The neurobiological mechanisms that underlie depression are not yet entirely clear, and their relationship to the psychological changes which accompany depression have not been explored. Cognitively, it appears that there is a change in the processing of information, often exacerbated by stressful events and loss of control. This leads to the overvaluing of negative interpretations and a sense of hopelessness and helplessness, which characterize the psychological state in depression. Antidepressant medications, electroconvulsive therapy (ECT), cognitive behavioral therapy and interpersonal psychotherapy can reverse this process.
Much of our knowledge about the potential neurobiological changes in depression results from studying the factors that change during the medical treatment of depression. Thus far a series of modulating neurotransmitter systems have been implicated. These mainly include norepinephrine (NE), serotonin (5-HT), and some peptides such as corticotropin-releasinghormone (CRH) (1), substance P (2) and the endorphins (3). At times the changes leading to depression appear to arise spontaneously, perhaps driven by genetic factors, while at other times they seem to be related to stressors involving loss and loss of control. This observation led Seligman to attempt to create an animal model of depression using aversive stressors in an unpredictable fashion that would lead to a sense of lost control for the animal. This resulted in the development of the learned helplessness model of depression (4). In this model, animals exposed to unpredictable and uncontrollable aversive stimuli alter their behavior in that they no longer try to escape from aversive situations from which they could escape. These animals also develop many of the signs of depression, including weight loss, sleep changes with decreased rapid eye movement (REM) sleep, decreased libido, and increased cortisol secretion. These changes persist for weeks but can be reversed with antidepressant treatment (3). Using this model we wanted to examine the neurobiological mechanisms by which learning reverses helplessness compared to antidepressant medications.
To date we have been able to show that in learned helplessness both the 5-HT and the NE systems show alterations when the animal becomes helpless. Reliable markers of helplessness in these systems are an increase in hippocampal NE beta receptor (5) and 5-HT-1b receptor density (6). Analogous to the situation seen clinically in depression, cortisol, while increased on average, does not show an increase in all helpless animals (7), so that it cannot be seen as a suitable marker. We followed the NE beta receptor density as a function of various treatments, in an effort to determine if the same neurobiological mechanisms came into play in all cases. Initially, we carried out an experiment to examine how closely the level of noradrenergic beta receptor would correlate with helpless behavior, in order to determine if it would serve as a valid marker of the neurobiological mechanisms brought into play in reversing depression. We used this marker in the current study to monitor neurobiological change as a function of pharmacological or behavioral treatment.
METHODS
Learned helplessness training and testing
The animals were initially exposed to a training session during which they experienced a series of random foot shocks of 0.8 mA intensity. The shocks were of varying lengths over 1.5 seconds and with random intervals between them. The sessions lasted for 40 minutes with the rats experiencing a total of 20 minutes of shocks. On the following day the animals were tested in a grid cage with a light cue. They received a 60 second shock of 0.8 mA pulsing on and off with a 35 ms frequency concurrently with a cue light. The shock could be terminated if the rat pressed a bar in the cage. Each trial in which the rat pressed the bar in under 20 seconds was counted as an escape. Initially, rats would press the bar inadvertently by jumping during the shock and then learn that bar pressing terminated the shock. Rats that had fewer than five escapes in 15 trials were termed helpless, while rats with more than ten were considered not helpless and served as similarly stressed control animals. During any training and testing session, 15 to 20% of the animals were normally found to develop helplessness. They maintained an unchanged level of helplessness for two weeks using this paradigm and then gradually remitted.
Behavioral training and medication treatment
Behavioral training was carried out using a rat harness, which encased the front paws and had threads to each paw whereby the rat could be walked around the cage without having to be touched. The helpless rats were given a signal light and then walked to the bar press to terminate the shock, which began 5 seconds after the light signal. After ten days, most rats had learned to anticipate the shock when being presented with the light signal and went to the bar without assistance. Medication treatment was carried out through daily i.p. injections. The course of treatment ran over five days, in which time a behavioral response was seen. The administered drug dosages were imipramine 18 mg/kg, fluvoxamine 12 mg/kg and mianserin 8 mg/kg all given once daily i.p.
Beta receptor binding
The binding was carried out using methods described by Martin et al (5). Two ligands, 3H-CGP12177 (8) and 125I-iodocyanopindolol (125ICYP) (9), were used and the results compared and found to be identical. Rat hippocampal membranes were homogenized, washed in sodium phosphate buffer, and centrifuged at 25,000 g at 4° C for 10 min. The pellet was resuspended and used for the binding assay. 125ICYP was utilized in a concentration range of 30 to 240 pM, while the CGP concentration ranged from 0.05 to 5.0 nM. In both cases non-specific binding was determined by adding 2 molar d-l propranolol. B-max was determined in fmol/mg protein as a measure of numbers of binding sites. The control values were taken as 100% and correspond to a value of 55 fmol/mg protein.
RESULTS
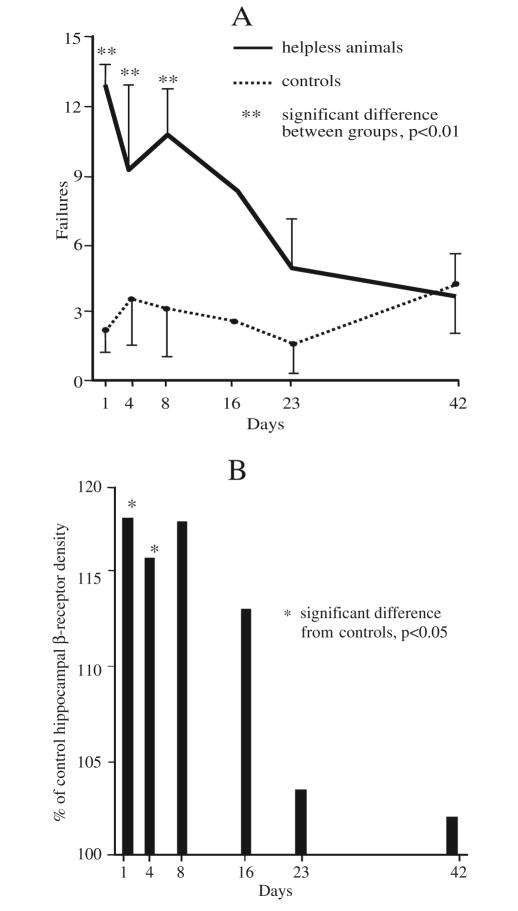
Figure 1 illustrates the correlation of helpless behavior and NE beta receptor levels in the hippocampus as a function of time. A large series of animals were trained and tested for helplessness. At selected time intervals after the training, a subset of the animals were then retested behaviorally and beta receptor levels in the hippocampus were determined. The curve of behavior vs. time shows that the animals spontaneously gradually lose their helpless behavior. In parallel the curve for the beta receptor levels as a function of time also reverts toward normal. The correlation between the two curves is very good and illustrates that the change in NE beta receptor is a valid marker of helpless behavior and can be used to track the neurobiological effects of antidepressant drugs and behavioral training in helpless animals. It should be noted that on day 3 the animals tested had a somewhat reduced behavioral score, indicating that in this group some animals were spontaneously reverting to non-helpless behavior. This is mirrored in the beta receptor levels, suggesting that the correlation between these variables is very tight.
Figure 1.
A: Number of failures to escape from footshock in 15 trials, in helpless animals compared to controls. B: Hippocampal beta receptor density in helpless animals at different time points.
Table 1 indicates that either the treatment with imipramine, fluoxamine or mianserin, or a behavioral training experience all lead to a rapid reversal of the helpless behavior. While the pharmacological treatments appear to work within five days, the behavioral approach generally showed its effectiveness within ten days and no attempt to optimize the training was attempted. When the animals were examined for levels of NE beta receptors, all three groups had receptor levels that had reverted to normal, non-helpless values. This suggests that in the central nervous system of helpless animals similar changes take place under the influence of antidepressant medication and targeted behavioral training. In the case of fluoxamine and mianserin, our experiments demonstrate that while pathologically up-regulated receptors are normalized, wild type receptors are not down-regulated, in contrast to imipramine, which down-regulates all NE beta receptors.
Table 1.
Neurochemical and behavioral response to treatment
| Beta receptors density | Behavior failures | |
|---|---|---|
| in % of control value | (out of 15 trials) | |
| Helpless | 122 ± 5 | 12.3 ± 0.2 |
| Imipramine | 98 ± 3 * | 3.8 ± 0.2 * |
| Mianserin | 93 ± 4 * | 4.2 ± 0.3 * |
| Fluvoxamine | 95 ± 2 * | 4.8 ± 0.4 * |
| Behavioral training | 101 ± 3 * | 3.9 ± 0.6 * |
* Significantly different from helpless value, p < 0.0001
DISCUSSION
These results suggest that, at the neurobiological level, both a psychological approach involving learning and a medical approach involving antidepressant medications may act through the same pathways. This suggests that no dichotomy exists between a psychotherapeutic approach and a medical one in terms of central mechanisms, indicating the necessity for developing measures to monitor such changes in the central nervous system of patients, which in turn could then be used to maximize response. The dichotomy between a biological approach and a psychological one appears artificial, as both are aimed at altering the functional organization of the central nervous system in such a way as to normalize responses to stress.
A first drawback of this study is the choice of one arbitrary step in a complex neurobiological mechanism to act as a marker of the complete neurobiological process. Secondly, the study uses methods that are only applicable to animal research.
The chain of events that trigger a depressive episode is thought to involve activation of c-AMP responsive binding element (CREB) and subsequent gene activation. In our view depression arises when specific structural changes involving decreasing synaptic contacts take place. The reversal of depression, as hypothesized by Duman et al (10), involves increasing synaptogenesis. This can be initiated by increasing beta receptor stimulation, among other modulators; this should lead to the down-regulation of receptor numbers, activation of CREB and induction of neurotrophic factors such as brain derived neurotrophic factor (BDNF), stimulating increased membrane synthesis, resulting in increased synaptic contacts. One way to monitor such effects in patients is to find a marker which reflects membrane synthesis. We believe that one such marker may be choline levels as measured by magnetic resonance spectroscopy. The levels measured are the sum of small choline containing molecules, that predominantly consist of phospholipids, which are the building blocks of cell membranes. In an initial attempt to use this marker, we found that choline levels were indeed decreased in the hippocampus of severely depressed patients and that these levels reverted to normal in all cases when the patients responded to a course of ECT (11). Thus, it should be possible to use this marker in patients receiving a variety of treatments, in order to determine whether the same final common pathway, namely new synapse formation, is increased in response to psychotherapy, antidepressant medication or ECT.
Acknowledgement
We would like to thank Diane Biale for performing the initial behavioral studies.
References
- 1.Keck ME. Holsboer F. Hyperactivity of CRH neuronal circuits as a target for therapeutic interventions in affective disorders. Peptides. 2001;22:835–844. doi: 10.1016/s0196-9781(01)00398-9. [DOI] [PubMed] [Google Scholar]
- 2.Kramer MS. Cutler N. Feighner J, et al. Distinct mechanisms for antidepressant activity by blockade of central substance P receptors. Science. 1998;281:1640–1645. doi: 10.1126/science.281.5383.1640. [DOI] [PubMed] [Google Scholar]
- 3.Henn FA. Edwards E. Muneyyirci J. Animal models of depression. Clin Neurosci. 1993;1:152–156. [Google Scholar]
- 4.Overmier JB. Seligman MEP. Effects of inescapable shock upon subsequent escape and avoidance responding. J Comp Physiol Psychol. 1967;64:28–33. doi: 10.1037/h0024166. [DOI] [PubMed] [Google Scholar]
- 5.Martin JV. Edwards E. Johnson JO, et al. Monoamine receptors in an animal model of affective disorder. J Neurochem. 1990;55:1142–1148. doi: 10.1111/j.1471-4159.1990.tb03117.x. [DOI] [PubMed] [Google Scholar]
- 6.Edwards E. Harkins K. Wright G, et al. Modulation of [3H] paroxetine binding to the 5-hydroxytryptamine uptake site in an animal model of depression. J Neurochem. 1991;56:1581–1585. doi: 10.1111/j.1471-4159.1991.tb02054.x. [DOI] [PubMed] [Google Scholar]
- 7.Greenberg L. Edwards E. Henn FA. Dexamethasone suppression test in helpless rats. Biol Psychiatry. 1989;26:530–532. doi: 10.1016/0006-3223(89)90074-7. [DOI] [PubMed] [Google Scholar]
- 8.Riva M. Creese I. Lack of serotonergic influence on desipramine-induced ß-adrenergic receptor down-regulation. Serotonin Symp. 1989;1:102. [Google Scholar]
- 9.Engel G. Identification of different subgroups of betareceptors by means of binding studies in guinea pig and human lung. Triangle. 1980;19:69–76. [PubMed] [Google Scholar]
- 10.Duman RS. Heninger GR. Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- 11.Ende G. Braus DF. Walter S, et al. The hippocampus in patients treated with electroconvulsive therapy. Arch Gen Psychiatry. 2000;57:937–943. doi: 10.1001/archpsyc.57.10.937. [DOI] [PubMed] [Google Scholar]