Abstract
The activities of ceftobiprole and other β-lactams were examined with 30 Streptococcus pneumoniae isolates containing multiple pbp1a, pbp2b, and pbp2x mutations. The highest ceftobiprole MIC was 1 μg/ml, while the comparator MICs were 16 to 64 μg/ml. Fifty percent inhibitory concentrations for penicillin-binding protein 2x were 0.5 μg/ml (ceftobiprole) and 4 μg/ml (ceftriaxone) in a penicillin- and ceftriaxone-resistant isolate.
Community-acquired pneumonia (CAP) is diagnosed in 4,000,000 patients per year in the United States. Streptococcus pneumoniae is the leading bacterial cause of CAP (20 to 60% of cases) (3, 18). Haemophilus influenzae, Staphylococcus aureus, and atypical pathogens are also causative agents (3 to 10%) (3, 18).
Combination therapy with an extended-spectrum cephalosporin and a macrolide is one of the CAP treatments recommended for adults in hospital settings (3, 18). Against S. pneumoniae and H. influenzae, the extended-spectrum cephalosporins are some of the most active β-lactam agents (19). However, against S. aureus, primarily methicillin-resistant strains, these cephalosporins are considered clinically ineffective (12, 13).
Ceftobiprole, a new parenteral cephalosporin in phase 3 clinical trials, exhibits a broad spectrum of activities against many clinically important gram-negative and gram-positive pathogens, including the common CAP pathogens S. pneumoniae, H. influenzae, and S. aureus (12-14, 24). Ceftobiprole is differentiated from other parenteral cephalosporins due to its low MICs for S. aureus, including methicillin-resistant strains, and for cefotaxime- or ceftriaxone-resistant S. pneumoniae (12-14).
β-Lactam resistance in S. pneumoniae is caused by mutations in the penicillin-binding domains of one or more of its six penicillin-binding proteins (PBPs) resulting from point mutations or mosaic genes (9-11, 15). Altered PBP 1a, PBP 2x, and PBP 2b are the most important PBPs for β-lactam resistance among clinical isolates (1, 2, 9, 22).
In this study, the activities of ceftobiprole against 30 recent clinical S. pneumoniae isolates from six U.S. states with varied penicillin susceptibilities and defined mutations in the penicillin-binding domains of pbp1a, pbp2b, and pbp2x were examined.
(This work was presented in part at the 45th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, D.C., 2005.)
All MICs except for ceftobiprole MICs were determined with panels manufactured by Trek Diagnostic Systems (Cleveland, OH), using CLSI methods (5, 6). Ceftobiprole-containing medium was prepared fresh for each assay.
Approximately 1-kb fragments of pbp1a, pbp2x, and pbp2b encompassing the penicillin-binding domain of each gene were PCR amplified as described by Nichol et al. (17) and sequenced by ACGT (Wheeling, IL). Competition assays were performed with a protocol based on the work of Sifaoui et al. (20), using Bocillin-FL (Invitrogen, Carlsbad, CA). PBPs were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and visualized using a LumiImager (Roche Diagnostics, Indianapolis, IN).
Seven of the 30 isolates were penicillin susceptible, with all β-lactam MICs being ≤0.03 μg/ml. These isolates had no mutations in the penicillin-binding motifs of pbp1a, pbp2b, and pbp2x (Table 1).
TABLE 1.
Ceftobiprole and comparator β-lactam MICs for S. pneumoniae clinical isolates with specific PBP genotypes
| Genotypea | Strain | MIC (μg/ml)b
|
Substitution in indicated motif
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PBP 1ac
|
PBP 2xd
|
PBP 2be
|
||||||||||||||
| BPR | CRO | CTX | AMC | PEN | CXM | 370STMK | 428SRNVP | 337STMK | 371I | 384R | 400M | 546LKSGT | 442SSNT | 614KTGTA | ||
| wt | 8865 | 0.008 | 0.03 | 0.03 | 0.03 | ≤0.015 | ≤0.03 | ---- | ----- | ---- | - | - | - | ----- | ---- | ----- |
| 8869 | 0.008 | 0.03 | 0.03 | 0.03 | ≤0.015 | ≤0.03 | ---- | ----- | ---- | - | - | - | ----- | ---- | ----- | |
| 8621 | 0.008 | 0.03 | 0.03 | 0.03 | ≤0.015 | ≤0.03 | ---- | ----- | ---- | - | - | - | ----- | ---- | ----- | |
| 8625 | 0.008 | 0.03 | ≤0.015 | 0.03 | ≤0.015 | ≤0.03 | ---- | ----- | ---- | - | - | - | ----- | ---- | ----- | |
| 7876 | ≤0.004 | 0.03 | 0.03 | 0.03 | ≤0.015 | ≤0.03 | ---- | ----- | ---- | - | - | - | ----- | ---- | ----- | |
| 8626 | ≤0.004 | ≤0.015 | ≤0.015 | 0.03 | ≤0.015 | ≤0.03 | ---- | ----- | ---- | - | - | - | ----- | ---- | ----- | |
| 8025 | ≤0.004 | 0.03 | 0.03 | 0.03 | 0.03 | ≤0.03 | ---- | ----- | ---- | - | - | - | ----- | ---- | ----- | |
| 1 | 8882 | 0.008 | 0.06 | 0.06 | 0.06 | 0.12 | 0.25 | ---- | ----- | ---- | - | - | - | ----- | ---A | ----- |
| 8009 | 0.015 | 0.06 | 0.06 | 0.12 | 0.12 | 0.25 | ---- | ----- | -A-- | - | G | - | ----- | ---A | ----- | |
| 7891 | 0.06 | 0.12 | 0.12 | 0.12 | 0.12 | 0.25 | ---- | ----- | ---- | - | - | - | ----- | ---A | ----- | |
| 2 | 7877 | 0.12 | 0.5 | 0.5 | 2 | 1 | 4 | -S-- | ----- | -A-- | T | G | - | V---- | ---A | ----- |
| 7879 | 0.25 | 0.5 | 0.5 | 1 | 1 | 4 | ---- | ----- | -A-- | T | G | - | V---- | ---A | ----- | |
| 8006 | 0.25 | 0.5 | 1 | 2 | 1 | 4 | ---- | ----- | -A-- | T | G | - | V---- | ---A | ----- | |
| 8010 | 0.25 | 0.5 | 0.5 | 1 | 1 | 4 | ---- | ----- | -A-- | T | G | - | V---- | ---A | ----- | |
| 8617 | 0.25 | 0.5 | 0.5 | 1 | 1 | 4 | ---- | ----- | -A-- | T | G | - | V---- | ---A | ----- | |
| 3 | 8032 | 0.12 | 0.5 | 0.5 | 2 | 2 | 2 | -A-- | ----T | -A-- | T | G | - | V---- | ---A | ----- |
| 8157 | 0.12 | 1 | 2 | 2 | 2 | 8 | -A-- | ----T | -A-- | T | G | - | V---- | ---A | ----- | |
| 8151 | 0.25 | 1 | 1 | 2 | 2 | 8 | -A-- | ----T | -A-- | T | G | - | V---- | ---A | ----- | |
| 7888 | 0.25 | 1 | 1 | 2 | 4 | 8 | -A-- | ----T | -A-- | T | G | - | V---- | ---A | ----- | |
| 7889 | 0.25 | 2 | 2 | 2 | 4 | 16 | -A-- | ----T | -A-- | T | G | - | V---- | ---A | ----- | |
| 8007 | 0.25 | 2 | 2 | 4 | 4 | 8 | -A-- | ----T | -A-- | T | G | - | V---- | ---A | ----- | |
| 8161 | 0.5 | 2 | 2 | 2 | 4 | 8 | -A-- | ----T | -A-- | T | G | - | V---- | ---A | ----- | |
| 4 | 8867 | 0.25 | 1 | 2 | 8 | 8 | 8 | -S-- | ----T | -A-- | T | G | - | V---- | ---A | ----G |
| 8024 | 0.25 | 2 | 2 | 8 | 8 | 16 | -S-- | ----T | -A-- | T | G | - | V---- | ---A | ----G | |
| 5 | 7771 | 0.5 | 4 | 8 | 16 | 8 | 32 | -A-- | ----T | -A F- | T | G | T | V---- | ---A | ----G |
| 8623 | 0.5 | 4 | 8 | 16 | 8 | 32 | -S-- | ----T | -A F- | T | G | T | V---- | ---A | ----G | |
| 8036 | 0.5 | 8 | 8 | 8 | 8 | 32 | -S-- | ----T | -A F- | T | G | T | V---- | ---A | ----G | |
| 8048 | 0.5 | 8 | 8 | 8 | 8 | 32 | -S-- | ----T | -A F- | T | G | T | V---- | ---A | ----G | |
| 8819 | 1 | 8 | 16 | 16 | 8 | 64 | -S-- | ----T | -A F- | T | G | T | V---- | ---A | ----G | |
| 8575 | 1 | 16 | 16 | 16 | 8 | 64 | -S-- | ----T | -A F- | T | G | T | V---- | ---A | ----- | |
wt, wild type.
BPR, ceftobiprole; CRO, ceftriaxone; CTX, cefotaxime; AMC, amoxicillin-clavulanic acid; PEN, penicillin; CXM, cefuroxime.
There were no substitutions in the 557KTG motif. Dashes indicate no change from the wild type; boldface amino acids are different from wild type.
There were no substitutions in the 393AHSSNV motif. Dashes indicate no change from the wild type; boldface amino acids are different from wild type.
There were no substitutions in the 385SVVK motif. Dashes indicate no change from the wild type; boldface amino acids are different from wild type.
For the eight penicillin-intermediate isolates, penicillin MICs were 0.12 μg/ml for three and 1 μg/ml for five isolates. The former group had a T-to-A substitution in the PBP 2b SSNT motif (genotype 1), with corresponding β-lactam MICs of ≤0.25 μg/ml (Table 1). In addition to the previous PBP 2b substitution, a penicillin MIC of 1 μg/ml was associated with a T-to-A change in the STMK motif and an L-to-V change in the LKSGT motif of PBP 2x (genotype 2). Although these isolates were resistant to cefuroxime, cefotaxime and ceftriaxone MICs were ≤1 μg/ml, and the ceftobiprole MICs were ≤0.25 μg/ml (Table 1).
The remaining 15 isolates were penicillin resistant. Seven isolates with genotype 3 had PBP 1a substitutions of T to A in the STMK motif and P to T in the SRNVP motif associated with penicillin MICs of 2 to 4 μg/ml, in addition to the PBP 2x and PBP 2b changes previously mentioned (Table 1). These seven isolates were not susceptible to cefuroxime, and some were not susceptible to ceftriaxone, cefotaxime, and amoxicillin-clavulanic acid; the ceftobiprole MICs for these isolates were ≤0.5 μg/ml (Table 1). Isolates with genotype 4 had an additional substitution in PBP 2b of A to G in the KTGTA motif and a T-to-S change in the STMK motif of PBP 1a, as opposed to the T-to-A change seen in genotype 3 (Table 1). These changes corresponded with an increased penicillin MIC (8 μg/ml), resistance to cefuroxime and amoxicillin-clavulanic acid, and an intermediate interpretation for cefotaxime, while ceftobiprole retained MICs of ≤0.25 μg/ml (Table 1). For six isolates resistant to all the comparator β-lactams (MICs, 4 to 64 μg/ml), ceftobiprole MICs were ≤1 μg/ml (Table 1). These isolates (genotype 5) had the same PBP substitutions as genotype 4 isolates, with the addition of an M-to-F change in the STMK motif of PBP 2x (Table 1).
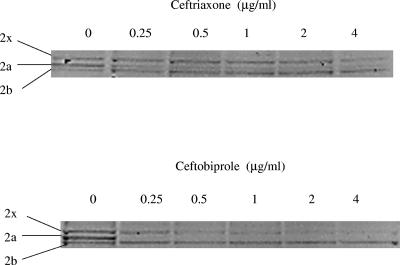
Against the penicillin- and ceftriaxone-resistant isolate 8819, ceftobiprole had a higher affinity for PBP 2x than did ceftriaxone, with 50% inhibitory concentrations (IC50s) of 0.5 μg/ml and 4 μg/ml for ceftobiprole and ceftriaxone, respectively (Fig. 1). Both drugs had low affinities (IC50s of >4 μg/ml) for PBP 2b (Fig. 1), as expected, since cephalosporins do not have PBP 2b as a primary target (8).
FIG. 1.
Binding of ceftobiprole and ceftriaxone to PBPs 2x, 2a, and 2b from S. pneumoniae isolate 8819 (penicillin resistant, ceftriaxone resistant).
In this study, the PBP 1a, 2b, and 2x substitutions found in and adjacent to the penicillin-binding motifs, SXXK, SXN, and KT/SG, were similar to substitutions reported by others (1, 7, 16, 17, 21). Recent reports identified additional PBP 2x substitutions important for the development of high-level β-lactam resistance in clinical isolates, with I371T, R384G, and M400T mutations having the greatest impact on resistance (4, 23). The I371T and R384G substitutions were found in all isolates with genotypes 2 to 5. These substitutions impede β-lactam binding by causing a conformational change in a loop that is adjacent to the entrance of the active-site cavity (4). The M400T substitution was found only in isolates with genotype 5. This substitution appears to be important for a low acylation efficiency and is found in association with the well-characterized PBP 2x substitution M339F (4). In our experiments, ceftobiprole was able to bind to this mutated PBP 2x protein (IC50, 0.5 μg/ml) and was the only β-lactam tested that had low MICs (0.5 to 1 μg/ml) for isolates with this PBP 2x substitution.
Previous reports by Kosowska et al. (14), who used agar dilution assays, and Hebeisen et al. (12) identified ceftobiprole MICs as high as 2 to 4 μg/ml for penicillin-resistant S. pneumoniae strains. However, like Jones et al. (13), we did not identify any ceftobiprole MICs of >1 μg/ml in microdilution broth assays. It will be important in the future to determine whether additional PBP mutations are associated with high ceftobiprole MICs or whether there were MIC differences due to different testing methodologies.
In summary, six PBP genotypes were found among the 30 pneumococcal isolates, with ceftobiprole having the lowest MICs for the organisms. The highest ceftobiprole MIC for our collection was 1 μg/ml for isolates that were resistant to the other β-lactams (MICs of 16 to 64 μg/ml). β-Lactam MIC increases correlated closely with increases in the numbers of PBP 1a, 2x, and 2b substitutions. These results explain why ceftobiprole retains activity against S. pneumoniae isolates resistant to extended-spectrum cephalosporins and penicillins.
Acknowledgments
This work was supported by Johnson & Johnson Pharmaceutical Research & Development, LLC.
REFERENCES
- 1.Asahi, Y., Y. Takeuchi, and K. Ubukata. 1999. Diversity of substitutions within or adjacent to conserved amino acid motifs of penicillin-binding protein 2X in cephalosporin-resistant Streptococcus pneumoniae isolates. Antimicrob. Agents Chemother. 43:1252-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asahi, Y., and K. Ubukata. 1998. Association of a Thr-371 substitution in a conserved amino acid motif of penicillin-binding protein 1A with penicillin resistance of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 42:2267-2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartlett, J. G., S. F. Dowell, L. A. Mandell, T. M. File, D. M. Musher, and M. J. Fine. 2000. Practice guidelines for the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 31:347-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carapito, R., L. Chesnel, T. Vernet, and A. Zapun. 2006. Pneumococcal β-lactam resistance due to a conformational change in penicillin-binding protein 2x. J. Biol. Chem. 281:1771-1777. [DOI] [PubMed] [Google Scholar]
- 5.Clinical Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard, 7th ed. M7-A7. Clinical Laboratory Standards Institute, Wayne, Pa.
- 6.Clinical Laboratory Standards Institute. 2006. Performance standards for antimicrobial susceptibility testing. Sixteenth informational supplement. M100-S16. Clinical Laboratory Standards Institute, Wayne, Pa.
- 7.Coffey, T. J., M. Daniels, L. K. McDougal, C. G. Dowson, F. C. Tenover, and B. G. Spratt. 1995. Genetic analysis of clinical isolates of Streptococcus pneumoniae with high-level resistance to expanded-spectrum cephalosporins. Antimicrob. Agents Chemother. 39:1306-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grebe, T., and R. Hakenbeck. 1996. Penicillin-binding proteins 2b and 2x of Streptococcus pneumoniae are primary resistance determinants for different classes of β-lactam antibiotics. Antimicrob. Agents Chemother. 40:829-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hakenbeck, R. 1999. β-Lactam-resistant Streptococcus pneumoniae. Epidemiology and evolutionary mechanism. Chemotherapy 45:83-94. [DOI] [PubMed] [Google Scholar]
- 10.Hakenbeck, R. 1998. Mosaic genes and their role in penicillin-resistant Streptococcus pneumoniae. Electrophoresis 19:597-601. [DOI] [PubMed] [Google Scholar]
- 11.Hakenbeck, R., A. Konig, I. Kern, M. Van Der Linden, W. Keck, D. Billot-Klein, R. Legrand, B. Schoot, and L. Gutmann. 1998. Acquisition of five high-Mr penicillin-binding protein variants during transfer of high-level β-lactam resistance from Streptococcus mitis to Streptococcus pneumoniae. J. Bacteriol. 180:1831-1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hebeisen, P., I. Heinze-Krauss, P. Angehrn, P. Hohl, M. G. P. Page, and R. L. Then. 2001. In vitro and in vivo properties of Ro 63-9141, a novel broad-spectrum cephalosporin with activity against methicillin-resistant staphylococci. Antimicrob. Agents Chemother. 45:825-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones, R. N., L. M. Deshpande, A. H. Mutnick, and D. J. Biedenbach. 2002. In vitro evaluation of BAL9141, a novel parenteral cephalosporin active against oxacillin-resistant staphylococci. J. Antimicrob. Chemother. 50:915-932. [DOI] [PubMed] [Google Scholar]
- 14.Kosowska, K., D. B. Hoellman, G. Lin, C. Clark, K. Credito, P. McGhee, B. Dewasse, B. Bozdogan, S. Shapiro, and P. C. Appelbaum. 2005. Antipneumococcal activity of ceftobiprole, a novel broad-spectrum cephalosporin. Antimicrob. Agents Chemother. 49:1932-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laible, G., B. G. Spratt, and R. Hakenbeck. 1991. Interspecies recombinational events during the evolution of altered PBP 2x genes in penicillin-resistant clinical isolates of Streptococcus pneumoniae. Mol. Microbiol. 5:1993-2002. [DOI] [PubMed] [Google Scholar]
- 16.Nagai, K., T. A. Davies, M. R. Jacobs, and P. C. Appelbaum. 2002. Effects of amino acid alterations in penicillin-binding proteins (PBPs) 1a, 2b, and 2x on PBP affinities of penicillin, ampicillin, amoxicillin, cefditoren, cefuroxime, cefprozil, and cefaclor in 18 clinical isolates of penicillin-susceptible, -intermediate, and -resistant pneumococci. Antimicrob. Agents Chemother. 46:1273-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nichol, K. A., G. G. Zhanel, and D. J. Hoban. 2002. Penicillin-binding protein 1A, 2B, and 2X alterations in Canadian isolates of penicillin-resistant Streptococcus pneumoniae. Antimicrob. Agents Chemother. 46:3261-3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niederman, M. S., L. A. Mandell, A. Anzueto, J. B. Bass, W. A. Broughton, G. D. Campbell, N. Dean, T. File, M. J. Fine, P. A. Gross, F. Martinez, T. J. Marrie, J. F. Plouffe, J. Ramirez, G. A. Sarosi, A. Torres, R. Wilson, and V. L. Yu. 2001. Guidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am. J. Respir. Crit. Care Med. 163:1730-1754. [DOI] [PubMed] [Google Scholar]
- 19.Sahm, D. F. 2003. Resistance issues and community-acquired respiratory infections. Clin. Cornerstone 5(Suppl. 3):S4-S11. [DOI] [PubMed] [Google Scholar]
- 20.Sifaoui, F., M.-D. Kitzis, and L. Gutmann. 1996. In vitro selection of one-step mutants of Streptococcus pneumoniae resistant to different oral β-lactam antibiotics is associated with alterations of PBP2x. Antimicrob. Agents Chemother. 40:152-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith, A. M., and K. P. Klugman. 1998. Alterations in PBP 1A essential for high-level penicillin resistance in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 42:1329-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith, A. M., and K. P. Klugman. 1995. Alterations in penicillin-binding protein 2B from penicillin-resistant wild-type strains of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 39:859-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith, A. M., and K. P. Klugman. 2005. Amino acid mutations essential to production of an altered PBP 2X conferring high-level β-lactam resistance in a clinical isolate of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 49:4622-4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zbinden, R., V. Punter, and A. Von Graevenitz. 2002. In vitro activities of BAL9141, a novel broad-spectrum pyrrolidinone cephalosporin, against gram-negative nonfermenters. Antimicrob. Agents Chemother. 46:871-874. [DOI] [PMC free article] [PubMed] [Google Scholar]