Abstract
Absorption, metabolism, and excretion of [14C]viramidine, a prodrug of ribavirin, were studied in humans following a single oral dose (600 mg). Viramidine was rapidly absorbed, with a time to maximum concentration of the drug in plasma of 1.5 h. Viramidine and ribavirin accounted for only 4.3% and 42% of plasma area under the concentration-time curve (AUC) for radioactivity, respectively, indicating extensive conversion of viramidine to ribavirin, followed by further metabolism of ribavirin. The drug was largely trapped in red blood cells (RBC), with an RBC-to-plasma radioactivity AUC0-∞ ratio of 108. Excretion of total radioactivity in urine and feces accounted for 50.8% and 26.1% of the dose, respectively. The metabolic profile in urine (0 to 24 h) indicated that viramidine was excreted primarily as triazole carboxamide (TCONH2), triazole carboxylic acid nucleoside (TCOOH), and ribavirin with a small amount of unchanged viramidine, which each accounted for 64.1%, 17.0%, 15.7%, and 3.2% of urinary radioactivity, respectively. The amounts of unchanged viramidine (3.4% of dose) and ribavirin (10% of dose) in urine were small after oral administration of viramidine.
Ribavirin (1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamide) is a purine nucleoside analog with activity against a variety of DNA and RNA viral infections (18, 19). At present, combination therapy of ribavirin and pegylated interferon alfa-2a/2b is the “gold standard” in the treatment of chronic hepatitis C (13, 14, 17). However, ribavirin has a dose-limiting side effect, hemolytic anemia (4, 7, 8). A ribavirin analog that retains those properties deemed critical in the treatment of chronic hepatitis C but with less potential for hemolytic anemia would be highly desirable.
Viramidine, a prodrug of ribavirin, is currently under development for the treatment of chronic hepatitis C virus (HCV) infections. After multiple oral dosings (10 mg/kg of body weight) of viramidine or ribavirin (10) in cynomolgus monkeys, the level of viramidine in the liver was 38% higher and the level in red blood cells (RBC) was only half compared to the results after ribavirin dosing. In addition, viramidine had a much better safety profile than ribavirin did in a 28-day toxicity study in monkeys (5). The results of these animal studies suggest that viramidine has potential for a better liver uptake and is safer than ribavirin in humans.
After a single oral dose of viramidine at 200, 600, or 1,200 mg in healthy volunteers, viramidine was absorbed and rapidly converted to ribavirin, with dose-proportional increases in the levels of ribavirin and viramidine in plasma (12). After oral dosing of viramidine at 400, 600, or 800 mg twice a day (BID) for 28 days in HCV patients, viramidine was rapidly absorbed and converted to ribavirin. Both viramidine and ribavirin were preferentially distributed in RBC rather than plasma (1).
In a phase 2 study (6), 180 patients received pegylated interferon alfa-2a at 180 μg/week subcutaneously in combination with viramidine at 400 mg (n = 47), 600 mg (n = 43), or 800 mg (n = 45) orally BID or ribavirin at 100 or 1,200 mg (n = 45) daily. Results indicate that viramidine had antiviral activity comparable to that of ribavirin when used in combination with pegylated interferon alfa-2a, but with a significantly lower incidence of hemolytic anemia. It was recently reported in a phase 3 study (2) that patients who received pegylated interferon alfa-2a at 180 μg/week subcutaneously in combination with viramidine at 600 mg (n = 646) orally BID or ribavirin at 100 or 1,200 mg daily (n = 324), the overall intent-to-treat sustained viral response rates were 38% for viramidine and 52% for ribavirin. However, the rate of anemia (hemoglobin of less than 10 g/dl) was statistically and significantly lower in patients treated with viramidine than in those treated with ribavirin (5% versus 24%).
The aim of this study is to determine the absorption, metabolism, and excretion of [14C]viramidine in humans.
MATERIALS AND METHODS
Compound.
The compound [5-14C]viramidine (Fig. 1) was synthesized using [14C]barium carbonate as a precursor. The labeled nucleoside was extensively purified by column chromatography and repetitive recrystallization. The chemical identity and purity were verified by mass spectrometry and proton magnetic resonance spectrometry. The radiopurity (>98%) of the preparation was confirmed by high-performance liquid chromatography (HPLC) coupled with radioflow detection. Ribavirin, triazole carboxamide (TCONH2), and triazole carboxylic acid nucleoside (TCOOH) were obtained from Valeant Research & Development (Costa Mesa, Calif.).
FIG. 1.
Chemical structures of viramidine, ribavirin, TCONH2, and TCOOH. The asterisk indicates the position of the 14C-labeled carbon.
Drug administration.
Six healthy adult male subjects participated in the study. Subject inclusion criteria included the following. (i) Subjects had to be healthy adult males aged 50 years or older. (ii) Subjects had to be sterile or willing to use an approved method of contraception from the time of administration of the first dose to 6 months following study completion or early termination. A fertile subject and his partner had to be willing to use a double-barrier method of contraception, if neither person had been made sterile surgically. (iii) Subjects had to have a body mass index within the range of 18 to 30 kg/m2. (iv) Subjects had to undergo clinical laboratory evaluations and have normal results or abnormal results considered by the investigator to be not clinically significant. These included pretreatment electrocardiogram; blood tests for hematology, liver, and renal function; and urinalysis and drug screening. (v) Subjects had to be able to understand and be willing to sign an informed consent form and be able to adhere to study schedules and requirements.
Subject exclusion criteria included the following: (i) history of clinically significant metabolic, hematological, pulmonary, cardiovascular, gastrointestinal, neurological, hepatic, renal, urological, or psychiatric disorder; (ii) history of hypersensitivity or allergies to ribavirin, viramidine, any formulation ingredients, or any drug compound, unless approved by the investigator; (iii) history of stomach or intestinal surgery (unless deemed acceptable by the investigator) (an appendectomy or cholecystectomy was reason for exclusion); (iv) history or presence of an abnormal electrocardiogram, which in the investigator's opinion, was clinically significant; (v) history of alcoholism or drug addiction within 6 months prior to study entry; (vi) subjects who had received another investigational drug within 3 months prior to the start of this study; (vii) subjects who had donated blood within the preceding 30 days; (viii) subjects who had poor peripheral venous access; (ix) subjects who used prescription or over-the-counter drugs within 1 week prior to study entry; (x) subjects who had a positive urine drug screen at the screening visit; and (xi) previous exposure to 14C or radioactive compounds with similarly long decay.
All volunteers provided written, informed consent to participate in the trial. They were informed that the study was for research only, that the study was not expected to provide them with any therapeutic benefit, and that they were free to withdraw from the study at any time without prejudice. The Guy's Hospital Research Ethics Committee provided formal approval for the study, which was conducted in accordance with the International Conference on Harmonization Guidelines and Good Clinical Practice Standards.
Following an overnight fast, each volunteer received a single oral dose of [14C]viramidine, given as three 200-mg capsules with a total radioactivity of 52.2 microcuries. All six subjects completed the study. All subjects were admitted to the unit approximately 24 h prior to receiving the drug and remained in the unit under clinical supervision for a period of 14 days postdose.
Sample collection.
Blood samples were collected via an indwelling intravenous cannula line and transferred to tubes containing heparin and centrifuged at 2,000 rpm for 15 min at 5°C to obtain plasma. The remaining red blood cell mass was washed twice with 1 ml of phosphate-buffered saline, pH 7.4, centrifuged at 200 rpm for 10 min at 4°C, and then stored at −70°C. Plasma and RBC samples were collected prior to dosing (0 h) and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 24, 48, 72, 96, 120, 144, 168, 216, 264, 336, 504, 576, and 672 h after dosing. Fecal and urine samples were collected at 0 to 24, 24 to 48, 48 to 72, 72 to 96, 96 to 120, 120 to 144, 144 to 168, 168 to 192, 192 to 216, 216 to 240, 240 to 264, 264 to 288, 288 to 312, and 312 to 336 h after dosing.
Measurement of radioactivity.
The radioactivity levels in plasma (0.5 ml) and urine (1.5 ml) were measured using an Ultima Gold XR scintillation cocktail and a liquid scintillation counter (model Tri-Carb 3100 TR; Packard Instrument Company, Meriden, Conn.). Fecal samples were homogenized in a suitable quantity of water. Weighed aliquots of homogenates and RBC were allowed to dry at least overnight and were then combusted using a Packard model 307 automatic sample oxidizer [PerkinElmer LAS (UK) Ltd.]. Carbo-SorbE and PermafluorE+ were used as absorbent and scintillator, respectively. Scintillation counting data were automatically corrected for counting efficiency on the basis of an external standard and an instrument-stored quench curve generated from a series of sealed quenched standards. Radioactivity was measured as disintegrations per minute per gram and converted to microgram equivalents per gram using the specific activity in the dosing solution. It is assumed that 1 ml of RBC and plasma weighs 1 g.
LC-MS/MS method for the determination of viramidine and ribavirin in plasma.
A sensitive and specific method of liquid chromatography and tandem mass spectrometry (LC-MS/MS) for the simultaneous analyses of viramidine and ribavirin in human plasma was used. The method involved the additions of [13C]viramidine and [13C]ribavirin as internal standards, protein precipitation with acetonitrile, HPLC separation, and quantification by an MS/MS system using positive electrospray ionization in the multiple reaction monitoring mode. The precursor→ product ion m/z transition was monitored at 245 → 113, 250 → 113, 244 → 112, and 249 → 112 for ribavirin, [13C]ribavirin, viramidine, and [13C]viramidine, respectively. The calibration curves for viramidine and ribavirin were linear over a concentration range from 10 to 10,000 ng/ml. For both viramidine and ribavirin, the lower limit of quantification was 10 ng/ml. For viramidine, intraday and interday analyses of quality control (QC) samples at 30, 500, 1,000, and 10,000 ng/ml indicated good precision (coefficient of variation [CV] between 3.32% and 6.11%) and accuracy (between 94.2% and 108%). For ribavirin, intraday and interday analyses of QC samples at 30, 500, 1,000, and 10,000 ng/ml indicated similar precision (CV between 3.53% and 5.96%) and accuracy (between 91.8% and 105%).
LC-MS/MS method for the determination of viramidine and ribavirin in RBC.
The concentrations of total viramidine and total ribavirin in human RBC were determined by an LC/MS-MS method. The method involves the additions of [13C]viramidine and [13C]ribavirin as internal standards, protein precipitation by perchloric acid, enzyme digestion to convert all phosphorylated metabolites to their corresponding nucleosides, solid-phase extraction (SPE) for sample clean up, and final analysis by liquid chromatography and tandem mass spectrometry. The MS/MS was selected to monitor m/z 244 → 112, 249 → 112, 245 → 113, and 250 → 113 for viramidine, [13C]viramidine, ribavirin, and [13C]ribavirin, respectively, using positive electrospray ionization. The calibration curves for viramidine and ribavirin covered the nominal concentration range of 100 to 100,000 ng/ml, with a nominal lower limit of quantification of 100 ng/ml. For QC samples, the mean accuracy of the back-calculated concentrations varied from 88.5% to 90.3% for viramidine and from 95.0% to 97.7% for ribavirin. The mean precision (CV) in the back-calculated concentrations ranged from 3.71% to 4.18% and from 4.02% to 5.69% for viramidine and ribavirin, respectively.
HPLC procedure for studying the metabolic profiles in urine.
A pooled urine sample was obtained by combining 5% (vol/vol) of each sample from all collection periods (up to 24 h) from all six subjects. Prior to HPLC analysis, an ENV SPE cartridge was used to clean up the sample. The collected eluant from SPE (94% recovery) was then analyzed using an Agilent 1100 HPLC (Agilent, Palo Alto, Calif.) coupled with a radioactivity detector (β-Ram model 3, IN/US Systems, Inc., Tampa, Fla.). An Atlantis dC18 column (4.6 × 150 mm; 5 mm; Waters, Milford, Mass.) was used for the separation. Mobile phase A was 10 mM ammonium acetate in water, and mobile phase B was 10 mM ammonium acetate in methanol. The gradient conditions were 100% mobile phase A between 0 and 3 min, ramped to 95% mobile phase A and 5% mobile phase B between 3 and 8 min, ramped to 50% mobile phase A and 50% mobile phase B between 8 and 9 min, maintained at 50% mobile phase A and 50% mobile phase B between 9 and 12 min, ramped to 100% mobile phase A between 12 and 13 min, and maintained at 100% mobile phase A between 13 and 20 min. The mobile phases were delivered at 1.0 ml/min.
To determine the structures of those metabolites, the fractions corresponding to different metabolites were collected, and their MS/MS spectra were compared to those of synthetic standards using different HPLC conditions.
Pharmacokinetic analysis.
The level of radioactivity and concentrations of viramidine and ribavirin in plasma and RBC were used to determine pharmacokinetic parameters using noncompartmental methods (WinNonlin; Pharsight Corp., Mountain View, Calif.). The maximum concentration of drug in serum (Cmax) and time to maximum concentration of drug in serum (Tmax) were the observed values. The area under the concentration-time curve (AUC) to the last quantifiable sampling time (tf), AUCtf, was computed by using the linear trapezoidal rule. The area under the concentration-time curve to infinity, AUC0-∞, was calculated as the sum of AUCtf and the quotient of the last measurable concentration and the elimination rate constant (kel). The kel was estimated as the negative slope of the regression of log concentration versus time. Half-life (t1/2) was calculated by dividing 0.693 by kel. fe (0 to 336 h) is the cumulative amount of the drug excreted in urine from time zero to 336 h, expressed as a percentage of the dose. Renal clearance (CLR) was calculated as the ratio of fe to AUC. Apparent total body clearance after oral dosing (CL/F) was calculated as the ratio of dose to AUC0-∞, where F is oral bioavailability. For the amount of viramidine-derived ribavirin expressed as a percentage of the dose, the dose administered was adjusted to represent an equivalent dose of ribavirin-free base. The equivalent ribavirin dose was estimated to be dose × (244.2/243.2), where 244.2 is the molecular weight of ribavirin-free base and 243.2 is the molecular weight of viramidine-free base.
RESULTS
Levels of radioactivity in plasma and RBC.
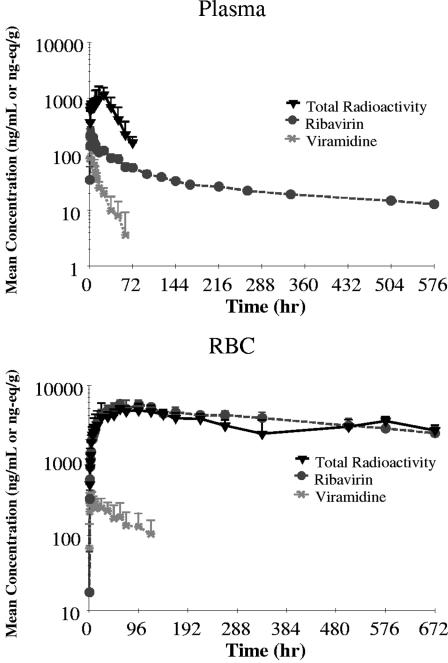
Following oral administration of [14C]viramidine, plasma levels of radioactivity reached a maximum of 1.30 μg-eq/ml at 20 h (median Tmax) and then decreased with time (Fig. 2), with an elimination t1/2 of 18.1 h. RBC levels of radioactivity reached a maximum of 5.15 μg-eq/ml at 60 h (median Tmax) and then decreased with time (Fig. 2), with an elimination t1/2 of 754 h. The ratio of RBC radioactivity AUC0-∞ to plasma radioactivity was 108, indicating extensive preferential distribution of radioactivity to RBC, rather than plasma.
FIG. 2.
Mean concentration-time profiles of radioactivity, viramidine, and ribavirin in human plasma and RBC following oral dosing of [14C]viramidine at 600 mg (n = 6).
Concentrations of viramidine in plasma and RBC.
Following oral administration of [14C]viramidine, plasma levels of viramidine reached a maximum of 0.228 μg/ml at 1.5 h (median Tmax) and then decreased with time (Fig. 2), with an elimination t1/2 of 27.8 h. RBC levels of radioactivity reached a maximum of 0.337 μg/ml at 4.0 h (median Tmax) and then decreased with time (Fig. 2), with an elimination t1/2 of 78.8 h, which was much longer than the t1/2 in plasma. The ratio of RBC viramidine AUC0-∞ to that of plasma was 14.8, again indicating preferential distribution of viramidine to RBC, rather than plasma.
Concentrations of ribavirin in plasma and RBC.
Following oral administration of [14C]viramidine, plasma levels of ribavirin reached a maximum of 0.260 μg/ml at 2.0 h (median Tmax) and then decreased with time (Fig. 2), with an elimination t1/2 of 418 h. RBC levels of radioactivity reached a maximum of 6.02 μg/ml at 66 h (median Tmax) and then decreased with time (Fig. 2), with an elimination t1/2 of 507 h, which was similar to t1/2 in plasma. The ratio of RBC ribavirin AUC0-∞ to that of plasma was 159, again indicating much preferential distribution of ribavirin to RBC, rather than plasma.
Urinary and fecal excretion of radioactivity, viramidine, and ribavirin.
Following oral dosing of [14C]viramidine, 50.8% of the dose was excreted in urine (0 to 336 h), and 26.1% of the dose was excreted in feces (0 to 336 h). Only a small amount of viramidine (3.39% of dose) was recovered in urine, indicating extensive metabolism of viramidine in humans. Renal clearance values were 114 ml/min for total radioactivity, 172 ml/min for viramidine, and 67.6 ml/min for ribavirin.
Metabolic profiles in urine.
Following oral administration of [14C]viramidine, it was excreted into urine primarily as TCONH2, TCOOH, and ribavirin, with a small amount of unchanged viramidine, which accounted for 64.1%, 17.0%, 15.7%, and 3.2% of urinary radioactivity, respectively.
DISCUSSION
Following administration of a single oral dose of [14C]viramidine in humans, viramidine was rapidly absorbed, with a Tmax of 1.5 h. Total radioactivity AUC0-∞ in RBC (4,860 μg-eq · h/ml) was much higher than that in plasma (48.6 μg-eq · h/ml), indicating that the drug was mostly trapped in RBC, rather than plasma. However, it should be noted that calculation of radioactivity AUC0-∞ in RBC is based on the accurate estimation of radioactivity t1/2 in RBC. Figure 2 shows a longer RBC radioactivity t1/2 than the sampling window, and the data do not appear to be in a terminal decline phase, rendering a questionable estimation of radioactivity t1/2 in RBC.
The ratios of viramidine AUC0-672 h to radioactivity AUC0-672 h were 0.0429 in plasma and 0.0105 in RBC, indicating extensive metabolism of viramidine. Furthermore, the ratios of viramidine AUC0-∞ to ribavirin AUC0-∞ were 0.103 in plasma and 0.00918 in RBC, indicating extensive conversion of viramidine to ribavirin in humans.
It is important to note that the t1/2 of plasma viramidine in humans (27.8 h) was much shorter than that of plasma ribavirin (418 h), indicating that the rate of conversion of viramidine to ribavirin was faster than the rate of conversion of ribavirin to other metabolites. These findings are also in good agreement with the observation that following oral dosing of [14C]viramidine, viramidine was the only minor radioactive peak in urine from 0 to 24 h.
Following oral dosing of [14C]viramidine, 50.8% of the dose was excreted in urine from 0 to 336 h and 26.1% of the dose was excreted in feces from 0 to 336 h feces, with a total recovery of 76.9% of dose. This is not unexpected, since RBC radioactivity AUC0-672 h is about 100 times plasma radioactivity AUC0-672 h (Table 1), indicating that most of the administered drug was trapped in the RBC. Furthermore, the ribavirin elimination half-life in RBC was estimated at approximately 40 days (3, 16).
TABLE 1.
Pharmacokinetic parameters in human plasma and RBC following oral dosing of [14C]viramidine
| Property, analyte, or ratio | Parameter (unit) | Value in:a
|
Ratio of values in RBC/plasmaa | |
|---|---|---|---|---|
| Plasma | RBC | |||
| Radioactivity (A) | AUC0-672 h (μg-eq · h/ml) | 46.6 ± 16.2 | 2,140 ± 389 | 51.5 ± 13.3 |
| AUC0-∞ (μg-eq · h/ml) | 48.6 ± 15.6 | 4,860 ± 629 | 108 ± 31.6 | |
| Cmax (μg-eq/ml) | 1.30 ± 0.342 | 5.15 ± 0.665 | 4.11 ± 0.729 | |
| Tmax (h) | 20 (2-24) | 60 (24-120) | NA | |
| t1/2 (h) | 18.1 ± 6.38 | 754 ± 179 | NA | |
| Viramidine (B) | AUC0-672 h (μg · h/ml) | 2.00 ± 0.512 | 22.5 ± 9.31 | 10.7 ± 3.68 |
| AUC0-∞ (μg · h/ml) | 2.38 ± 0.784 | 34.9 ± 13.1 | 14.8 ± 5.02 | |
| Cmax (μg/ml) | 0.228 ± 0.0498 | 0.337 ± 0.0751 | 1.50 ± 0.325 | |
| Tmax (h) | 1.50 (1.5-6.0) | 4.0 (2.0-6.0) | NA | |
| t1/2 (h) | 27.8 ± 18.2 | 78.8 ± 31.3 | NA | |
| Ribavirin (C) | AUC0-672 h (μg · h/ml) | 19.5 ± 1.63 | 2,450 ± 386 | 129 ± 21.9 |
| AUC0-∞ (μg · h/ml) | 26.4 ± 2.37 | 4,230 ± 1320 | 159 ± 4.50 | |
| Cmax (μg/ml) | 0.260 ± 0.0530 | 6.02 ± 0.873 | 23.8 ± 5.06 | |
| Tmax (h) | 2.0 (1.5-6.0) | 66 (60-96) | NA | |
| t1/2 (h) | 418 ± 66.6 | 507 ± 169 | NA | |
| B/A | AUC0-672 h | 0.0429 | 0.0105 | NA |
| Cmax | 0.175 | 0.0654 | NA | |
| C/A | AUC0-672 h | 0.418 | 1.14 | NA |
| Cmax | 0.200 | 1.16 | NA | |
| B/C | AUC0-∞ | 0.103 | 0.00918 | NA |
| Cmax | 0.877 | 0.0560 | NA | |
Values are shown as means ± standard deviations, except for Tmax, which is expressed as mean (range) (n = 6). NA, not applicable.
In the current study, renal clearance values were 172 ml/min for viramidine and 67.6 ml/min for ribavirin, which were similar to those reported previously for viramidine (113 ml/min) and ribavirin (84.0 ml/min) in a single, rising-dose study (12).
Percent absorption can be estimated from the percentage of radioactivity excreted in urine after oral dosing, corrected for total recovery. On the basis of this approach, the absorption of viramidine was estimated to be 66.1%, which is similar to that reported for rats (61.7%) but higher than that in monkeys (43.9%).
Following oral administration of [14C]viramidine, viramidine was excreted into urine primarily as TCONH2 with smaller amounts of ribavirin, TCOOH, and unchanged viramidine. Similar metabolic profiles in urine were also obtained in rats and monkeys following oral dosing of [14C]viramidine (11). These data confirmed that regardless of species, oral viramidine is absorbed and extensively converted to ribavirin, followed by further metabolism of ribavirin to TCONH2 and TCOOH, which do not possess any antiviral activity (9, 15).
It was clear from the study that following oral administration to humans, viramidine is absorbed and extensively converted to ribavirin. The drug was largely trapped in the RBC. Excretion of total radioactivity in urine and feces accounted for 50.8% and 26.1% of the oral dose, respectively (Table 2). Viramidine was excreted in urine, primarily as TCONH2, with smaller amounts of ribavirin, TCOOH, and unchanged viramidine.
TABLE 2.
Excretion in human urine and feces
| Property, analyte, or ratio | Parameter (unit) | Value in urine (mean ± SD) (n = 6) | Value in feces (mean ± SD) (n = 6) | Total value (mean ± SD) (n = 6) |
|---|---|---|---|---|
| Radioactivity (A) | fe (% of dose) | 50.8 ± 14.0 | 26.1 ± 13.8 | 76.9 ± 5.46 |
| CLR (ml/min) | 114 ± 14.6 | NAa | NA | |
| CL/F (ml/min) | 227 ± 80.0 | NA | NA | |
| Viramidine (B) | fe (% of dose) | 3.39 ± 0.682 | NA | NA |
| CLR (ml/min) | 172 ± 24.3 | NA | NA | |
| CL/F (ml/min) | 4610 ± 1530 | NA | NA | |
| Ribavirin (C) | fe (% of dose) | 10.0 ± 0.965 | NA | NA |
| CLR (ml/min) | 67.6 ± 3.90 | NA | NA | |
| CL/F (ml/min) | NA | NA | NA | |
| B/A | fe | 0.0667 | NA | NA |
| C/A | fe | 0.197 | NA | NA |
| B/C | fe | 0.339 | NA | NA |
NA, not applicable.
Acknowledgments
This study was supported by Valeant Research & Development, Costa Mesa, Calif.
REFERENCES
- 1.Aora, S., C. Xu, A. Teng, J. Peterson, L. T. Yeh, R. Gish, D. Lau, S. Rossi, and C. C. Lin. 2005. Ascending multiple-dose pharmacokinetics of viramidine, a prodrug of ribavirin, in adult subjects with compensated hepatitis C infection. J. Clin. Pharmacol. 45:275-285. [DOI] [PubMed] [Google Scholar]
- 2.Benhamou, Y., P. Pockros, M. Rodriguez-Torres, S. Gordon, M. Shiffman, Y. Lurie, N. Afdhal, K. Lamon, Y. Kim, and B. Murphy. 2006. The safety and efficacy of Viramidine® plus pegylated interferon alfa-2b versus ribavirin plus pegylated interferon alfa-2b in therapy-naïve patients infected with HCV: phase 3 results, abstr. 741. Abstr. 41st Eur. Assoc. Study of Liver. Vienna, Austria, European Association for the Study of the Liver, Geneva, Switzerland.
- 3.Catlin, D. H., R. A. Smith, and A. I. Samuels. 1980. 14C-ribavirin: distribution and pharmacokinetic studies in rats, baboons and man, p. 83-98. In R. A. Smith and R. A. Kirkpatrick (ed.), Ribavirin: a broad spectrum antiviral agent. Academic Press Inc., New York, N.Y.
- 4.Connor, E., S. Morrison, J. Lane, J. Oleske, R. L. Sonke, and J. Connor. 1993. Safety, tolerance, and pharmacokinetics of systemic ribavirin in children with human immunodeficiency virus infection. Antimicrob. Agents Chemother. 37:532-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dadgostari, S., C. Xu, L. Yeh, C. C. Lin, and D. Vitarella. 2004. Viramidine demonstrates better safety than ribavirin in monkeys but not rats. Drug Chem. Toxicol. 27:191-211. [DOI] [PubMed] [Google Scholar]
- 6.Gish, R., S. Arora, N. Nelson, H. Fernandez, and K. Lamon. 2004. Safety and efficacy of viramidine in combination with pegylated interferon alfa-2a for treatment of hepatitis C therapy-naïve patients, abstr. 479. Abstr. 39th Eur. Assoc. Study of Liver. Berlin, Germany. European Association for the Study of the Liver, Geneva, Switzerland.
- 7.Laskin, O. L., J. A. Longstreth, C. C. Hart, D. Scavuzzo, C. M. Kalman, J. D. Connor, and R. B. Roberts. 1987. Ribavirin disposition in high-risk patients for acquired immunodeficiency syndrome. Clin. Pharmacol. Ther. 41:546-555. [DOI] [PubMed] [Google Scholar]
- 8.Lertora, J. J., A. B. Rege, J. T. Lacour, N. Ferencz, W. J. George, R. B. VanDyke, K. C. Agrawal, and N. E. Hyslop, Jr. 1991. Pharmacokinetics and long-term tolerance to ribavirin in asymptomatic patients infected with human immunodeficiency virus. Clin. Pharmacol. Ther. 50:442-449. [DOI] [PubMed] [Google Scholar]
- 9.Lin, C. C., L. T. Yeh, T. Luu, D. Lourenco, and J. Y. Lau. 2003. Pharmacokinetics and metabolism of [14C]ribavirin in rats and cynomolgus monkeys. Antimicrob. Agents Chemother. 47:1395-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin, C. C., D. Lourenco, G. Xu, and L. T. Yeh. 2004. Disposition and metabolic profiles of [14C]viramidine and [14C]ribavirin in rat and monkey red blood cells and liver. Antimicrob. Agents Chemother. 48:1872-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin, C. C., K. Luu, D. Lourenco, and L. Yeh. 2003. Pharmacokinetics and metabolism of [14C]viramidine in rats and cynomolgus monkeys. Antimicrob. Agents Chemother. 47:2458-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin, C. C., L. Philips, C. Xu, and L. T. Yeh. 2004. Pharmacokinetics and safety of viramidine, a prodrug of ribavirin, in healthy volunteers. J. Clin. Pharmacol. 44:265-275. [DOI] [PubMed] [Google Scholar]
- 13.Manns, M. P., J. G. McHutchison, S. C. Gordon, V. K. Rustgi, M. Shiffman, R. Reindollar, Z. D. Goodman, K. Koury, M. Ling, J. K. Albrecht, and the International Hepatitis Interventional Therapy Group. 2001. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 358:958-965. [DOI] [PubMed] [Google Scholar]
- 14.McHutchison, J. G., S. C. Gordon, E. R. Schiff, M. L. Shiffmann, W. M. Lee, V. K. Rustgi, Z. D. Goodman, M. H. Ling, S. Cort, and J. K. Albrecht. 1998. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N. Engl. J. Med. 339:1485-1492. [DOI] [PubMed] [Google Scholar]
- 15.Miller, J. P., L. J. Kigwana, D. G. Streeter, R. K. Robins, L. N. Simon, and J. Roboz. 1977. The relationship between the metabolism of ribavirin and its proposed mechanism of action. Ann. N. Y. Acad. Sci. 284:211-229. [DOI] [PubMed] [Google Scholar]
- 16.Page, T., and J. D. Connor. 1990. The metabolism of ribavirin in erythrocytes and nucleated cells. Int. J. Biochem. 22:379-383. [DOI] [PubMed] [Google Scholar]
- 17.Poynard, T., P. Marcellin, S. S. Lee, C. Niederau, G. S. Minuk, G. Ideo, V. Bain, J. Heathcote, S. Zeuzem, C. Trepo, and J. Albrecht. 1998. Randomised trial of interferon alpha-2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha-2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. Lancet 352:1426-1432. [DOI] [PubMed] [Google Scholar]
- 18.Sidwell, R. W., J. H. Huffman, G. P. Khare, L. B. Allen, J. T. Witkowski, and R. K. Robins. 1972. Broad-spectrum antiviral activity of virazole: 1-beta-d-ribofuranosyl-1,2,4-triazole-3-carboxamide. Science 177:705-706. [DOI] [PubMed] [Google Scholar]
- 19.Stephen, E. L., D. E. Jones, C. J. Peters, G. A. Eddy, P. S. Loizeaux, and P. B. Jahrling. 1980. Ribavirin treatment of Toga-, Aren-, and Bunyavirus infections in subhuman primates and other laboratory animal species, p. 169-183. In R. A. Smith and R. A. Kirkpatrick (ed.), Ribavirin: a broad spectrum antiviral agent. Academic Press Inc., New York, N.Y.