regulator of qacA (5, 14), was also present on these plasmids. Therefore, to determine if qacR directly contributes to tPMP-1r, we constructed pSK5754, a pSK1 derivative, in which qacA was replaced with a chloramphenicol resistance gene. This plasmid lacked qacA but still possessed a fully intact qacR determinant (and all other determinants of pSK1) and did not confer tPMP-1r (tested only at 2 μg/ml) (Table 2). Additionally, the qacA-negative plasmid vector-bearing strain SK5871 was tPMP-1s, whereas the qacA-qacR plasmid-bearing construct (SK5872) and the qacA-alone plasmid-bearing construct (SK5873) each had approximately twofold-increased tPMP-1 resistance. These data support the notion that this is a qacA-specific effect and that the presence or absence of qacR has no bearing on the observed tPMP-1 resistance phenotype.
Effect of QacA efflux pump inhibition on S. aureus Et and tPMP-1 susceptibility phenotypes.
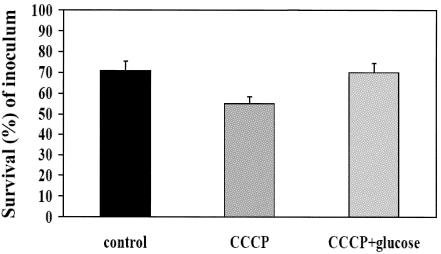
The QacA membrane protein uses the PMF to drive substrate export and is inhibitable by the membrane energy uncoupler CCCP (28). Figure 1 depicts the impact of CCCP (in the presence or absence of glucose) on the Et susceptibility profiles of the qacA-bearing strain SK2355. As noted, preexposure to CCCP reduced the Et MIC by approximately fourfold (P < 0.05 versus the MIC for non-CCCP-treated cells), an effect that was reversed by coexposures to both CCCP and glucose (Fig. 1). In contrast, CCCP preexposures had only modest impacts on the tPMP-1r phenotype of the strain SK2355 (Fig. 2). In addition, exposing the plasmid-free parental control strain SK982 to CCCP (12.5 to 100 mM) caused no changes in its tPMP-1s profile (data not shown).
FIG. 1.
Effects of efflux pump inhibition by CCCP upon the Et MICs (±SD) and potential reversal by coincubation with glucose in SK2355. See the text for details.
FIG. 2.
Effect of efflux pump inhibition by CCCP on tPMP-1 susceptibility in SK2355 (percentage of survival ± SD after 2 h of incubation) and potential reversal by glucose coincubation. See the text for details.
Impact of QacA substrate-tPMP-1 coincubations on net antimicrobial activity.
Simultaneous exposure of qacA-bearing strains to two or more substrates microbicidal for the efflux system should saturate this pump and lead to a reduced efflux of the individual substrate molecules. This, in turn, should lead to enhanced inhibition of growth of the organism. This effect has been confirmed for a number of QacA-substrate combinations (27). To test whether Et and tPMP-1 are cotransported by QacA, we performed an FIC analysis using combinations of the two QacA substrates, Et and Chlx, or tPMP-1. The coincubation of Et and Chlx reduced the MICs for each compound by approximately fourfold compared to those for each compound alone (Fig. 3a) (FIC index, 1; P < 0.05). This interaction was interpreted as additive. In contrast, the coincubation of Et with tPMP-1 had little effect on the antistaphylococcal activity of either agent alone (Fig. 3b) (FIC index, 2 [indifference]).
FIG. 3.
Et-Chlx and Et-tPMP-1 checkerboard synergy assays with SK2355. Impact of Et and Chlx alone and in combination (comb) (a) and of Et and tPMP-1 alone and in combination (b) on the single-agent MICs. See the text for details.
Fatty acid analyses.
There were no substantive differences observed in total fatty acid contents, unsaturation distributions, iso- or anteiso-branched-chain fatty acid contents, or ratios between the study strains (data not shown).
Phospholipid analyses.
Fluorescamine, a fluorescent probe which specifically labels CM surface-exposed (outer-leaflet) positively charged amino PLs was used to assay the CM LPG distribution (2, 15). For both the parental SK982 and qacA-bearing SK2355 strains, PG was the predominant CM phospholipid (∼67% for each strain). This mirrors data generated from our laboratory using other genetic background strains of S. aureus (41). There were no substantial differences in the relative proportions among the three major membrane phospholipids, PG, CL (∼7% for each strain), and LPG (∼23% for each strain), in a comparison of the parental strain, SK982, with qacA-bearing SK2355. Moreover, the distributions of LPG within the outer versus the inner CM leaflets did not differ between strains (∼5 to 6% of the total LPG was distributed to the outer CM leaflet).
CM fluidity measurements.
DPH is a probe that localizes in the hydrophobic core of the lipid bilayer and is highly fluorescent when bound therein. Fluorescence polarization studies using DPH revealed that the CMs of the qacA-bearing strain SK2355 (p.i., 0.293 ± 0.01) were more fluid than those of the parental strain (p.i., 0.31 ± 0.13) and those of the strains carrying qacB- and qacC-containing plasmids (p.i., 0.315 ± 0.02 and 0.308 ± 0.01, respectively).
Cytochrome c binding.
Cytochrome c is a cationic protein derived from equine heart. It has been used previously to estimate the relative surface charge of the cell envelope of isogenic S. aureus strain pairs (34). Cytochrome c binding assays showed that there were no differences in levels of binding to the cell surface of the parental strain SK9892, SK2725, or the tPMP-1r strain SK2355 (28% ± 3%, 31% ± 14%, and 30% ± 4%, respectively). Although the binding of cytochrome c to the qacC-bearing strain SK2416 was somewhat higher than that of the other three constructs (46% ± 14%), this difference did not reach statistical significance.
DISCUSSION
We have previously shown that the capacity of S. aureus strains to acquire low-level resistance to tPMP-1 enables the organism to persist in both experimental infections and human endovascular infections (1, 6, 7, 11). Five mechanisms have been described which are associated with reduced in vitro susceptibility to tPMPs in S. aureus. (i) Small-colony variants, either gentamicin-induced or created by the mutagenesis of respiratory chain components (e.g., the hemB or menD locus) (19), maintain a substantially lowered Δψ than parental strains due to such electron transport chain defects (19, 20). (ii) Variants selected following serial passages of tPMP-1r S. aureus strains in this peptide (43), like small-colony variants, exhibit a diminished Δψ (A. S. Bayer, M. R. Yeaman, H.-G. Sahl, D. Brar, and R. A. Proctor, Abstr. 97th Gen. Meet. Am. Soc. Microbiol., abstr. A-106, p. 19, 1997). (iii) Strains with transposon disruptions in the complex I enzyme function result in the defective generation of the Δψ. We have recently shown that mutations within the snoA-snoG operon (which encodes an NADH oxidoreductase) render such strains relatively tPMP-1r (2). Importantly for CAMPs, a threshold Δψ appears to be critical for their interactions with target microbial membranes. Thus, the diminished Δψ observed in these three strain types likely contributes to their tPMP-1r phenotype. (iv) Strains showing relative increases in cell surface positive charge, as demonstrated by Peschel et al. (33, 34), have cell membranes and cell walls that contain high proportions of positively charged phospholipids and teichoic acids species, respectively. This renders such strains relatively resistant to killing by a number of CAMPs, including tPMP-1. Lysinylation of phosphotidylglycerol and d-alanylation of teichoic acid appear to be responsible for this phenotype. These data suggest that the charge-based surface repulsion of CAMPs by a less negatively charged surface envelope could contribute to CAMP resistance (33, 34). (v) We also recently showed that S. aureus strains carrying plasmid determinants (e.g., pSK1) encoding the PMF-dependent, multidrug export pump QacA of the major facilitator superfamily of transport proteins also exhibit reduced susceptibility to tPMP-1 in vitro, compared to that of the isogenic parental or a qacA mutant construct (21). The current study was designed to further investigate the mechanisms by which qacA carriage confers relative tPMP-1 resistance and whether this phenotype is specific for qacA or is a general mechanism conferred by the presence of other multidrug exporter proteins.
Several interesting observations emanated from this study. First, among the three multidrug export proteins QacA, QacB, and QacC, only QacA was associated with low-level tPMP-1 resistance at multiple peptide concentrations. These three multidrug export proteins all confer resistance to monovalent cationic chemicals, for example Et. In addition, QacA confers resistance to bivalent cationic compounds, such as Chlx (24, 27). In fact, studies have shown that QacA mediates resistance to more than 30 cationic, lipophilic antimicrobials belonging to 12 distinct chemical families, although no resistance to trivalent cations or to anionic compounds has been observed (5, 27, 28). QacC is a small-membrane protein that belongs to a separate family of transport proteins, the small, multidrug resistance family (31), and shares no structural homology to QacA or QacB. In contrast, the QacA and QacB export proteins are highly related; there are only seven amino acid differences between them, and one of these differences, at position 323, is responsible for the resistance to bivalent cations demonstrated by QacA but not QacB (29). Second, resistance to CAMPs via active efflux has been described to exist in a number of bacteria. For example, in Yersinia enterocolitica, the Ros system, composed of RosA and RosB, a major facilitator superfamily multidrug export protein and a potassium efflux protein, respectively, mediates resistance to CAMPs using an energy-dependent process (4). Similarly, in Neisseria spp., Shafer et al. and Tzeng et al. have demonstrated the presence of the PMF-driven Mtr multidrug efflux system which modulates susceptibility to a range of CAMPs (36, 38). However, despite the fact that tPMP-1 is a lysine-rich cationic peptide (net charge, +5 [46]), making it a potential candidate for export by QacA, two lines of evidence reported here are against this hypothesis. (i) Interference with QacA pump function via the PMF inhibitor CCCP did not significantly impact the tPMP-1 susceptibility phenotype and (ii) coincubation of Et and tPMP-1 in the qacA-bearing strain did not substantially affect the in vitro activity of either agent, suggesting that QacA-mediated cotransport of these two antimicrobials did not occur. Furthermore, CCCP might induce a relative tPMP-1r phenotype by its nonspecific reduction in the PMF, which might be compensated for by a putative QacA-mediated tPMP-1 efflux in strain SK2355. Therefore, we evaluated the impacts of CCCP on the parental strain's (SK982) tPMP-1 susceptibility profile. The finding that CCCP did not affect the tPMP-1 susceptibility of strain SK982 strongly suggests that the QacA-associated tPMP-1r phenotype observed in SK2355 is not attributable to a combined specific efflux and a nonspecific PMF reduction.
As the cytoplasmic membrane is a principal target for initiating the staphylocidal pathway of tPMP-1, we carried out several analyses of the membrane structure and function in a comparison of the parental strain with strains expressing the three different multidrug export pumps. Only the qacA-bearing strain exhibited an increase in membrane fluidity compared to that of the other members of this isogenic strain set. Of note, enhanced membrane fluidity has been a consistent feature of several other tPMP-1r S. aureus strains, including the above-mentioned transposon and serial-passage variants. We recently studied the potential biophysical mechanisms of increased membrane fluidity in the transposon-derived sno mutant of S. aureus (K. Mukhopadhyay, W. Whitmire, J. Molden, Y. Q. Xiong, A. Peschel, P. McNamara, R. A. Procotor, J. Adler-Moore, M. R. Yeaman, and A. S. Bayer, Abstr. 105th Gen. Meet. Am. Soc. Microbiol., abstr. A-002, p. 1, 2005). In this construct, the basis for enhanced fluidity was related to an increased proportion of shorter acyl chain length fatty acids and a shift in branched-chain fatty acids from iso-branched to anteiso-branched species (16). Of particular importance, more-fluid membranes tend to leak protons, with a resulting decrease in Δψ (17). Since a threshold Δψ appears to be required for the optimal microbicidal efficacy of tPMP-1 (20, 44), this is one potential explanation for the linkage between enhanced membrane fluidity and relative tPMP-1 resistance. In addition, extremes of CM fluidity or rigidity are likely to impact the interactions of CAMPs with the target membrane (26, 41). It should be emphasized that relatively small changes in CM characteristics (such as fluidity) have a major impact upon interactions with CAMPs (35). Of note, we could not detect any substantial differences in membrane fatty acid composition in the current stain set.
An additional mechanism by which CM structure may influence tPMP-1 susceptibility profiles relates to its PL content and distribution (17). Several laboratories have shown that the overall content of the unique positively charged membrane phospholipid species, LPG, may substantially affect the overall surface charge of the organism. Peschel et al. (33, 34) have recently shown that knockouts of the genes responsible for the lysinylation of PG (mprF) or the d-alanylation of cell wall teichoic acid (dltA) render the surface envelope of such constructs more negatively charged. This enables a number of CAMPs to bind to the surfaces of these strains more avidly, making them more peptide susceptible in vitro. In recent studies, we confirmed that the transposon-derived tPMP-1r snoD mutant displayed a significantly increased proportion of outer-leaflet-localized LPG compared to that of its tPMP-1s parental strain. These characteristics provided the mutant with an enhanced repulsive cell surface toward the cationic molecule, cytochrome c, and perhaps tPMP-1. Similar analyses in the current study could not confirm either the increased overall LPG content or the enhanced outer-leaflet LPG asymmetry for the qacA-bearing construct.
In summary, our investigations delineate a strong correlation between the presence of the QacA transmembrane efflux pump and a tPMP-1r phenotype that is not explicable by the transport of this cationic peptide. Ongoing studies in our laboratories are focusing upon (i) analyses of tPMP-1 binding among these distinct constructs and (ii) investigations to define the mechanism(s) of enhanced membrane fluidity in tPMP-1r strains in the context of qacA carriage.
Acknowledgments
This research was supported by grants from the National Institutes of Health to A.S.B. (AI-39108) and M.R.Y. (AI-48031 and RR-13004) and from the National Health and Medical Research Council (Australia) to M.H.B. and R.A.S. (301938). T. J. was supported in part by the Harbor-UCLA Initiative for Minority Student Development program (NIH1R25GM56902) funded by the National Institute of General Medical Science.
REFERENCES
- 1.Bayer, A. S., D. Cheng, M. R. Yeaman, G. R. Corey, R. S. McClelland, L. J. Harrel, and V. G. Fowler, Jr. 1998. In vitro resistance to thrombin-induced platelet microbicidal protein among clinical bacteremic isolates of Staphylococcus aureus correlates with an endovascular infectious source. Antimicrob. Agents Chemother. 42:3169-3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayer, A. S., P. McNamara, M. R. Yeaman, N. Lucindo, T. Jones, A. L. Cheung, H.-G. Sahl, and R. A. Proctor. 2006. Transposon disruption of the complex I NADH oxidoreductase gene (snoD) in Staphylococcus aureus is associated with reduced susceptibility to the microbicidal activity of thrombin-induced platelet microbicidal protein 1. J. Bacteriol. 188:211-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bayer, A. S., R. Prasad, J. Chandra, A. Koul, M. Smriti, A. Varma, R. A. Skurray, N. Firth, M. H. Brown, S.-P. Koo, and M. R. Yeaman. 2000. In vitro resistance of Staphylococcus aureus to thrombin-induced platelet microbicidal protein is associated with alterations in cytoplasmic membrane fluidity. Infect. Immun. 68:3548-3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bengoechea, J. A., and M. Skurnik. 2000. Temperature-regulated efflux pump/potassium antiporter system mediates resistance to cationic antimicrobial peptides in Yersinia. Mol. Microbiol. 37:67-80. [DOI] [PubMed] [Google Scholar]
- 5.Brown, M. H., and R. A. Skurray. 2001. Staphylococcal multidrug efflux protein QacA. J. Mol. Microbiol. Biotechnol. 3:163-170. [PubMed] [Google Scholar]
- 6.Dhawan, V. K., A. S. Bayer, and M. R. Yeaman. 1998. In vitro resistance to thrombin-induced platelet microbicidal protein is associated with enhanced progression and hematogenous dissemination in experimental Staphylococcus aureus infective endocarditis. Infect. Immun. 66:3476-3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhawan, V. K., M. R. Yeaman, A. L. Cheung, E. Kim, P. M. Sullam, and A. S. Bayer. 1997. Phenotypic resistance to thrombin-induced platelet microbicidal protein in vitro is correlated with enhanced virulence in experimental endocarditis due to Staphylococcus aureus. Infect. Immun. 65:3293-3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dixit, B. L., and C. M. Gupta. 1998. Role of the actin cytoskeleton in regulating the outer phosphatidylethanolamine levels in yeast plasma membrane. Eur. J. Biochem. 254:202-206. [DOI] [PubMed] [Google Scholar]
- 9.Dogra, S., S. Krishnamurthy, V. Gupta, B. L. Dixit, C. M. Gupta, D. Sanglard, and R. Prasad. 1999. Asymmetric distribution of phosphatidylethanolamine in C. albicans: possible mediation by CDR1, a multidrug transporter belonging to ATP binding cassette (ABC) superfamily. Yeast 15:111-121. [DOI] [PubMed] [Google Scholar]
- 10.Eliopoulos, G. M., and R. C. Moellering, Jr. 1991. Antimicrobial combinations, p. 432-492. In V. Lorian (ed.), Antibiotics in laboratory medicine, 3rd ed. Williams & Wilkins, Baltimore, Md.
- 11.Fowler, V. G., Jr., L. M. McIntyre, M. R. Yeaman, G. E. Peterson, L. Barth Reller, G. R. Corey, D. Wray, and A. S. Bayer. 2000. In vitro resistance to thrombin-induced platelet microbicidal protein in isolates of Staphylococcus aureus from endocarditis patients correlates with an intravascular device source. J. Infect. Dis. 182:1251-1254. [DOI] [PubMed] [Google Scholar]
- 12.Fowler, V. G., Jr., G. Sakoulas, L. M. McIntyre, V. G. Meka, R. D. Arbeit, C. H. Cabell, M. E. Stryjewski, G. M. Eliopoulos, L. B. Reller, G. R. Corey, T. Jones, N. Lucindo, M. R. Yeaman, and A. S. Bayer. 2004. Persistent bacteremia due to methicillin-resistant Staphylococcus aureus infection is associated with agr dysfunction and low-level in vitro resistance to thrombin-induced platelet microbicidal protein. J. Infect. Dis. 190:1140-1149. [DOI] [PubMed] [Google Scholar]
- 13.Grkovic, S., M. H. Brown, K. M. Hardie, N. Firth, and R. A. Skurray. 2003. Stable low-copy-number Staphylococcus aureus shuttle vectors. Microbiology 149:785-794. [DOI] [PubMed] [Google Scholar]
- 14.Grkovic, S., K. M. Hardie, M. H. Brown, and R. A. Skurray. 2003. Interactions of the QacR multidrug-binding protein with structurally diverse ligands: implications for the evolution of the binding pocket. Biochemistry 42:15226-15236. [DOI] [PubMed] [Google Scholar]
- 15.Ibrahim, A. S., and M. A. Ghannoum. 1996. Chromatographic analysis of lipids, p. 52-79. In R. Prasad (ed.), Manual on membrane lipids. Springer-Verlag, Berlin, Germany.
- 16.Klein, W., M. H. W. Weber, and M. A. Marahiel. 1999. Cold shock response of Bacillus subtilis: isoleucine-dependent switch in the fatty acid branching pattern for membrane adaptation to low temperatures. J. Bacteriol. 181:5341-5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kol, M. A., A. I. de Kroon, J. A. Killian, and B. de Kruijff. 2004. Transbilayer movement of phospholipids in biogenic membranes. Biochemistry 43:2673-2681. [DOI] [PubMed] [Google Scholar]
- 18.Kol, M. A., A. van Dalen, A. I. de Kroon, and B. de Kruijff. 2003. Translocation of phospholipids is facilitated by a subset of membrane-spanning proteins of the bacterial cytoplasmic membrane. J. Biol. Chem. 278:24586-24593. [DOI] [PubMed] [Google Scholar]
- 19.Koo, S.-P., A. S. Bayer, H.-G. Sahl, R. A. Proctor, and M. R. Yeaman. 1996. Staphylocidal action of thrombin-induced platelet microbicidal protein is not solely dependent on transmembrane potential. Infect. Immun. 64:1070-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koo, S.-P., A. S. Bayer, B. L. Kagan, and M. R. Yeaman. 1999. Membrane permeabilization by thrombin-induced platelet microbicidal protein 1 is modulated by transmembrane voltage polarity and magnitude. Infect. Immun. 67:2475-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kupferwasser, L. I., R. A. Skurray, M. H. Brown, N. Firth, M. R. Yeaman, and A. S. Bayer. 1999. Plasmid-mediated resistance to thrombin-induced platelet microbicidal protein in staphylococci: role of the qacA locus. Antimicrob. Agents Chemother. 43:2395-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kupferwasser, L. I., M. R. Yeaman, S. M. Shapiro, C. C. Nast, and A. S. Bayer. 2002. In vitro susceptibility to thrombin-induced platelet microbicidal protein is associated with reduced disease progression and complication rates in experimental Staphylococcus aureus endocarditis: microbiological, histopathologic, and echocardiographic analyses. Circulation 105:746-752. [DOI] [PubMed] [Google Scholar]
- 23.Leelaporn, A., N. Firth, I. T. Paulsen, A. Hettiaratchi, and R. A. Skurray. 1995. Multidrug resistance plasmid pSK108 from coagulase-negative staphylococci: relationships to Staphylococcus aureus qacC plasmids. Plasmid 34:62-67. [DOI] [PubMed] [Google Scholar]
- 24.Littlejohn, T. G., D. DiBerardino, L. J. Messerotti, S. J. Spiers, and R. A. Skurray. 1991. Structure and evolution of a family of genes encoding antiseptic and disinfectant resistance in Staphylococcus aureus. Gene 101:59-66. [DOI] [PubMed] [Google Scholar]
- 25.Littlejohn, T. G., I. T. Paulsen, M. T. Gillespie, J. M. Tennent, M. Midgley, I. G. Jones, A. S. Purewal, and R. A. Skurray. 1992. Substrate specificity and energetics of antiseptic and disinfectant resistance in Staphylococcus aureus. FEMS Microbiol. Lett. 95:259-266. [DOI] [PubMed] [Google Scholar]
- 26.Lohner, K., and S. E. Blondelle. 2005. Molecular mechanisms of membrane perturbation by antimicrobial peptides and the use of biophysical studies in the design of novel peptide antibiotics. Comb. Chem. High Throughput Screen. 8:241-256. [DOI] [PubMed] [Google Scholar]
- 27.Mitchell, B. A., M. H. Brown, and R. A. Skurray. 1998. QacA multidrug efflux pump from Staphylococcus aureus: comparative analysis of resistance to diamidines, biguanidines, and guanylhydrazones. Antimicrob. Agents Chemother. 42:475-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitchell, B. A., I. T. Paulsen, M. H. Brown, and R. A. Skurray. 1999. Bioenergetics of the staphylococcal multidrug export protein QacA: identification of distinct binding sites for monovalent and divalent cations. J. Biol. Chem. 274:3541-3548. [DOI] [PubMed] [Google Scholar]
- 29.Paulsen, I. T., M. H. Brown, T. G. Littlejohn, B. A. Mitchell, and R. A. Skurray. 1996. Multidrug resistance proteins QacA and QacB from Staphylococcus aureus: membrane topology and identification of residues involved in substrate specificity. Proc. Natl. Acad. Sci. USA 93:3630-3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paulsen, I. T., M. H. Brown, and R. A. Skurray. 1998. Characterization of the earliest known Staphylococcus aureus plasmid encoding a multidrug efflux system. J. Bacteriol. 180:3477-3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paulsen, I. T., R. A. Skurray, R. Tam, M. H. Saier, Jr., R. J. Turner, J. H. Wiener, E. B. Goldberg, and L. L. Grinius. 1996. The SMR family: a novel family of multidrug efflux proteins involved with the efflux of lipophilic drugs. Mol. Microbiol. 19:1167-1175. [DOI] [PubMed] [Google Scholar]
- 32.Pendland, S. L., S. C. Piscitelli, P. C. Schreckenberger, and L. H. Danziger. 1994. In vitro activities of metronidazole and its hydroxy metabolite against Bacteroides spp. Antimicrob. Agents Chemother. 38:2106-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peschel, A., R. W. Jack, M. Otto, L. V. Collins, P. Staubitz, G. Nicholson, H. Kalbacher, W. F. Nieuwenhuizen, G. Jung, A. Tarkowski, K. P. van Kessel, and J. A. van Strijp. 2001. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with l-lysine. J. Exp. Med. 193:1067-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peschel, A., M. Otto, R. W. Jack, H. Kalbacher, G. Jung, and F. Gotz. 1999. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J. Biol. Chem. 274:8405-8410. [DOI] [PubMed] [Google Scholar]
- 35.Pokorny, A., and P. F. Almeida. 2004. Kinetics of dye efflux and lipid flip-flop induced by delta-lysin in phosphatidylcholine vesicles and the mechanism of graded release by amphipathic, alpha-helical peptides. Biochemistry 43:8846-8857. [DOI] [PubMed] [Google Scholar]
- 36.Shafer, W. M., X.-D. Qu, A. J. Waring, and R. I. Lehrer. 1998. Modulation of Neisseria gonorrhoeae susceptibility to vertebrate antibacterial peptides due to a member of the resistance/nodulation/division efflux pump family. Proc. Natl. Acad. Sci. USA 95:1829-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang, Y.-Q., M. R. Yeaman, and M. E. Selsted. 2002. Antimicrobial peptides from human platelets. Infect. Immun. 70:6524-6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tzeng, Y.-L., K. D. Ambrose, S. Zughaier, X. Zhou, Y. K. Miller, W. M. Shafer, and D. S. Stephens. 2005. Cationic antimicrobial peptide resistance in Neisseria meningitidis. J. Bacteriol. 187:5387-5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu, T., M. R. Yeaman, and A. S. Bayer. 1994. In vitro resistance to platelet microbicidal protein correlates with endocarditis source among bacteremic staphylococcal and streptococcal isolates. Antimicrob. Agents Chemother. 38:729-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiong, Y. Q., A. S. Bayer, and M. R. Yeaman. 2002. Inhibition of intracellular macromolecular synthesis in Staphylococcus aureus by thrombin-induced platelet microbicidal proteins. J. Infect. Dis. 185:348-356. [DOI] [PubMed] [Google Scholar]
- 41.Xiong, Y. Q., K. Mukhopadhyay, M. R. Yeaman, J. Adler-Moore, and A. S. Bayer. 2005. Functional interrelationships between cell membrane and cell wall in antimicrobial peptide-mediated killing of Staphylococcus aureus. Antimicrob. Agents Chemother. 49:3114-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiong, Y.-Q., M. R. Yeaman, and A. S. Bayer. 1999. In vitro antibacterial activities of platelet microbicidal protein and neutrophil defensin against Staphylococcus aureus are influenced by antibiotics differing in mechanism of action. Antimicrob. Agents Chemother. 43:1111-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yeaman, M. R. 1997. The role of platelets in antimicrobial host defense. Clin. Infect. Dis. 25:951-968. [DOI] [PubMed] [Google Scholar]
- 44.Yeaman, M. R., A. S. Bayer, S.-P. Koo, W. Foss, and P. M. Sullam. 1998. Platelet microbicidal proteins and neutrophil defensin disrupt the Staphylococcus aureus cytoplasmic membrane by distinct mechanisms of action. J. Clin. Investig. 101:178-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yeaman, M. R., Y.-Q. Tang, A. J. Shen, A. S. Bayer, and M. E. Selsted. 1997. Purification and in vitro activities of rabbit platelet microbicidal proteins. Infect. Immun. 65:1023-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yount, N. Y., K. D. Gank, Y. Q. Xiong, A. S. Bayer, T. Pender, W. H. Welch, and M. R. Yeaman. 2004. Platelet microbicidal protein 1: structural themes of a multifunctional antimicrobial peptide. Antimicrob. Agents Chemother. 48:4395-4404. [DOI] [PMC free article] [PubMed] [Google Scholar]