Abstract
Ionizing radiation therapy (RT) is an important local modality for the treatment of cancer. The current rationale for its use is based largely on the ability of RT to kill the cancer cells by a direct cytotoxic effect. Nevertheless, considerable evidence indicates that RT effects extend beyond the mere elimination of the more radio-sensitive fraction of cancer cells present within a tumor at the time of radiation exposure. For instance, a large body of evidence is accumulating on the ability of RT to modify the tumor microenvironment and generate inflammation. This may have far reaching consequences on the response of a patient to treatment, especially if radiation-induced tumor cell kill were to translate into the generation of effective anti-tumor immunity. Although much remains to be learned about how radiation can impact tumor immunogenicity, data from pre-clinical studies provide the proof of principle that different immunotherapeutic strategies can be combined with RT to enhance anti-tumor effects. Conversely, RT could reveal a useful tool to combine with immunotherapy.
This article will briefly summarize what is known about the impact of RT on tumor immunity, including tumor-associated antigens, antigen presenting cells, and effector mechanisms. In addition, the experimental evidence supporting the contention that RT can be used as a tool to induce anti-tumor immunity is discussed, and a new approach to radio-immunotherapy of cancer is proposed.
Keywords: Ionizing radiation, immunotherapy, cross-priming, tumor-associated antigens
Introduction
Conventional cytotoxic therapies like radiation and chemotherapy have been generally viewed as immunosuppressive. However, advances in the understanding of the mechanisms that regulate the development of anti-tumor immunity, as well as improved knowledge of the complex effects of radiation on tissues 1, have revived interest in the possibility of combining radiation and immune-based therapies to achieve a better local and systemic tumor control 2.
The concept that the immune system can distinguish the neoplastic from the normal self has been proposed almost a century ago 3. Since William Coley started treating patients at the end of the 19th century with bacterial toxins, there have been waves of enthusiasm for immunotherapy for treatment of cancer. The introduction of cytokines, in particular interleukin-2 (IL-2), for cancer treatment was a major clinical effort that had modest success. Until recently, however, these efforts have been hampered by a lack of molecular definition of tumor antigens, a means of delivering them effectively, and a sensitive and reliable way to measure responses. This situation changed with the molecular cloning of human tumor-associated antigens that could be recognized by T cells, the ability to culture powerful antigen presenting cells (APC) in the form of dendritic cells (DC), and to assess immune responses to specific tumor epitopes using tetramer and ELISPOT assays 4. These advances allied to the development of genetically modified mouse models have led to a deeper understanding of the interactions between cancer and the immune system of the host 5. The available experimental evidence supports the hypothesis that once tumors have become clinically apparent their immunogenicity has been modified by the selective pressure of the immune system, resulting in the growth of tumors that are characteristically poorly immunogenic, being able to escape immune detection and/or to actively inhibit immune effectors 5. Furthermore, it is clear that, although T cells become tolerant to many self antigens in the thymus, which depletes the pool that might react to cancer, tolerance to many self components is actively maintained in the periphery by several mechanisms. For example, immature DC presenting self antigens to T cells are tolerogenic. This peripheral tolerance can be broken by “maturation” of DC in local sites. The evolutionary purpose of this is to generate responses to invading pathogens but it leads to the belief that T cells can respond to “self” antigens on tumors, something for which there is now considerable evidence 6. The recognition of the fact that the host can break a state of tolerance that has developed to its own tumor offers many possibly effective immunotherapeutic strategies, some being currently tested in clinical trials.
In this paper, the basic knowledge about the interactions between tumors and the immune system, and the mechanisms that regulate the activation of cell-mediated immunity will be briefly reviewed, as will evidence for a possible role of radiation therapy in enhancing overall tumor immunogenicity and homing of effector immune cells to the tumor site. Strategies for combining the use of ionizing radiation and immunomodulators are proposed.
1. Tumor Antigens
The antigen specificity of T and B cells, i.e., their ability to recognize with extreme specificity the subtle differences that occur in normal cells upon infection or transformation, is one of the major appeals of immunotherapy. Truly tumor-specific antigens are rare. They can arise from point mutations 7 or other genetic alterations specific to a given tumor or group of tumors, such as fusion proteins generated by translocations 8, or sometimes from alterations in post-translational modification 9. Most of the tumor antigens that are targets for the immune system are more properly defined as tumor associated antigens (TAA) (Table 1). This definition includes antigens that are not mutated but are differentially expressed by neoplastic and normal cells, either in time, quantity, location or cellular context, resulting in a preferential or exclusive recognition of the tumor by the immune system. For example, carcinoembryonic antigens are normally expressed only during embryonic development 10, p53 and HER-2/neu are overexpressed in some cancer cells 11, 12, and a growing family of Cancer Testes (CT) antigens are expressed only in male germ cells, and sometimes placenta and fetal ovary 13. TAA with a tissue-restricted expression can be legitimate targets for immunotherapy, especially when the tumor arises from non-essential tissues, such as differentiation antigens expressed by melanoma 14, and prostate cancer 15. A special class of TAA is derived from oncogenic viruses associated with some types of cancer, such as human papilloma virus E6 and E7 proteins in cervical cancer, and Epstein-Barr virus-derived antigens in lymphomas 16, 17. Importantly, TAA-specific T cells are frequently detected in the peripheral blood and within the tumor of cancer patients 13. Tumor-infiltrating lymphocytes have on many occasions been used to define TAA that have then been successfully cloned. Obviously, these are by themselves ineffective at causing tumor regression and the aim of immunotherapy is to boost and harness these existing resources to convert them into an effective anti-tumor response.
Table 1.
Examples of human tumor-associated antigens recognized by T cells*.
| Category | Gene‡ | Tumor expression |
|---|---|---|
| Cancer Testis | BAGE | Melanoma, myeloma, lung, bladder and breast carcinoma |
| GAGE-1 | Melanoma, myeloma, lung, bladder, prostate and breast carcinoma, esophageal and head/neck SCC, sarcoma | |
| MAGE-A1 | Melanoma, myeloma, lung, bladder, prostate, colorectal and breast carcinoma, esophageal and head/neck SCC, sarcoma | |
| NY-ESO-1 | Melanoma, myeloma, lung, bladder, prostate, and breast carcinoma, esophageal and head/neck SCC, sarcoma | |
| Differentiation | Gp100 | Melanoma |
| Melan-A/MART-1 | Melanoma | |
| Prostate-specific antigen | Prostate carcinoma | |
| Mammoglobin-A | Breast carcinoma | |
| Overexpressed | Alpha-fetoprotein | Hepatocellular carcinoma and yolk-sac tumors. |
| HER-2/neu | Melanoma, ovarian, gastric, pancreatic and breast carcinoma | |
| P53 | Esophageal, gastric, colon, pancreatic, and other carcinomas | |
| Mutated (shared) ‡ | K-ras | Pancreatic and colorectal adenocarcinomas |
| TRP-2/INT2 | Melanoma, high grade gliomas |
This table lists only some examples of the more common tumor antigens identified. For references about individual antigens listed and for a comprehensive review see Novellino et al.128.
Mutated antigens are tumor-specific. However, few mutations common to more than one patient and sometimes more than one tumor type have been identified. These mutations are usually crucial in the process of neoplastic transformation.
2. Antigen Presentation
a. Dendritic cells
In the last decade the crucial role played by the innate immune system, and in particular by DC in determining T cell activation has been better understood. DC are lineage-negative, bone marrow-derived mononuclear cells found in blood and many peripheral tissues (reviewed in 18). They can be broadly divided into myeloid or plasmacytoid DC (MDC and PDC, respectively) based on phenotypic, morphological and functional differences. MDC are comprised of additional subsets, e.g. Langerhans cells of the epidermis, and dermal or interstitial DC. PDC are the major interferon-alpha (IFNα) producing cells in the body and play a role in mediating antiviral and tumor-specific immune responses. MDC in particular are capable of capturing antigens with high efficiency by phagocytosis, macropinocytosis, and adsorptive endocytosis mediated by an array of receptors. Antigens acquired both endogenously (i.e., synthesized within the DC cytosol), or exogenously (acquired from the extracellular environment) are processed into peptides, which are loaded onto Major Histocompatibility Complex class I and II (MHC I and II) molecules and transported to the cell surface for recognition by antigen-specific T cells.
DC most efficiently capture antigens when they are “immature”. The terminal process of differentiation termed “maturation” transforms DC from poorly immunostimulatory cells specialized for antigen capture into cells specialized for T cell stimulation. This process is accompanied by cytoskeletal reorganization, loss of adhesiveness, acquisition of cellular motility with development of characteristic cytoplasmic extensions or “veils”, migration to lymphoid tissues, reduced phagocytic uptake, and enhanced T cell activation potential 18. Mature DC can secrete chemokines and cytokines that attract other immune cells and activate resting T cells. For the latter an important phenotypic change during DC maturation is a marked increase in expression of MHC II, CD40, and CD80 and CD86 co-stimulatory molecules. This is important because, in order to become activated T cells need not only the signals mediated by engagement of the T cell receptors (TCR) with peptide antigen bound to MHC molecules, but also co-stimulatory signals. The latter are provided by interaction of CD28 on the T cell with CD80 and CD86 co-stimulatory molecules on the DC (Figure 1). Importantly, a cross-talk exists between DC and T cells whereby ligation of CD40 on DC by CD40-Ligand on T cells stimulates the release of IL-12 by DC, while activation of CD4+ T cells by interaction with DC induces them to produce IL-2. Both of these cytokines are required for the development of an effective cytolytic T cell (CTL) response.
Figure 1. A model for the role of ionizing radiation in promoting cross-presentation of TAA and activation of anti-tumor T cells.
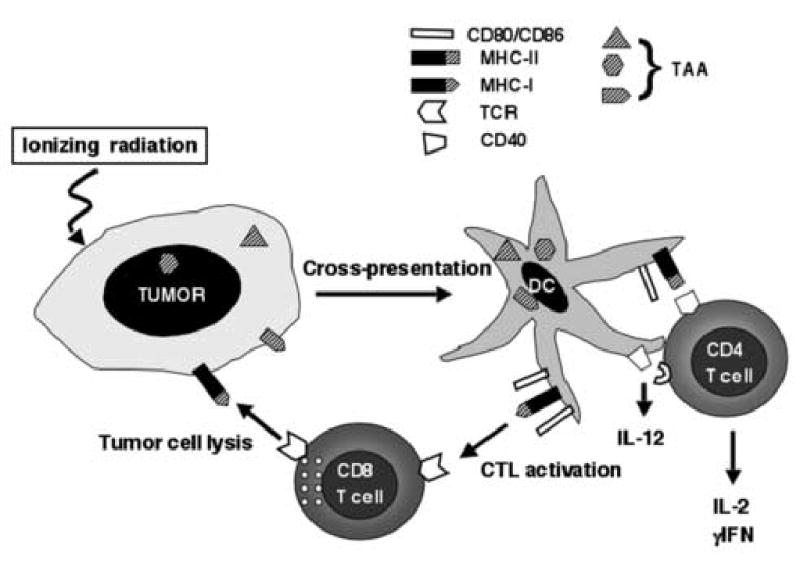
It is well-established that dendritic cells (DC) can efficiently uptake tumor associated antigens (TAA) from apoptotic and necrotic tumor cells and present them to both CD4+ and CD8+ cytolytic T cells (CTL), a process termed cross-presentation. By killing tumor cells ionizing radiation can promote this process. In the presence of adequate “danger signals” that induce DC maturation and up-regulation of co-stimulatory molecules CD80 and CD86, tumor-specific T cells are activated to produce pro-inflammatory cytokines and become effectors capable of killing the tumor cells. Recognition and killing of tumor cells by CTL may be further enhanced by the radiation-induced up-regulation of Fas and/or MHC I molecules on the tumor cells.
Maturation of DC can be induced by a growing number of exogenous and endogenous molecular signals generally referred to as “danger signals” following the model of Matzinger who proposed that the immune system could be activated non only by infectious agents but also by self components that are released during cellular stress and damage 19. Danger signals include host-derived pro-inflammatory cytokines such as tumor necrosis factor (TNF) α, IL-1, IL-6 and IFNα, and a variety of molecules released not only by microbes but also by damaged host tissues20. These non-cytokine molecules signal primarily through transmembrane receptors related to Drosophila Toll protein known as Toll-like receptors (TLRs) 21 which are expressed by DC.
b. Cross-presentation of cell associated antigens
One of the features of DC is their capacity to process captured exogenous antigens. These include apoptotic and necrotic virus-infected or tumor cells, immune complexes, opsonized tumor cells, heat shock proteins (HSPs), DNA- or RNA-encoded antigens, organisms, e.g. bacteria, viruses, virus-like particles, exosomes, soluble proteins 22 and even ”bits” of live cells “nibbled off” by phagocytes, onto MHC I 23. These phenomena, termed “cross-presentation,” permits DC to elicit CD8+ as well as CD4+ T cell responses to exogenous antigens. Several antigen uptake receptors target exogenous antigens to DC, including FcRs, C-type lectins, scavenger receptors, integrins and heat shock protein receptors 20.
Cross-presentation is considered to be a major mechanism by which tumor antigens are presented to T cells. TAA can access DC through the uptake of cellular components, in particular cell fragments (e.g., apoptotic bodies), perhaps opsonized by antibody, necrotic cells and possibly proteins and nucleic acids released from dying tumor cells. The interesting question is when is this process associated with danger signals that induce maturation of DC to become efficient APC. Apoptosis, a physiological form of cell death, occurs during normal tissue turnover, during embryogenesis and following infection or inflammation of tissues. The safe disposal of apoptotic cells by surrounding phagocytes including DC and macrophages, prevents the activation of bystander cells and tissue damage following the release of cellular constituents. Indeed, Huang et al. have shown that mouse DC subsets can constitutively acquire apoptotic cells in the intestine and transport the ingested material to mesenteric lymph nodes 24. Cells undergoing apoptosis are characterized by altered distribution of membrane lipids and exposure of modified carbohydrates on the plasma membrane enabling rapid recognition of apoptotic cells by specific receptors on phagocytes and DC. The uptake of apoptotic cells under normal conditions fails to elicit DC maturation, hence, significant autoimmune responses. In fact, this process allows immature DC to remain immunosuppressive and induce “cross-tolerance” in contrast to cross-priming 25. A similar mechanism may occur in tumor settings where DC having captured dying tumor cells, but not exposed to a maturation stimulus, may induce tolerance rather than immunity. However, when apoptotic cells are delivered to DC together with inflammatory or other “danger” signals they are an excellent source of antigen for priming of effector T cells. Several studies have examined the effects of DC pre-pulsed with apoptotic cells and administered in vivo to mice, and to humans (reviewed in ref. 26). Significantly, the efficiency of cross-presentation of cell-associated antigens is substantially greater (many hundred fold) than cross-presentation of soluble antigen 27.
Necrotic cells may also be sources of tumor antigens for DCs while simultaneously providing maturation signals. This can also occur as secondary necrosis when there is failure to clear apoptotic cell death 28. Thus, exposure of immature DC to necrotic but not apoptotic tumor cells, both in vitro and in vivo, results in their maturation 29. Indeed, cells rendered necrotic by freeze-thawing were shown to have immunostimulatory activity when injected in vivo as they enhanced T cell responses to co-injected antigens 30, 31.
The issue is whether ionizing radiation-induced apoptosis can increase tumor immunogenicity. The immunostimulatory activity associated with cell lysates (endogenous adjuvant activity) was heightened if the cells were first stressed by UV radiation, indicating that injury can modulate this effect 30, 31. Although, the prevailing view is that apoptotic cells induce tolerance when captured by DC, it is worth pointing out that there are examples in the literature where apoptotic cells are immunostimulatory (reviewed in 26). For example, immunization with apoptotic cells or in situ induction of tumor cell apoptosis 32–34 induced T cell responses in vivo. The discrepancies between the different results are likely to be due to the cell type tested and apoptosis pathway induced 35. Of note, it has been reported that immature DCs injected into tumors following ionizing radiation-induced tumor cell apoptosis were able to stimulate a strong anti-tumor immunity 36. This suggests that, at least in some circumstances, radiation-induced tumor cell death may be associated with the production of maturation signals for DC.
The relative contribution of apoptosis versus necrosis to cross-presentation remains unknown but when monitored in vitro, the efficiency of antigen presentation is not dramatically different 37. Endogenous factors that are released from or are associated with necrotic cells may account for the ability of necrotic cells to activate DC (reviewed in 20). Examples include immunostimulatory self DNA that binds TLR9, self ssRNA that stimulates TLR7 and TLR8, secondary structures of mRNA that activate TLR3, and HSP that stimulate TLR4. Recently, uric acid was identified as another factor associated with cell death and activation of DC 38. And lastly, the immune system is alerted to massive cell death not only by factors released from dying cells, but also by factors emanating from disruption of tissue architecture e.g. fibrinogen 39, such as oligosaccharides of hyaluronan 40, EDA-containing fibronectin 41 and heparan sulfate proteoglycan that stimulate phagocytes. Therefore, the induction of necrosis in vivo may not only be accompanied by the release of self antigens, but also inflammatory factors which may enhance DC maturation and the immune response. Some of these factors may facilitate the coordination and generation of spontaneous anti-tumor immune responses.
The form of cell associated antigens being cross presented from dying cells could include HSPs-associated proteins, native proteins 42, peptides 43 or other constituents. The relative contribution of each, which may depend upon the system/antigen being studied, remains to be established. It is generally considered that maturation signals are essential to convert cross-tolerance to cross-priming 44. Signals from virus- or bacteria-infected apoptotic cells (e.g. dsRNA, and inflammatory cytokines such as TNF alpha, type I IFNs) can provide the maturation stimuli (reviewed in 20). In the case of tumors, however, unless there is extensive necrosis and/or release of endogenous adjuvants that activate DC, the end result may be cross-tolerance rather than cross priming. Although the ability of ionizing radiation to generate the signals required for DC maturation remains controversial, a combined approach of inducing cell death by radiation, in concert with administration of synthetic oligodeoxynucleotides with unmethylated CpG motifs that activate DC by binding to TLR9, can lead to the priming or enhancement of anti-tumor responses 45.
3. Effector mechanisms
Although antibodies directed against abnormally expressed or activated receptors on cancer cells (e.g., trastuzumab, or Herceptin) 46, have a role in the therapy of cancer, in general antibodies against most TAA are not very effective at causing tumor regression. Non-specific effector mechanisms also operate in a cancer setting, such as NK, NKT cells, macrophages and neutrophils. However, the evidence is overwhelming that T cell-mediated immunity is far more effective, in particular in terms of tumor cell killing (reviewed in ref. 47). Much is known about the mechanisms of activation of T cells and how they cause tumor cell lysis.
Specific T cells bind through their TCR to a molecular complex composed of MHC-encoded glycoproteins called in humans Human Leukocyte Antigens (HLA) that are loaded with a short antigenic peptides derived from partially degraded proteins. CD8+ T cells recognize MHC I molecules that have antigenic peptides of 8 to 10 amino acids in length that are derived from intracellular proteins by proteasome cleavage 48. CD4+ T cells recognize MHC II molecules with bound peptides of 15 to 20 amino acids in length derived from extracellular proteins that are processed through the endocytic compartment 49. Whereas MHC I molecules are widely expressed in the organism, MHC II expression is normally restricted to cells of hematopoietic origin capable of functioning as APC such as macrophages, DCs, and B cells. Since activated T cells have to recognize and kill cancer cells that, for the most part, will not express MHC II, it is not surprising that CD8+ CTL are the main effectors against cancer cells 50, whereas CD4+ T cells play a role in induction and maintenance of the CD8+ T cell response mainly by providing help via the production of cytokines 51.
Following activation, it takes about 48–72 hours for CD8+ T cells to differentiate into a CTL, a process that requires the expression of effector molecules capable of inducing the death of target cells. CTL use two major independent pathways to kill, mediated through release of cytotoxic granule contents, and by ligation of death receptors, respectively (reviewed in reference 52). TCR-mediated recognition of a target cell triggers degranulation of the CTL and release of effector molecules perforin and granzymes into a cleft formed between the two cells. Granzymes enter the target cell by endocytosis and are released into the cytoplasm by the activity of the pore-forming protein perforin. Granzyme B triggers apoptosis mainly by cleavage of Bid and caspase activation through the intrinsic mitochondrial pathway, whereas granzyme A causes single-strand DNA breaks and apoptosis by a slower pathway. Alternatively, CTL express the ligands for death receptors such as Fas, TNFR, and TRAIL-R and can trigger apoptotic death of the target cells expressing them. Overall, the ability of CTL to use multiple pathways to kill cancer cells contributes to their effectiveness as anti-cancer effectors.
4. Regulation of T cell activation
Activated T cells are powerful effectors that can destroy cells. Since anti-cancer responses are in many cases directed against self-antigens, the aim of immunotherapy may be construed as an attempt to develop a directed pathogenic autoimmune response and will always carry with it a risk of autoimmunity. In fact this is sometimes seen in patients responding to immunotherapy of melanoma when they develop vitiligo associated with tumor regression 53. It is therefore not surprising that multiple mechanisms are in place to regulate T cell activation and that these mechanisms will also regulate attempts at tumor immunotherapy.
As described above, in order to be able to optimally stimulate T cells, DC require to undergo “maturation”, and in its absence DC instead induce tolerance to the captured antigens 54, 55. The functional state of DC therefore plays a crucial role in activation of T cell-mediated immunity. Dysregulation of DC maturation and function has been reported in tumor-bearing patients and in experimental mouse models, and is recognized as an important mechanism of suppression of anti-tumor immunity 56–58.
Another important resource for maintaining peripheral tolerance and controlling T cell activation lies in a recently defined T cell subset, namely regulatory CD4+CD25+ T-cells (T-reg) 59. Depletion of T-reg cells can cause organ-specific autoimmunity, and also can induce rejection of some tumors 60. Noticeably, the efficacy of cancer vaccines is enhanced when T-reg are depleted 61.
Finally, T cell activation is regulated also at the level of the effector T cells themselves. One of the better-understood mechanisms is that mediated via the CTLA-4 molecule, which is up regulated on the surface of T-cells during the early stages of activation. CTLA-4 down-regulates T-cell responses by competing with CD28 for binding to co-stimulatory molecules (reviewed in reference 62). In physiological conditions, CTLA-4–mediated inhibition is important for the maintenance of peripheral tolerance. However, in conditions of suboptimal APC function such as in tumor-bearing hosts it is an obstacle to the development of effective anti-tumor immunity 63, 64.
4. Effects of ionizing radiation on the immune system
Ionizing radiation therapy (RT) has a well-established ability to kill cancer cells, and other cells within the tumor stroma, including endothelial cells and intratumoral lymphocytes 65. Tumor cells killed by RT should be a very good source of antigens for DC uptake and presentation to T cells 66 (Figure 1). Understanding this process has important implications for the effects of RT on development of anti-tumor immunity. The possibility of using RT to promote tumor antigen-presentation by DC has been explored by us and others in pre-clinical studies showing that anti-tumor immunity can be elicited in vivo when tumor irradiation is combined with administration of DC or a DC growth factor to increase DC numbers in tumor-bearing mice 67–69. However, no direct evidence that RT on its own is able to enhance tumor immunity is currently available. As mentioned above, optimal activation of T cells by DC presenting tumor-derived antigens can be achieved only in the presence of inflammatory or “danger” signals. Danger signals are generated upon radiation exposure, although their nature remains largely undefined 1. Pro-inflammatory cytokines IL-1β and TNFα can be induced by radiation both in vitro and in vivo 70–73, and may act to signal danger. In addition, production of other inducers of DC maturation such as prostaglandin E2 is upregulated in tumor cells following radiation 74, 75. Overall, the available in vivo data support the hypothesis that RT may provide at least some of the necessary maturation signals. In vitro, irradiation of DC does not block their ability to undergo maturation in response to appropriate signals. However, irradiated DC, even though they are not killed, do lose some of their ability to process antigens and generate anti-tumor T cell-mediated immunity 76.
Other effects of RT may influence the effector phase of the anti-tumor immune response. RT can up-regulate death receptors such as Fas/CD95, MHC I, and co-stimulatory molecules on certain tumor cells, and this may enhance their tendency to either die or be recognized 2, 77–83. Radiation-mediated up-regulation of Fas on mouse colon adenocarcinoma cells has been shown to sensitize tumor cells to killing by anti-tumor CTL adoptively transferred or elicited by vaccination of mice with recombinant Pox viruses 82, 84. In this system, improved killing was mediated by Fas on tumor cells being cross-linked by Fas-Ligand on T cells. It will be interesting to determine whether the same mechanisms can play a role in other tumor models.
Finally, radiation has complex effects on the tumor microenvironment and vessels that have been shown to facilitate homing of both antigen presenting and effector T cells to the tumor 68, 85. Homing may be facilitated by radiation-induced inflammatory signals, and by changes in Extracellular matrix proteins86, and in the expression of adhesion molecules by endothelial cells 87–90. Interestingly, a differential response of tumor and normal vessels to radiation-induced localization of P-selectin to the vascular lumen has been reported 91 and may increase the entry of effector T cells into tumors 92.
5. Pre-clinical studies of radiotherapy in combination with immunotherapy
While clearly the potential is present for RT to generate anti-tumor immunity, the evidence that it does so in the clinical situation is lacking. However, strategies tested in recent pre-clinical studies have shown some promise in enhancing anti-tumor immunity in the RT setting, by combining radiation with some of the approaches recently developed in the immunotherapy field 93.
a. Strategies based on cytokines
Fifteen years ago Cameron et al. 94 showed that local tumor irradiation could be successfully combined with the T-cell growth factor IL-2 and/or adoptive transfer of tumor-infiltrating lymphocytes to obtain a synergistic anti-tumor effect. Unfortunately, the toxicity of systemic IL-2 administration has limited its clinical application. Since then, many other cytokines have been characterized and shown to induce powerful anti-tumor effects 93. Among them, IL-3, IL-12, and TNFα have been tested in combination with radiation.
IL-3 can expand hematopoietic precursors and enhance antigen presentation by DC 95. The intratumoral expression of IL-3 in a mouse fibrosarcoma and prostate carcinoma increased the tumor response to radiation by eliciting anti-tumor immunity 96, 97. Irradiation was also shown to increase the response of non-transduced tumors to anti-tumor immunity elicited by systemic vaccination with IL-3 gene transduced tumor cells 98. This supports the hypothesis that local radiation enhances the susceptibility of solid tumors to immune-mediated destruction, perhaps by facilitating the penetration and/or function of DC and effector T cells.
IL-12 is secreted by activated DC and is required for the development of effective anti-tumor T cell-mediated immunity 99. The combination of IL-12 and local radiation was tested in a poorly immunogenic mouse fibrosarcoma model 100. Intratumoral delivery of an adenoviral vector encoding IL-12 combined with fractionated RT improved both, local and systemic tumor control via local anti-angiogenic effects of IL-12, and IL-12 elicited anti-tumor immune responses, respectively. A similar approach was used to induce the expression of IL-12 and the costimulatory molecule CD80 in the poorly immunogenic 4T1 mammary carcinoma and B16 melanoma models101. In both tumors the growth delay was significantly better when RT and adenoviral-mediated gene transduction were used in combination. The therapeutic effect was mediated by T and NK cells, in addition to other detected effects, such as inhibition of angiogenesis.
TNFα is produced by many cell types, including activated T cells, and is a pro-inflammatory cytokine with powerful anti-tumor effects. Although TNFα can inhibit the proliferation and/or induce apoptosis of many tumor cell types102, its in vivo anti-tumor effects are mostly mediated by direct cytotoxicity on the tumor endothelium 103. In pre-clinical studies TNFα has been shown to increase the anti-tumor effects of local radiation 104. However, the contribution of the immune response to the therapeutic effects of this combination remain to be established105.
b. Strategies based on dendritic cells
Fms-like tyrosine kinase receptor 3 ligand (Flt3-L), is a growth factor that stimulates production of DC and has been shown to induce anti-tumor immunity to several mouse tumors, although its effects as a single agent are limited to early and more immunogenic tumors 106, 107. The first study to test the combination of Flt3-L with local RT employed the Lewis lung model of metastatic carcinoma 67. When Flt3-L was administered following the ablation of the primary tumor by high dose (60 Gy) local RT, lung metastases were inhibited and disease-free survival enhanced compared to that of mice treated with RT or Flt3-L alone. Importantly, the anti-metastatic effect required T cells, since it was not observed in nude (T cell deficient) mice. These results provide preliminary evidence in support to the hypothesis that RT-induced tumor cell death can release antigens for DC to present to T cells. The high single dose of radiation used in this study limits its clinical applicability, in addition to the fact that the intrinsic tumor immunogenicity could explain these responses. Nevertheless, these studies provide initial proof of principle, and stimulated our group to further investigate whether more clinically relevant radiation doses could be used to elicit systemic anti-tumor immunity in combination with Flt3-L. We used the mouse mammary carcinoma 67NR, a moderately immunogenic syngeneic tumor. A radiation dose sufficient to cause growth delay of the irradiated tumor, in this case 2Gy, was able to induce a systemic anti-tumor effect only in combination with Flt3-L administration. Inhibition of tumor growth outside of the field of radiation was specific and required T cells, confirming that it was immune-mediated 69.
Other groups have used a slightly different approach based around on the same hypothesis, that RT can free tumor-derived antigens for DC uptake and presentation. Nikitina et al., 68 used in vitro bone marrow-derived DCs injected i.v. and s.c. around the tumor following local irradiation, whereas Teitz-Tennenbaum et al. 108 used intratumoral injection of DC. In both cases, the administration of DC after RT was able to induce a potent anti-tumor immune response.
c. Strategies based on vaccination
Vaccination with autologous tumor cells modified to be more immunogenic by selection or cytokine-transduction has been combined successfully with local tumor radiation in a rat and mouse glioma model, respectively 109, 110. The vaccines were shown to induce anti-tumor T cells and to synergize with local brain radiation in enhancing survival and cure of mice, but the mechanism of this synergy remains to be established. In this respect, Lumniczky et al. speculated that radiation-mediated reduction in tumor burden would allow the immune cells to overcome the decreased tumor mass 110.
Similar synergy between local tumor radiation and vaccination with vaccinia and avipox recombinant viruses expressing TAA and co-stimulatory molecules was reported recently in a mouse adenocarcinoma model84. In this model, the up-regulation of Fas induced by radiation on tumor cells was shown to be responsible for the improved therapeutic efficacy of vaccination 84. Importantly, mice that were cured of their tumor following treatment with the combination of vaccination and radiation showed development of CD4+ and CD8+ T cells specific for TAA not present in the vaccine, indicating the broadening of the immune response, a phenomenon also called antigenic spread or antigen cascade 84.
d. Strategies based on targeting Toll-like receptors
As described above, activation of DC and other immune cells via TLR receptors can induce potent immune responses. Oligodeoxynucleotides containing CpG motifs bind to TLR9 and have been shown to induce anti-tumor immunity 111. Local peritumoral or intratumoral injection of CpG oligodeoxynucleotides was recently shown to synergize with local radiation in controlling tumor growth in an immunogenic mouse tumor model 45. Importantly, mice cured of their tumors following treatment with RT and CpG oligodeoxynucleotides were resistant to subsequent tumor challenge indicating the development of a strong protective immune response 112. CpG oligodeoxynucleotides administration also enhanced the radioresponse of a non immunogenic tumor, although the enhancement was more modest 112.
e. Radiation and antibody-mediated blockade of CTLA-4
As mentioned above, one of the main obstacles to the success of immunotherapy is the fact that the immune system is tolerant to antigens on growing tumors. Therefore, strategies aimed at breaking this tolerance have become a main focus of tumor immunotherapy 113. Monoclonal antibody-mediated blockade of the CTLA-4 molecule on T cells was shown to be sufficient to elicit effective anti-tumor immunity to relatively immunogenic tumors by facilitating tumor-specific T cell activation 63. For poorly immunogenic tumors, CTLA-4 blockade was effective if used in combination with vaccination with irradiated tumor cells modified to produce GM-CSF 114–116. In a pre-clinical model of metastatic breast cancer, the mouse 4T1 adenocarcinoma, we tested the combination of local RT and CTLA-4 blockade. Similarly to other poorly immunogenic tumors, 4T1 primary tumor growth and metastatic spread were not affected by CTLA-4 blockade alone. However, radiation in combination with CTLA-4 blockade was able to induce a CD8+ T cell mediated anti-tumor response capable of inhibiting the metastases outside the field of radiation and extending the survival of the mice 117. These results indicate that, at least in some cases, radiation directed to the primary tumor can increase in situ the tumor immunogenicity and potentially become an alternative to vaccination with irradiated tumor cells. The relative simplicity and low cost of this approach make it an attractive candidate for translation into the clinic.
6. Clinical studies combining radiation therapy and immunomodulators
Some of the strategies combining radiation with modifiers of the immune response have been tested in the clinic. The administration of TNFα during RT was tested in a phase I trial in patients with locally advanced primary tumors or metastatic disease. Although a tumor response was observed in some patients, TNFα showed severe systemic toxicity limiting its use 118. To overcome this problem while maximizing the local anti-tumor effect, an adenovector expressing TNFα under the control of a radiation-inducible promoter was developed 119. A recently published phase I study in patients with solid tumors demonstrated safety and a greater response in lesions treated with the TNFα-expressing adenovector and RT compared to RT alone 120. However, no abscopal effect was observed. Although the potential is there for TNFα to induce/enhance anti-tumor immunity 121, the synergism between TNFα and RT seems to involve mechanisms other than immunity 120.
A recent phase II clinical trail was designed to examine whether vaccination with poxvirus encoding prostate-specific antigen (PSA) could be combined with standard external beam radiation therapy in patients with prostate cancer 122. The initial vaccine dose was given before RT with boosts both before and after the radiation treatment. Although RT alone did not generate immune responses, the results of the combined RT plus vaccination are encouraging in that patients were able to make responses to the vaccine. In addition to demonstrating the feasibility of combining radio- and immuno-therapy, this trial also suggests that, like in the pre-clinical studies 84, this combination can generate an antigen cascade with development of T cells directed against other TAA not present in the vaccine122, a phenomenon recently proposed to play a crucial role in determining the therapeutic efficacy of immunotherapy123.
7. Future directions
Traditionally considered an immune-suppressive treatment modality, ionizing radiation has started to reveal its potential to enhance immunity. However, the role of radiation as an independent immune-enhancer remains under investigation. While encouraging preclinical data are emerging their translation to the clinic is just beginning. Several reasons justify this delay. First of all, the exact mechanisms for this new application of RT remain quite elusive. They are likely to be context-dependent and relative to the degree of tumor immunogenicity. Literally, no clinical data are available to indicate what is the optimal radiation dose/technique and fractionation to optimize its application as a form of immunotherapy. In fact, scant data are available with regards to the specific radiosensitivity of the different cellular components of the immune system. Original studies describing the metachronous effect of RT on circulating immune cells are available, but desperately need to be revisited and updated to reflect the enormous progress made by immunology. Specifically, modern accurate monitoring of changes in the circulating immune cells during standard fractionated radiotherapy is warranted.
Conversely, the rapid parallel growth of tumor vaccine strategies makes it compelling to explore a renewed partnership, especially in tumor settings where chemotherapy and radiation have failed and immunotherapy is showing promising initial results 124, 125. Therefore, although it is premature to use RT as an immunomodulator outside of its scope as a cytocidal agent, the combination of radiotherapy with vaccination and with other strategies shown to be effective in pre-clinical studies can be tested in the clinic122. For instance, clinical trials assessing the efficacy of CTLA-4 blockade in combination with some types of vaccination are ongoing and have shown some promise 126, 127. The ability of local RT and CTLA-4 blockade to activate anti-tumor T cells could be explored in the setting of metastatic disease, and both local and systemic (outside of the field of radiation) responses could be monitored. In such setting it would be possible to gather preliminary evidence as to whether RT used to control tumor growth also works as an “in situ vaccination”.
In conclusion, more investigations about a potentially novel application of ionizing radiation as a component of immunotherapy are warranted. If clinical efficacy is demonstrated it could open a completely new approach to cancer management, with the advantage of using an established modality and treatment equipment commonly available in the community.
Acknowledgments
S.D. is supported by a clinical investigator award from NIH/NCI (K08 CA89336), by a grant from the Speaker’s Fund for Biomedical research: Towards the Science of Patient Care, awarded by the City of New York, and by a Breast Cancer Research Award from The Chemotherapy Foundation made possible by The Joyce and Irving Goldman Family Foundation. N.B. is supported by grants from the CRI, the Doris Duke Charitable Foundation, the Burroughs Wellcome Fund, the Ludwig Institute and NIH AI061684, 52731. W.H.M. is supported by research grants from the U.S. Army (#W81XWH-04-1-0126) and the NIH/NCI (RO1 CA-101752). SCF is supported by Department of Defense grant DAMD17-01-1-0345 and Center of Excellence award BC030282, by ACS grant TURSG CCE 103174, and by a grant from the Breast Cancer Research Foundation. NYU Cancer Institute is supported by NIH/NCI grant 5 P30 CA16087-25.
Footnotes
CONFLICT OF INTEREST NOTIFICATION PAGE:
There are no potential conflicts of interest involving any of the authors.
References
- 1.McBride WH, Chiang C-S, Olson JL, et al. A sense of danger from radiation. Radiat Res. 2004;162:1–19. doi: 10.1667/rr3196. [DOI] [PubMed] [Google Scholar]
- 2.Friedman EJ. Immune modulation by ionizing radiation and its implications for cancer immunotherapy. Curr Pharm Des. 2002;8:1765–1780. doi: 10.2174/1381612023394089. [DOI] [PubMed] [Google Scholar]
- 3.Ehrlich P. Ueber den jetzigen stand der Karzinomforschung. Ned Tijdschr Geneeskd. 1909;5:273–290. [Google Scholar]
- 4.Yee C, Riddell SR, Greenberg PD. In vivo tracking of tumor-specific T cells. Curr Opin Immunol. 2001;13:141–146. doi: 10.1016/s0952-7915(00)00196-5. [DOI] [PubMed] [Google Scholar]
- 5.Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nature Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 6.Spiotto MT, Fu YX, Schreiber H. Tumor immunity meets autoimmunity: antigen levels and dendritic cell maturation. Curr Opin Immunol. 2003;15:725–730. doi: 10.1016/j.coi.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 7.Wolfel T, Hauer M, Schneider J, et al. A p16INK4a-insensitive CDK4 mutant targeted by cytolytic T lymphocytes in a human melanoma. Science. 1995;269:1281–1284. doi: 10.1126/science.7652577. [DOI] [PubMed] [Google Scholar]
- 8.Clark RE, Dodi IA, Hill SC, et al. Direct evidence that leukemic cells present HLA-associated immunogenic peptides derived from the BCR-ABL β 3α 2 fusion protein. Blood. 2001;98:2887–2893. doi: 10.1182/blood.v98.10.2887. [DOI] [PubMed] [Google Scholar]
- 9.Finn OJ, Jerome KR, Henderson RA, et al. MUC-1 epithelial tumor mucin-based immunity and cancer vaccines. Immunol Rev. 1995;145:61–89. doi: 10.1111/j.1600-065x.1995.tb00077.x. [DOI] [PubMed] [Google Scholar]
- 10.Tsang KY, Zaremba S, Nieroda CA, et al. Generation of human cytotoxic T cells specific for human carcinoembryonic antigen epitopes from patients immunized with recombinant vaccinia-CEA vaccine. J Natl Cancer Inst. 1995;87:982–990. doi: 10.1093/jnci/87.13.982. [DOI] [PubMed] [Google Scholar]
- 11.Theobald M, Biggs J, Dittmer D, et al. Targeting p53 as a general tumor antigen. Proc Natl Acad Sci U S A. 1995;92:11993–11997. doi: 10.1073/pnas.92.26.11993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ioannides CG, Fisk B, Fan D, et al. Cytotoxic T cells isolated from ovarian malignant ascites recognize a peptide derived from the HER-2/neu proto-oncogene. Cell Immunol. 1993;151:225–234. doi: 10.1006/cimm.1993.1233. [DOI] [PubMed] [Google Scholar]
- 13.Scanlan MJ, Gure AO, Jungbluth AA, et al. Cancer/testis antigens: an expanding family of targets for cancer immunotherapy. Immunol Rev. 2002;188:22–32. doi: 10.1034/j.1600-065x.2002.18803.x. [DOI] [PubMed] [Google Scholar]
- 14.Engelhard VH, Bullock TN, Colella TA, et al. Antigens derived from melanocyte differentiation proteins: self-tolerance, autoimmunity, and use for cancer immunotherapy. Immunol Rev. 2002;188:136–146. doi: 10.1034/j.1600-065x.2002.18812.x. [DOI] [PubMed] [Google Scholar]
- 15.Heiser A, Coleman D, Dannull J, et al. Autologous dendritic cells transfected with prostate-specific antigen RNA stimulate CTL responses against metastatic prostate tumors. J Clin Invest. 2002;109:409–417. doi: 10.1172/JCI14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Konya J, Dillner J. Immunity to oncogenic human papillomaviruses. Adv Cancer Res. 2001;82:205–238. doi: 10.1016/s0065-230x(01)82007-8. [DOI] [PubMed] [Google Scholar]
- 17.Rooney CM, Smith CA, Ng CY, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92:1549–1555. [PubMed] [Google Scholar]
- 18.O’Neill DW, Adams S, Bhardwaj N. Manipulating dendritic cell biology for the active immunotherapy of cancer. Blood. 2004;104:2235–2246. doi: 10.1182/blood-2003-12-4392. [DOI] [PubMed] [Google Scholar]
- 19.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 20.Skoberne M, Beignon AS, Bhardwaj N. Danger signals: a time and space continuum. Trends Mol Med. 2004;10:251–257. doi: 10.1016/j.molmed.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Kopp E, Medzhitov R. Recognition of microbial infection by Toll-like receptors. Curr Opin Immunol. 2003;15:396–401. doi: 10.1016/s0952-7915(03)00080-3. [DOI] [PubMed] [Google Scholar]
- 22.Yewdell JW, Norbury CC, Bennink JR. Mechanisms of exogenous antigen presentation by MHC class I molecules in vitro and in vivo: implications for generating CD8+ T cell responses to infectious agents, tumors, transplants, and vaccines. Adv Immunol. 1999;73:1–77. doi: 10.1016/s0065-2776(08)60785-3. [DOI] [PubMed] [Google Scholar]
- 23.Harshyne LA, Watkins SC, Gambotto A, et al. Dendritic cells acquire antigens from live cells for cross-presentation to CTL. J Immunol. 2001;166:3717–3723. doi: 10.4049/jimmunol.166.6.3717. [DOI] [PubMed] [Google Scholar]
- 24.Huang FP, Platt N, Wykes M, et al. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J Exp Med. 2000;191:435–444. doi: 10.1084/jem.191.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hugues S, Mougneau E, Ferlin W, et al. Tolerance to islet antigens and prevention from diabetes induced by limited apoptosis of pancreatic beta cells. Immunity. 2002;16:169–181. doi: 10.1016/s1074-7613(02)00273-x. [DOI] [PubMed] [Google Scholar]
- 26.Rock KL, Hearn A, Chen CJ, et al. Natural endogenous adjuvants. Springer Semin Immunopathol. 2005;26:231–246. doi: 10.1007/s00281-004-0173-3. [DOI] [PubMed] [Google Scholar]
- 27.Li M, Davey GM, Sutherland RM, et al. Cell-associated ovalbumin is cross-presented much more efficiently than soluble ovalbumin in vivo. J Immunol. 2001;166:6099–6103. doi: 10.4049/jimmunol.166.10.6099. [DOI] [PubMed] [Google Scholar]
- 28.Rovere P, Vallinoto C, Bondanza A, et al. Cutting edge: bystander apoptosis triggers dendritic cell maturation and antigen-presenting function. J Immunol. 1998;161:4467–4471. [PubMed] [Google Scholar]
- 29.Sauter B, Albert ML, Francisco L, et al. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–433. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nature Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 31.Shi Y, Zheng W, Rock KL. Cell injury releases endogenous adjuvants that stimulate cytotoxic T cell responses. Proc Natl Acad Sci U S A. 2000;97:14590–14595. doi: 10.1073/pnas.260497597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ronchetti A, Rovere P, Iezzi G, et al. Immunogenicity of apoptotic cells in vivo: role of antigen load, antigen-presenting cells, and cytokines. J Immunol. 1999;163:130–136. [PubMed] [Google Scholar]
- 33.Kotera Y, Shimizu K, Mule JJ. Comparative analysis of necrotic and apoptotic tumor cells as a source of antigen(s) in dendritic cell-based immunization. Cancer Res. 2001;61:8105–8109. [PubMed] [Google Scholar]
- 34.Nowak AK, Lake RA, Marzo AL, et al. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J Immunol. 2003;170:4905–4913. doi: 10.4049/jimmunol.170.10.4905. [DOI] [PubMed] [Google Scholar]
- 35.Demaria S, Santori FR, Ng B, et al. Select forms of tumor cell apoptosis induce dendritic cell maturation. J Leukoc Biol. 2005;77:361–368. doi: 10.1189/jlb.0804478. [DOI] [PubMed] [Google Scholar]
- 36.Kim KW, Kim SH, Shin JG, et al. Direct injection of immature dendritic cells into irradiated tumor induces efficient antitumor immunity. Int J Cancer. 2004;109:685–690. doi: 10.1002/ijc.20036. [DOI] [PubMed] [Google Scholar]
- 37.Larsson M, Fonteneau JF, Somersan S, et al. Efficiency of cross presentation of vaccinia virus-derived antigens by human dendritic cells. Eur J Immunol. 2001;31:3432–3442. doi: 10.1002/1521-4141(200112)31:12<3432::aid-immu3432>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 38.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 39.Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol. 2001;167:2887–2894. doi: 10.4049/jimmunol.167.5.2887. [DOI] [PubMed] [Google Scholar]
- 40.Termeer C, Benedix F, Sleeman J, et al. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okamura Y, Watari M, Jerud ES, et al. The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem. 2001;276:10229–10233. doi: 10.1074/jbc.M100099200. [DOI] [PubMed] [Google Scholar]
- 42.Shen L, Rock KL. Cellular protein is the source of cross-priming antigen in vivo. Proc Natl Acad Sci U S A. 2004;101:3035–3040. doi: 10.1073/pnas.0308345101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neijssen J, Herberts C, Drijfhout JW, et al. Cross-presentation by intercellular peptide transfer through gap junctions. Nature. 2005;434:83–88. doi: 10.1038/nature03290. [DOI] [PubMed] [Google Scholar]
- 44.Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci U S A. 2002;99:351–358. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Milas L, Mason KA, Ariga H, et al. CpG oligodeoxynucleotide enhances tumor response to radiation. Cancer Res. 2004;64:5074–5077. doi: 10.1158/0008-5472.CAN-04-0926. [DOI] [PubMed] [Google Scholar]
- 46.Vogel C, Cobleigh MA, Tripathy D, et al. First-line, single-agent Herceptin(trastuzumab) in metastatic breast cancer: a preliminary report. Eur J Cancer. 2001;37 (Suppl 1):S25–29. [PubMed] [Google Scholar]
- 47.Blattman JN, Greenberg PD. Cancer immunotherapy: a treatment for the masses. Science. 2004;305:200–205. doi: 10.1126/science.1100369. [DOI] [PubMed] [Google Scholar]
- 48.Rock KLGA. Degradation of cell proteins and the generation of MHC class I-presented peptides. Annu Rev Immunol. 1999;17:739–779. doi: 10.1146/annurev.immunol.17.1.739. [DOI] [PubMed] [Google Scholar]
- 49.Watts C. The exogenous pathway for antigen presentation on major histocompatibility complex class II and CD1 molecules. Nat Immunol. 2004;5:685–692. doi: 10.1038/ni1088. [DOI] [PubMed] [Google Scholar]
- 50.Melief CJM. Tumor eradication by adoptive transfer of cytotoxic T lymphocytes. Adv Cancer Res. 1992;58:143–175. doi: 10.1016/s0065-230x(08)60294-8. [DOI] [PubMed] [Google Scholar]
- 51.Ossendorp F, Mengede E, Camps M, et al. Specific T helper cell requirement for optimal induction of cytotoxic class II negative tumors. J Exp Med. 1998;187:693–702. doi: 10.1084/jem.187.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Waterhouse NJ, Trapani JA. CTL: Caspases terminate life, but that's not the whole story. Tissue Antigens. 2002;59:175–183. doi: 10.1034/j.1399-0039.2002.590301.x. [DOI] [PubMed] [Google Scholar]
- 53.Yee C, Thompson JA, Roche P, et al. Melanocyte destruction after antigen-specific immunotherapy of melanoma: direct evidence of T cell-mediated vitiligo. J Exp Med. 2000;192:1637. doi: 10.1084/jem.192.11.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hawiger D, Inaba K, Dorsett Y, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dhodapkar MV, Steinman RM, Krasovsky J, et al. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–238. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gabrilovich DI, Chen HL, Girgis KR, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2:1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 57.Gabrilovich DI, Corak J, Ciernik IF, et al. Decreased antigen presentation by dendritic cells in patients with breast cancer. Clin Cancer Res. 1997;3:483–490. [PubMed] [Google Scholar]
- 58.Kusmartsev S, Gabrilovich DI. Immature myeloid cells and cancer-associated immune suppression. Cancer Immunol Immunother. 2002;51:293–298. doi: 10.1007/s00262-002-0280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163:5211–5218. [PubMed] [Google Scholar]
- 60.Onizuka S, Tawara I, Shimizu J, et al. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor-α) monoclonal antibody. Cancer Res. 1999;59:3128–3133. [PubMed] [Google Scholar]
- 61.Sutmuller RP, van Duivenvoorde LM, van Elsas A, et al. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25+ regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194:823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol. 2002;3:611–618. doi: 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- 63.Leach D, Krummel M, Allison JP. Enhancement of anti-tumor immunity By CTLA4-blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 64.Yang Y-F, Zou J-P, Mu J, et al. Enhanced induction of antitumor T-cell responses by cytotoxic T lymphocyte-associated molecule-4 blockade: the effect is manifested only at the restricted tumor-bearing stages. Cancer Res. 1997;57:4036–4041. [PubMed] [Google Scholar]
- 65.Watters D. Molecular mechanisms of ionizing radiation-induced apoptosis. Immunol Cell Biol. 1999;77:263–271. doi: 10.1046/j.1440-1711.1999.00824.x. [DOI] [PubMed] [Google Scholar]
- 66.Larsson M, Fonteneau JF, Bhardwaj N. Dendritic cells resurrect antigens from dead cells. Trends Immunol. 2001;22:141–148. doi: 10.1016/s1471-4906(01)01860-9. [DOI] [PubMed] [Google Scholar]
- 67.Chakravarty PK, Alfieri A, Thomas EK, et al. Flt3-Ligand administration after radiation therapy prolongs survival in a murine model of metastatic lung cancer. Cancer Res. 1999;59:6028–6032. [PubMed] [Google Scholar]
- 68.Nikitina EY, Gabrilovich DI. Combination of gamma-irradiation and dendritic cell administration induces a potent antitumor response in tumor-bearing mice: approach to treatment of advanced stage cancer. Int J Cancer. 2001;94:825–833. doi: 10.1002/1097-0215(20011215)94:6<825::aid-ijc1545>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 69.Demaria S, Ng B, Devitt M-L, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–870. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 70.Ishihara H, Tsuneoka K, Dimchev AB, et al. Induction of the expression of the interleukin-1 beta gene in mouse spleen by ionizing radiation. Radiat Res. 1993;133:321–326. [PubMed] [Google Scholar]
- 71.Hallahan DE, Spriggs DR, Beckett MA, et al. Increased tumor necrosis factor alpha mRNA after cellular exposure to ionizing radiation. Proc Natl Acad Sci U S A. 1989;86:10104–10107. doi: 10.1073/pnas.86.24.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hong JH, Chiang CS, Campbell IL, et al. Induction of acute phase gene expression by brain irradiation. Int J Radiat Oncol Biol Phys. 1995;33:619–626. doi: 10.1016/0360-3016(95)00279-8. [DOI] [PubMed] [Google Scholar]
- 73.Hong JH, Chiang CS, Tsao CY, et al. Rapid induction of cytokine gene expression in the lung after single and fractionated doses of radiation. Int J Radiat Biol. 1999;75:1421–1427. doi: 10.1080/095530099139287. [DOI] [PubMed] [Google Scholar]
- 74.Rieser C, Bock G, Klocker H, et al. Prostaglandin E2 and tumor necrosis factor alpha cooperate to activate human dendritic cells: synergistic activation of interleukin 12 production. J Exp Med. 1997;186:1603–1608. doi: 10.1084/jem.186.9.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Steinauer KK, Gibbs I, Ning S, et al. Radiation induces upregulation of cyclooxygenase-2 (COX-2) protein in PC-3 cells. Int J Radiat Oncol Biol Phys. 2000;48:325–328. doi: 10.1016/s0360-3016(00)00671-4. [DOI] [PubMed] [Google Scholar]
- 76.Liao YP, Wang CC, Butterfield LH, et al. Ionizing radiation affects human MART-1 melanoma antigen processing and presentation by dendritic cells. J Immunol. 2004;173:2462–2469. doi: 10.4049/jimmunol.173.4.2462. [DOI] [PubMed] [Google Scholar]
- 77.Sheard MA, Vojtesek B, Janakova L, et al. Up-regulation of Fas (CD95) in human p53wild-type cancer cells treated with ionizing radiation. Int J Cancer. 1997;73:757–762. doi: 10.1002/(sici)1097-0215(19971127)73:5<757::aid-ijc24>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 78.Sheard MA. Ionizing radiation as a response-enhancing agent for CD95-mediated apoptosis. Int J Cancer. 2001;96:213–220. doi: 10.1002/ijc.1020. [DOI] [PubMed] [Google Scholar]
- 79.Klein B, Loven D, Lurie H, et al. The effect of irradiation on expression of HLA class I antigens in human brain tumors in culture. J Neurosurg. 1994;80:1074–1077. doi: 10.3171/jns.1994.80.6.1074. [DOI] [PubMed] [Google Scholar]
- 80.Santin AD, Hiserodt JC, Fruehauf J, et al. Effects of irradiation on the expression of surface antigens in human ovarian cancer. Gynecol Oncol. 1996;60:468–474. doi: 10.1006/gyno.1996.0075. [DOI] [PubMed] [Google Scholar]
- 81.Vereecque R, Buffenoir G, Gonzalez R, et al. Gamma-ray irradiation induces B7.1 expression in myeloid leukaemic cells. Br J Haematol. 2000;108:825–831. doi: 10.1046/j.1365-2141.2000.01967.x. [DOI] [PubMed] [Google Scholar]
- 82.Chakraborty M, Abrams SI, Camphausen K, et al. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol. 2003;170:6338–6347. doi: 10.4049/jimmunol.170.12.6338. [DOI] [PubMed] [Google Scholar]
- 83.Garnett CT, Palena C, Chakarborty M, et al. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 2004;64:7985–7994. doi: 10.1158/0008-5472.CAN-04-1525. [DOI] [PubMed] [Google Scholar]
- 84.Chakraborty M, Abrams SI, Coleman CN, et al. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res. 2004;64:4328–4337. doi: 10.1158/0008-5472.CAN-04-0073. [DOI] [PubMed] [Google Scholar]
- 85.Ganss R, Ryschich E, Klar E, et al. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res. 2002;62:1462–1470. [PubMed] [Google Scholar]
- 86.Brooks PC, Roth JM, Lymberis SC, et al. Ionizing radiation modulates the exposure of the HUIV26 cryptic epitope within collagen type IV during angiogenesis. Int J Radiat Oncol Biol Phys. 2002;54:1194–1201. doi: 10.1016/s0360-3016(02)03748-3. [DOI] [PubMed] [Google Scholar]
- 87.Hallahan D, Kuchibhotla J, Wyble C. Cell adhesion molecules mediate radiation-induced leukocyte adhesion to the vascular endothelium. Cancer Res. 1996;56:5150–5155. [PubMed] [Google Scholar]
- 88.Hallahan DE, Virudachalam S. Accumulation of P-selectin in the lumen of irradiated blood vessels. Radiat Res. 1999;152:6–13. [PubMed] [Google Scholar]
- 89.Ryschich E, Harms W, Loeffler T, et al. Radiation-induced leukocyte adhesion to endothelium in normal pancreas and in pancreatic carcinoma of the rat. Int J Cancer. 2003;105:506–511. doi: 10.1002/ijc.11073. [DOI] [PubMed] [Google Scholar]
- 90.Hallahan D, Geng L, Qu S, et al. Integrin-mediated targeting of drug delivery to irradiated tumor blood vessels. Cancer Cell. 2003;3:63–74. doi: 10.1016/s1535-6108(02)00238-6. [DOI] [PubMed] [Google Scholar]
- 91.Hallahan DE, Staba-Hogan MJ, Virudachalam S, et al. X-ray-induced P-selectin localization to the lumen of tumor blood vessels. Cancer Res. 1998;58:5216–5220. [PubMed] [Google Scholar]
- 92.Tanigawa K, Takeshita N, Craig RA, et al. Tumor-specific responses in lymph nodes draining murine sarcomas are concentrated in cells expressing P-selectin binding sites. J Immunol. 2001;167:3089–3098. doi: 10.4049/jimmunol.167.6.3089. [DOI] [PubMed] [Google Scholar]
- 93.Antonia S, Mule JJ, Weber JS. Current developments of immunotherapy in the clinic. Curr Opin Immunol. 2004;16:130–136. doi: 10.1016/j.coi.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 94.Cameron RB, Spiess PJ, Rosenberg SA. Synergistic antitumor activity of tumor-infiltrating lymphocytes, interleukin 2, and local tumor irradiation. Studies on the mechanism of action. J Exp Med. 1990;171:249–263. doi: 10.1084/jem.171.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Eder M, Geissler G, Ganser A. IL-3 in the clinic. Stem Cells. 1997;15:327–333. doi: 10.1002/stem.150327. [DOI] [PubMed] [Google Scholar]
- 96.Chiang CS, Syljuasen RG, Hong JH, et al. Effects of IL-3 gene expression on tumor response to irradiation in vitro and in vivo. Cancer Res. 1997;57:3899–3903. [PubMed] [Google Scholar]
- 97.Oh YT, Chen DW, Dougherty GJ, et al. Adenoviral interleukin-3 gene-radiation therapy for prostate cancer in mouse model. Int J Radiat Oncol Biol Phys. 2004;59:579–583. doi: 10.1016/j.ijrobp.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 98.Chiang CS, Hong JH, Wu YC, et al. Combining radiation therapy with interleukin-3 gene immunotherapy. Cancer Gene Ther. 2000;7:1172–1178. doi: 10.1038/sj.cgt.7700217. [DOI] [PubMed] [Google Scholar]
- 99.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 100.Seetharam S, Staba MJ, Schumm LP, et al. Enhanced eradication of local and distant tumors by genetically produced interleukin-12 and radiation. Int J Oncol. 1999;15:769–773. doi: 10.3892/ijo.15.4.769. [DOI] [PubMed] [Google Scholar]
- 101.Lohr F, Hu K, Haroon Z, et al. Combination treatment of murine tumors by adenovirus-mediated local B7/IL12 immunotherapy and radiotherapy. Mol Ther. 2000;2:195–203. doi: 10.1006/mthe.2000.0114. [DOI] [PubMed] [Google Scholar]
- 102.Sugarman BJ, Aggarwal BB, Hass PE, et al. Recombinant human tumor necrosis factor-alpha: effects on proliferation of normal and transformed cells in vitro. Science. 1985;230:943–945. doi: 10.1126/science.3933111. [DOI] [PubMed] [Google Scholar]
- 103.Gerlach H, Lieberman H, Bach R, et al. Enhanced responsiveness of endothelium in the growing/motile state to tumor necrosis factor/cachectin. J Exp Med. 1989;170:913–931. doi: 10.1084/jem.170.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Weichselbaum RR, Hallahan DE, Beckett MA, et al. Gene therapy targeted by radiation preferentially radiosensitizes tumor cells. Cancer Res. 1994;54:4266–4269. [PubMed] [Google Scholar]
- 105.Weichselbaum RR, Kufe DW, Hellman S, et al. Radiation-induced tumour necrosis factor-alpha expression: clinical application of transcriptional and physical targeting of gene therapy. Lancet Oncol. 2002;3:665–671. doi: 10.1016/s1470-2045(02)00900-2. [DOI] [PubMed] [Google Scholar]
- 106.Maraskovsky E, Brasel K, Teepe M, et al. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med. 1996;184:1953–1962. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lynch DH, Andreasen A, Maraskovsky E, et al. Flt3 ligand induces tumor regression and anti-tumor immune responses in vivo. Nat Med. 1997;3:625–631. doi: 10.1038/nm0697-625. [DOI] [PubMed] [Google Scholar]
- 108.Teitz-Tennenbaum S, Li Q, Rynkiewicz S, et al. Radiotherapy potentiates the therapeutic efficacy of intratumoral dendritic cell administration. Cancer Res. 2003;63:8466–8475. [PubMed] [Google Scholar]
- 109.Graf MR, Prins RM, Hawkins WT, et al. Irradiated tumor cell vaccine for treatment of an established glioma. I. Successful treatment with combined radiotherapy and cellular vaccination. Cancer Immunol Immunother. 2002;51:179–189. doi: 10.1007/s00262-002-0269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lumniczky K, Desaknai S, Mangel L, et al. Local tumor irradiation augments the antitumor effect of cytokine-producing autologous cancer cell vaccines in a murine glioma model. Cancer Gene Ther. 2002;9:44–52. doi: 10.1038/sj.cgt.7700398. [DOI] [PubMed] [Google Scholar]
- 111.Krieg AM. Antitumor applications of stimulating toll-like receptor 9 with CpG oligodeoxynucleotides. Curr Oncol Rep. 2004;6:88–95. doi: 10.1007/s11912-004-0019-0. [DOI] [PubMed] [Google Scholar]
- 112.Mason KA, Ariga H, Neal R, et al. Targeting toll-like receptor 9 with CpG oligodeoxynucleotides enhances tumor response to fractionated radiotherapy. Clin Cancer Res. 2001;11:361–369. [PubMed] [Google Scholar]
- 113.Yu Z, Restifo NP. Cancer vaccines: progress reveals new complexities. J Clin Invest. 2002;110:289–294. doi: 10.1172/JCI16216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hurwitz AA, Yu TF-Y, Leach DR, et al. CTLA-4 blockade synergize with tumor-derived granulocyte-macrophage colony stimulating-factor for treatment of an experimental mammary carcinoma. Proc Natl Acad Sci USA. 1998;95:10067–10071. doi: 10.1073/pnas.95.17.10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hurwitz AA, Foster BA, Kwon ED, et al. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000;60:2444–2448. [PubMed] [Google Scholar]
- 117.Demaria S, Kawashima N, Yang AM, et al. Immune-mediated inhibition of metastases following treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11:728–734. [PubMed] [Google Scholar]
- 118.Hallahan DE, Vokes EE, Rubin SJ, et al. Phase I dose-escalation study of tumor necrosis factor-alpha and concomitant radiation therapy. Cancer J Sci Am. 1995;1:204–209. [PubMed] [Google Scholar]
- 119.Rasmussen HS, Rasmussen CS, Lempicki M, et al. TNFerade biologic: preclinical toxicology of a novel adenovector with a radiation-inducible promoter, carrying the human tumor necrosis factor alpha gene. Cancer Gene Ther. 2002;9:951–957. doi: 10.1038/sj.cgt.7700518. [DOI] [PubMed] [Google Scholar]
- 120.Senzer N, Mani S, Rosemurgy A, et al. TNFerade biologic, an adenovector with a radiation-inducible promoter, carrying the human tumor necrosis factor alpha gene: a phase I study in patients with solid tumors. J Clin Oncol. 2004;22:592–601. doi: 10.1200/JCO.2004.01.227. [DOI] [PubMed] [Google Scholar]
- 121.Kianmanesh A, Hackett NR, Lee JM, et al. Intratumoral administartion of low dose of an adenovirus vector encoding tumor necrosis factor alpha together with naive dendritic cells elicits significant suppression of tumor growth without toxicity. Hum Gene Ther. 2001;12:2035–2049. doi: 10.1089/10430340152677395. [DOI] [PubMed] [Google Scholar]
- 122.Gulley JL, Arlen PM, Bastian N, et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res. 2005;11:3353–3362. doi: 10.1158/1078-0432.CCR-04-2062. [DOI] [PubMed] [Google Scholar]
- 123.Lurquin C, Lethe B, De Plaen E, et al. Contrasting frequencies of antitumor and anti-vaccine T cells in metastases of a melanoma patient vaccinated with a MAGE tumor antigen. J Exp Med. 2005;201:249–257. doi: 10.1084/jem.20041378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Steiner HH, Bonsanto MM, Beckhove P, et al. Antitumor vaccination of patients with glioblastoma multiforme: a pilot study to assess feasibility, safety, and clinical benefit. J Clin Oncol. 2004;22:4272–4281. doi: 10.1200/JCO.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 125.Ramanathan RK, Lee KM, McKolanis J, et al. Phase I study of a MUC1 vaccine composed of different doses of MUC1 peptide with SB-AS2 adjuvant in resected and locally advanced pancreatic cancer. Cancer Immunol Immunother. 2005;54:254–264. doi: 10.1007/s00262-004-0581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hodi FS, Mihm MC, Soiffer RJ, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A. 2003;100:4712–4717. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Novellino L, Castelli C, Parmiani G. A listing of human tumor antigens recognized by T cells: March 2004 update. Cancer Immunol Immunother. 2005;54:187–207. doi: 10.1007/s00262-004-0560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


