Abstract
We investigated the distribution of the broad-spectrum antibiotic fosfomycin in infected soft tissue of patients with uncomplicated cellulitis of the lower extremities or diabetic foot infection using in vivo microdialysis. Our findings suggest that fosfomycin exhibits good and similar penetration into the fluid in the interstitial space in inflamed and noninflamed soft tissue in patients.
Severe soft tissue infections are a frequent problem in clinical practice. In diabetic patients, the combination of peripheral neuropathy and peripheral arterial occlusive disease (PAOD), and the increased likelihood of traumatic foot lesions predispose patients to soft tissue infections (3). Immediate empirical antibiotic therapy is mandatory long before information about the causal bacteria and their in vitro sensitivity against antimicrobial agents is available to prevent progressive tissue destruction or even life-threatening complications, such as septicemia (2). The successful treatment of bacterial infections depends not only on the choice of an appropriate antimicrobial agent for the microorganisms involved but also on sufficient levels of the antimicrobial agent in the interstitial fluid of the involved peripheral tissue. In a recent study, Frossard et al. (5) showed that fosfomycin, a drug used for the treatment of uncomplicated lower urinary tract infections (9), exhibits a strong ability to penetrate into interstitial fluid of unaltered human soft tissue. The concentration of fosfomycin in the fluid in interstitial space was high enough to efficiently eradicate selected bacterial isolates, such as Staphylococcus aureus, in vitro (5). The excellent tissue penetration of fosfomycin into noninflamed tissue of young volunteers and the fact that S. aureus is one of the bacteria most often isolated in severe soft tissue infections prompted us to investigate the penetration of fosfomycin in inflamed tissue of elderly patients with severe uncomplicated cellulitis of lower extremities or diabetic foot infection, respectively, using in vivo microdialysis (MD).
This was an open study performed at one center. It was approved by the ethics committee of the University Hospital Graz and performed in accordance with the Declaration of Helsinki and the Good Clinical Practice Guidelines of the European Commission. Informed consent was obtained from all patients. The first group included six patients (three females and three males; mean age, 61.7 ± 3.9 years; mean weight, 69.0 ± 7.8 kg; mean height, 165.3 ± 7.6 cm [mean ± standard deviation given for each variable for each group]) with severe uncomplicated cellulitis. One patient was diabetic and had PAOD stage IIa (according to Fontaine). The second group was six patients (three females and three males; mean age, 62.5 ± 7.1 years; mean weight, 76.3 ± 20.8 kg; mean height, 168 ± 11.8 cm) with diabetic foot infections. These six patients had insulin-dependent (three of six) or non-insulin-dependent (three of six) diabetes mellitus for 12.9 ± 9.6 years. Five patients had PAOD stage I (three of six) or IIa (two of six). Upon admission to the hospital, intravenous (i.v.) antibacterial therapy with fosfomycin plus clindamycin (1,800 mg/day) (in two patients with cellulitis and four patients with diabetic foot infection) or fosfomycin plus ceftriaxone (2 g/day) (in four patients with cellulitis and two patients with diabetic foot infection) was initiated. Antimicrobial therapy with fosfomycin was combined with either ceftriaxone or clindamycin to broaden the antimicrobial spectrum and to prevent rapid development of drug resistance reported for fosfomycin monotherapy (14). The numbers of other nonantibacterial drugs used per patient were 4 ± 3.2 and 5.2 ± 2.9 in patients with cellulitis and diabetic foot infection, respectively.
Each patient received fosfomycin (Biochemie GmbH, Vienna, Austria) in a daily dosage of about 200 mg/kg of body weight divided into three equal i.v. doses over 30 min every 8 h. To be practicable, the dose of each fosfomycin application was rounded to the nearest gram. Thus, the applied daily doses of fosfomycin were 14 ± 1.5 g (204.4 ± 14.7 mg/kg) in patients with cellulitis and 15.5 ± 3.9 g (203.3 ± 12.7 mg/kg) in patients with diabetic foot infection, i.e., 4.7 ± 0.5 g (68 ± 4.9 mg/kg) and 5.2 ± 1.2 g (67.8 ± 4.9 mg/kg) per single i.v. infusion, respectively. To ensure consistent experimental conditions, administration of fosfomycin was synchronized after the first dose to the usual time schedule with infusions starting at 0800, 1600, and 2400 h.
On day 3 of treatment, fosfomycin concentrations in the interstitial fluid of human soft tissues in vivo were measured by in vivo MD (13). Briefly, MD is based on sampling of analytes from the interstitial space by means of a semipermeable membrane at the tip of a MD probe. The probe is constantly perfused with a physiological solution at a constant flow rate (1.5 μl/min). Once the probe is implanted into the tissue, substances present in the interstitial fluid (concentration in tissue [Ctissue]) are filtered by diffusion out of the interstitial fluid into the probe, resulting in a concentration (Cdialysate) in the perfusion medium. For most analytes, equilibrium between the concentration in interstitial fluid of human soft tissue and the concentration in the perfusion medium is incomplete; therefore, Ctissue is greater than Cdialysate. The process by which these concentrations are interrelated is termed in vivo recovery. Therefore, to obtain absolute concentrations in interstitial fluid from concentrations in the dialysate, MD probe calibration was assessed by the retrodialysis method (8, 11). This method is based on the assumption that the diffusion process is quantitatively equal in both directions through the semipermeable membrane (15). Thus, during retrodialysis, fosfomycin was added to the perfusion medium, and the rate of disappearance through the membrane was taken as the in vivo recovery value. In vivo recovery was calculated as follows: percent recovery = 100 − (100 × fosfomycin Cdialysate × fosfomycin Cperfusate−1). In our study, in vivo recovery was assessed for each experiment by dialyzing inflamed or noninflamed subcutaneous tissue with a perfusion medium containing 20 μg of fosfomycin per ml. In vivo recovery was assessed before the i.v. application of fosfomycin at 1600 h on day 3 of fosfomycin treatment. At this time point, the presence of low concentrations of fosfomycin in interstitial fluid could be assumed. We cannot exclude the possibility that this may have affected the in vivo recovery assessment and the eventual calculation of the absolute concentrations of fosfomycin in interstitial fluid in our experiments. Theoretically, it could have caused a reduction of the calculated in vivo recovery, leading to an overestimation of the concentrations of fosfomycin in interstitial fluid. We believe, however, that this potential error is not large enough to affect the conclusions of this study.
In this study, MD probes (CMA 10; CMA Microdialysis AB, Stockholm, Sweden) (molecular size cutoff, 20 kDa; outer diameter, 0.5 mm; membrane length, 16 mm) were inserted into the upper subcutaneous layer of the thigh (noninflamed area) and into the upper subcutaneous layer within the area of inflammation by a previously described method (13). The MD system was connected and perfused at a flow rate of 1.5 μl/min with Ringer's solution except during the in vivo calibration period. Continuous perfusion was performed with a microinfusion pump (CMA/100; CMA Microdialysis AB). Thirty minutes after the probe was inserted, an in vivo probe calibration was performed for 40 min, which was followed by a 30-min washout period. Fosfomycin was then administered i.v. over a 30-min period at the aforementioned dose. Sampling was continued at 20-min intervals for up to 8 h. Microdialysates were immediately frozen and stored at −80°C until analysis. Venous blood was simultaneously taken at 20-min intervals according to the time of sampling of the microdialysates and was centrifuged at 1,500 × g for 3 min. Venous plasma was then pipetted into polypropylene tubes, immediately frozen, and stored at −80°C. Fosfomycin was analyzed by a previously published modified gas chromatographic method (4, 5). The limit of detection was 1 μg/ml.
For pharmacokinetic analysis, data were fitted by a commercially available computer program (Kinetica version 3.0; InnaPhase Corp., Philadelphia, Pa.) according to a two-compartment model for plasma and peripheral compartment values. The time to the maximal concentration (Tmax) and the maximal concentration of the drug (Cmax) were calculated for plasma and subcutaneous tissue. The area under the concentration-time curve (AUC) from 0 to 8 h (AUC0-8) was determined for plasma (AUC0-8, plasma) and noninflamed subcutaneous tissue (AUC0-8, tissue) and inflamed subcutaneous tissue (AUC0-8, inflamed) by the trapezoid rule. As a measure of drug penetration into noninflamed and inflamed tissue, the AUC0-8, tissue/AUC0-8, plasma and AUC0-8, inflamed/ AUC0-8, plasma ratios were determined. Concentrations in interstitial fluid were calculated from the dialysate as described previously (8). For comparisons between pharmacokinetic parameters for different compartments, the Wilcoxon signed rank test was used and P < 0.05 was set as the level of statistical significance (StatView version 5.0; SAS Institute Inc., Cary, N.C.).
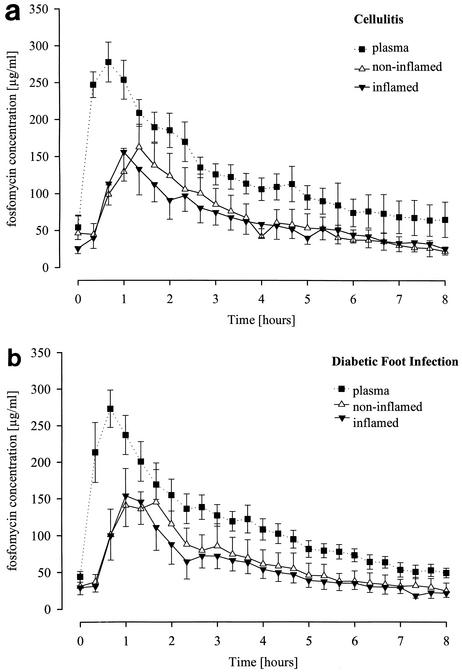
The time-versus-concentration profiles and the pharmacokinetic parameters of fosfomycin following the i.v. infusion of fosfomycin were determined for plasma and fluid in the interstitial space in inflamed and noninflamed subcutaneous tissue in patients with cellulitis (Fig. 1a and Table 1) and in patients with diabetic foot infection (Fig. 1b and Table 1). In both groups of patients, the fosfomycin AUC0-8 values in plasma (AUC0-8, plasma) were significantly greater than the AUC0-8 values in noninflamed tissue (AUC0-8, tissue) and the AUC0-8 values in inflamed tissue (AUC0-8, inflamed). In patients with cellulitis, the AUC0-8, inflamed/AUC0-8, plasma and AUC0-8, tissue/AUC0-8, plasma ratios were 0.70 ± 0.27 and 0.60 ± 0.22, respectively. In patients with diabetic foot infection, the AUC0-8, inflamed/AUC0-8, plasma and AUC0-8, tissue/AUC0-8, plasma ratios were 0.62 ± 0.35 and 0.73 ± 0.61, respectively. However, in patients with cellulitis and in patients with diabetic foot infection, the AUC0-8, tissue and AUC0-8, inflamed values were not significantly different from each other.
FIG. 1.
Profiles of time versus fosfomycin concentration in plasma and fluid in interstitial space in noninflamed and inflamed subcutaneous soft tissue in patients with cellulitis (a) or diabetic foot infection (b). Patients with severe uncomplicated cellulitis of lower extremities were given 68 ± 4.9 mg of fosfomycin per kg of body weight, and patients with diabetic foot infection were given 67.8 ± 4.9 mg/kg of body weight. Fosfomycin was infused i.v. for 30 min starting at time zero (t = 0 min). Results are given as means ± SEMs (six patients in each group).
TABLE 1.
Pharmacokinetic parameters for fosfomycin in plasma and subcutaneous tissue, noninflamed and inflamed, in patients with cellulitis or diabetic foot infection
| Fluida | Fosfomycin pharmacokinetic parametersb
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Patients with cellulitis
|
Patients with diabetic foot infection
|
|||||||
| Cmax (μg/ml) | C8h (μg/ml) | Tmax (h) | AUC0-8 (μg · h/ml) | Cmax (μg/ml) | C8h (μg/ml) | Tmax (h) | AUC0-8 (μg · h/ml) | |
| Plasma | 344 ± 53.6 | 65.0 ± 58.4 | 1,050 ± 139 | 320 ± 67.4 | 49.2 ± 15.9 | 1,331 ± 429 | ||
| s.c. tissue fluid | ||||||||
| Noninflamed | 141 ± 68.6 | 22.0 ± 15.1 | 1.13 ± 0.29 | 742 ± 483 | 136 ± 106.6 | 24.8 ± 26.2 | 1.15 ± 0.47 | 937 ± 848 |
| Inflamed | 150 ± 70.6 | 25.2 ± 19.2 | 0.78 ± 0.31 | 757 ± 492 | 139 ± 76.7 | 21.7 ± 13.7 | 0.90 ± 0.22 | 782 ± 524 |
s.c. tissue fluid, fluid in interstitial space in subcutaneous tissue.
Data are means ± standard deviations (six patients per group). See text for dosage and administration schedules. C8h, concentration at 8 h.
Inflammation induced by bacterial invasion into previously healthy peripheral soft tissues alters the microenvironment as a consequence of plasma protein extravasation and edema formation (7). In patients with long-lasting diabetes mellitus, the microenvironment in peripheral tissues is already altered by peripheral neuropathy as well as by macro- and microangiopathy (3). Thus, in diabetic patients, peripheral soft tissue infections, in particular of the feet, further disturb the microenvironment at the inflammation site. In addition, different degrees of accompanying PAOD in patients with diabetes mellitus might reduce the tissue penetration of antimicrobial agents, a phenomenon that was shown by Joukhadar et al. (6). Considering these factors, a reduced tissue penetration of fosfomycin in patients with cellulitis or diabetic foot infection could be expected. In our patients suffering from severe uncomplicated cellulitis or diabetic foot infection, the AUC values determined for inflamed subcutaneous tissue after the i.v. infusion of fosfomycin were not significantly different from those for noninflamed tissue. Thus, a similar distribution of fosfomycin in inflamed and noninflamed subcutaneous tissue is suggested. The mean AUC for subcutaneous tissue (AUCtissue)/AUCplasma ratios of 0.6 to 0.73 obtained in our patients are similar to the AUCtissue/AUCplasma ratios of 0.7 to 0.75 obtained in young healthy volunteers by Frossard et al. (5). These data indicate that the soft tissue penetration for fosfomycin in patients with bacterial tissue infections is similar to soft tissue penetration in healthy volunteers. The AUC ratios for inflamed and noninflamed subcutaneous tissue of our patients further indicate similar distributions of fosfomycin in inflamed and noninflamed tissues. The 30% lower AUC values for subcutaneous tissues compared to the AUC values for plasma in both healthy volunteers and our patients is probably due to diffusion barriers and not to protein binding of fosfomycin in plasma, which is negligible (14). The slightly lower AUCtissue/AUCplasma ratios in our patients compared to the values in young healthy volunteers found by Frossard et al. (5) might result from the different ages and concomitant medications taken by the two sets of patients, which reportedly can affect drug kinetics in patients (12). A possible release of a variety of systemically active mediators during inflammatory processes (1, 10) could also affect drug distribution from plasma to the fluid in the interstitial space.
In this study, in vivo MD was performed on the third day of fosfomycin treatment, and steady-state conditions for the concentration measurements can be assumed. The mean fosfomycin concentration in interstitial fluid in inflamed subcutaneous tissue 8 h after a mean dose of 5 g of fosfomycin (given i.v.) was about 22 to 25 μg/ml (Table 1), and in none of our patients was it below 6 μg/ml. Frossard et al. (5) reported mean fosfomycin concentrations of about 5 and 14 μg/ml in unaltered subcutaneous tissue of volunteers 8 h after single doses of 4 or 8 g of fosfomycin given i.v. The AUC values for plasma and the interstitial fluid of inflamed and noninflamed subcutaneous tissues of our patients were also higher than those obtained by Frossard et al. (5) after single i.v. infusions of 4 or 8 g of fosfomycin, although the AUCtissue/AUCplasma ratios were similar in both studies. In their study, Frossard et al. (5) could show that the in vitro exposure of different isolates of bacteria, e.g., S. aureus, to fosfomycin concentrations according to the time-versus-concentration profile in interstitial fluid obtained by MD after these single i.v. infusions of 4 or 8 g of fosfomycin efficiently inhibited bacterial growth (5). S. aureus was also the bacterium most often isolated (50% of cases) from soft tissue infection sites by swab culture technique in our patients.
Taken together, these findings suggest that in our patients the i.v. application of fosfomycin in a daily dose of 200 mg/kg of body weight (divided into three equal doses; i.e., 4 to 5 g per i.v. infusion) were sufficient to reach and maintain fosfomycin concentrations in the fluid in interstitial space in inflamed tissue to inhibit the growth of relevant bacteria like S. aureus. Thus, fosfomycin might qualify as an alternative candidate for the treatment of soft tissue infections, such as severe uncomplicated cellulitis and diabetic foot infection.
Acknowledgments
We thank Susanne Siedler and Robert Müllegger for their help in recruiting patients and for fruitful discussions during the preparation and performance of the study.
This study was supported in part by Biochemie GmbH, Vienna, Austria.
REFERENCES
- 1.Bellomo, R. 1992. The cytokine network in the critically ill. Anaesth. Intensive Care 20:288-302. [DOI] [PubMed] [Google Scholar]
- 2.Caputo, G. M., P. R. Cavanagh, J. S. Ulbrecht, G. W. Gibbons, and A. W. Karchmer. 1994. Assessment and management of foot disease in patients with diabetes. N. Engl. J. Med. 331:854-860. [DOI] [PubMed] [Google Scholar]
- 3.Chantelau, E. 1999. Zur Pathogenese der diabetischen Podopathie. Internist 40:994-1001. [DOI] [PubMed] [Google Scholar]
- 4.Dios-Vieitez, M. C., M. M. Goni, M. J. Renedo, and D. Fos. 1996. Determination of fosfomycin in human urine by capillary gas chromatography: application to clinical pharmacokinetic studies. Chromatographia 43:293-295. [Google Scholar]
- 5.Frossard, M., C. Joukhadar, B. M. Erovic, P. Dittrich, P. E. Mrass, M. van Houte, H. Burgmann, A. Georgopoulos, and M. Müller. 2000. Distribution and antimicrobial activity of fosfomycin in the interstitial fluid of human soft tissues. Antimicrob. Agents Chemother. 44:2728-2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joukhadar, C., N. Klein, M. Frossard, E. Minar, H. Stass, E. Lackner, M. Herrmann, E. Riedmüller, and M. Müller. 2001. Angioplasty increases target site concentrations of ciprofloxacin in patients with peripheral arterial occlusive disease. Clin. Pharmacol. Ther. 70:532-539. [DOI] [PubMed] [Google Scholar]
- 7.Maeda, H., T. Akaike, J. Wu, Y. Noguchi, and Y. Sakata. 1996. Bradykinin and nitric oxide in infectious disease and cancer. Immunopharmacology 33:222-230. [DOI] [PubMed] [Google Scholar]
- 8.Müller, M., O. Haag, T. Burgdorff, A. Georgopoulos, W. Weninger, B. Jansen, G. Stanek, E. Agneter, H. Pehamberger, and H. G. Eichler. 1996. Characterization of peripheral compartment kinetics of antibiotics by in vivo microdialysis in humans. Antimicrob. Agents Chemother. 40:2703-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel, S. S., J. A. Balfour, and H. M. Bryson. 1997. Fosfomycin tromethamine. A review of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy as a single dose oral treatment for acute uncomplicated lower urinary tract infections. Drugs 53:637-656. [DOI] [PubMed] [Google Scholar]
- 10.Sachs, M. K. 1991. Cutaneous cellulitis. Arch. Dermatol. 127:1845-1846. [PubMed] [Google Scholar]
- 11.Stahle, L., P. Arner, and U. Ungerstedt. 1991. Drug distribution studies with microdialysis. III. Extracellular concentration of caffeine in adipose tissue in man. Life Sci. 49:1853-1858. [DOI] [PubMed] [Google Scholar]
- 12.Swift, C. G. 1994. Pharmacokinetics and prescribing in the elderly. J. Antimicrob. Chemother. 34(Suppl. A):25-32. [DOI] [PubMed] [Google Scholar]
- 13.Ungerstedt, U. 1991. Microdialysis-principles and applications for studies in animal and man. J. Intern. Med. 230:365-373. [DOI] [PubMed] [Google Scholar]
- 14.Wildling, E., F. Stauffer, S. Breyer, O. Janata, H. Burgmann, A. Georgopoulos, and W. Graninger. 1992. Fosfomycin, eine therapeutische Alternative bei schwer zu behandelnden Infektionen. Antibiot. Monit. 8:87-90. [Google Scholar]
- 15.Zhao, Y., X. Liang, and C. E. Lunte. 1995. Comparison of recovery and delivery in vitro for calibration of microdialysis probes. Anal. Chim. Acta 316:403-410. [Google Scholar]