Abstract
An inflammation of the airway of patients with diffuse panbronchiolitis (DPB), is characterized by dense neutrophil infiltration. Resolution of the inflammation can be achieved by the removal of apoptotic neutrophils by human alveolar macrophages (AM) without liberating neutrophil proteases in the airway. To understand clinical efficacy for the treatment of DPB by 14- or 15-member macrolides, their effects on the phagocytosis of apoptotic neutrophils by AM were examined. Treatment of AM with erythromycin (ERY) or clarithromycin at clinically achievable levels significantly increased the levels of phagocytosis of apoptotic neutrophils. A serum factor was not essential for the enhancement by these 14-member macrolides. Of the antibiotics tested, these effects were specific for the 14-member macrolides and a 15-member macrolide, azithromycin, but not for the 16-member macrolides, clindamycin or β-lactam antibiotics. The enhanced phagocytosis of apoptotic neutrophils by ERY had no effect on the levels of interleukin-8 or tumor necrosis factor alpha production by lipopolysaccharide-stimulated AM after phagocytosis of the apoptotic neutrophils. The increased phagocytosis of apoptotic neutrophils by ERY was also found to be phosphatidylserine receptor-dependent for AM. These data indicate a novel anti-inflammatory action of 14-member and 15-member macrolides, and suggest that such antibiotics achieve clinical efficacy for patients with DPB, in part, through enhancing the nonphlogistic phagocytosis of apoptotic neutrophils by AM.
Inflammation of the airway of patients with diffuse panbronchiolitis (DPB), is characterized by dense neutrophil infiltration (14). We previously reported on a perpetual cycle of interleukin-8 (IL-8) production and neutrophil accumulation in the airway of such patients (29). Neutrophils that accumulate in the airways of patients with DPB also undergo apoptosis (H. Yoshimine, K Oishi, Y. Tsuchihashi, K. Matsushima, and T. Nagatake, abstract from the 2000 International Conference of the American Thoracic Society, Am. J. Respir. Crit. Care. Med. 161:A338, 2000), although the rates of apoptosis are delayed in the case of neutrophils that have migrated into the airways (20, 45).
Long-term therapy, with low doses of erythromycin (ERY) has been shown to be effective in treating patients with DPB (14, 17, 18, 29, 38). Similar clinical effects of other 14-member macrolides, such as clarithromycin (CLR) and roxithromycin (RXM), or a 15-member macrolide, azithromycin (AZM), for patients with DPB have also been reported (15, 16, 37). Several actions of 14-member macrolide antibiotic provide support for these clinical effects (12, 14, 29, 38-40). We, as well as other investigators, previously reported on the inhibitory effect of ERY on IL-8 production by bronchial epithelial cells or activated neutrophils (29, 38, 41). In addition, two other investigators reported that 14-member macrolides suppress transcription factors which regulate IL-8 gene expression in bronchial epithelial cells (1, 3). These lines of evidence suggest that the observed clinical effects of 14-member macrolide antibiotics may be due to their inhibitory effect on IL-8 gene expression in respiratory cells.
The mechanisms of apoptotic cell clearance by macrophages in human cells are not well understood. Several ligands and receptors have been shown to play a role in the phagocytosis of apoptotic neutrophils in vitro. These include the αVβ3/CD36/thrombospondin recognition system, phosphatidylserine (PS) receptor, complement receptors, scavenger receptors, CD14, and lectins (4, 5, 7, 22, 30, 35, 36). Among these, the mechanism of apoptotic neutrophil clearance has been studied only in human monocyte-derived macrophages (HMDM) (22, 35, 36). Apoptotic neutrophils in the airway, however, are ingested by human alveolar macrophages (AM) without releasing inflammatory mediators by surface recognition mechanisms, while necrotic cells liberate neutrophil serine proteinases and exacerbate the inflammatory response (13, 24, 27). It is generally accepted that this process is a crucial mechanism in the resolution of inflammatory lung diseases. The mechanism of apoptotic neutrophil clearance by AM, however, is not well understood.
On the other hand, it has recently been reported that glucocorticoids promote the phagocytosis of apoptotic neutrophils by HMDM (19). These investigators proposed novel therapeutic approaches with respect to the use of glucocorticoids in achieving an efficient and safe resolution of inflammation. It would, therefore, be of interest to determine whether 14-member or 15-member macrolide antibiotics affect the phagocytosis of apoptotic neutrophils by human AM. We report herein on a novel effect of 14-member and 15-member macrolides on the phagocytosis of neutrophils which are undergoing apoptosis by human AM.
MATERIALS AND METHODS
Reagents.
Dexamethasone 21-acetate (DEX) (Sigma Chemical Co., St. Louis. Mo), ERY (Dainippon Pharmaceutical Co., Ltd., Osaka, Japan), CLR (Taisho Pharmaceutical Co., Tokyo, Japan), RXM (Eisai Co., Ltd., Tokyo, Japan), oleandomycin (OLM) (Wako Pure Chemical Industries, Ltd., Osaka, Japan), josamycin (JOS) (Yamanouchi Pharmaceutical Co., Osaka, Japan), spiramycin (SPM) (Kyowa Hakko Kogyo Co., Ltd., Tokyo), AZM (Pfizer Pharmaceutical Co., Tokyo, Japan), ampicillin (AMP) (Pfizer Pharmaceutical Co.), cefaclor (CEC) (Shionogi & Co. Ltd., Osaka, Japan), and clindamycin (CLDM) (Japan Upjohn Co., Ltd., Tokyo, Japan) were dissolved at a concentration of 10 mg/ml in dimethyl sulfoxide (DMSO) (Wako Pure Chemical Industries) and subsequently diluted in RPMI 1640 (Asahi Techno Glass Co, Funabashi, Japan). A lipopolysaccharide (LPS) preparation from Pseudomonas aeruginosa serotype 10 (Sigma Chemical) was also used.
Neutrophil culture.
Neutrophils were purified from the peripheral blood of a normal volunteer by dextran sedimentation and density gradient centrifugation, and suspended at a concentration of 5 × 106 cells/ml in Iscove's modified Dulbecco's medium (IMDM) (Life Technologies, Grand Island, N.Y.) in a 96-well flat-bottom flexible plate (Becton Dickinson, Oxnard, United Kingdom) for 24 h at 37°C in 5% CO2 (44). After incubation, cultured neutrophils were collected and used in the phagocytosis assay.
Neutrophil apoptosis.
Neutrophils were collected from the flexible plate after 24 h of culture and were labeled with annexin V-FITC (Beckman Coulter Co., Marseille, France), which labels membrane PS residues, in order to measure cell membrane changes associated with apoptosis. Analyses were performed by means of flow cytometry (Beckman Coulter Co.) (44). Aged human neutrophils when cultured for 24 h in IMDM after neutrophil separation were determined to be 85% apoptotic.
Preparation of AM.
AM were collected from nonsmoking, healthy volunteers by bronchoalveolar lavage using broncofiberscopy as previously described (28). For AM recovered from bronchoalveolar lavage fluids, the AM were washed, suspended in RPMI 1640, seeded at 3 × 105 cells per 24-well plate or 1.2 × 105 cells per 96-well plate with 10% heat-inactivated autologous serum, and cultured for 1 to 3 days at 37°C in an atmosphere of 5% CO2.
Phagocytosis of apoptotic neutrophils by AM.
For each assay of phagocytosis, AM were collected from different donors. Autologous apoptotic neutrophils were added to the cultured AM in the presence of autologous fresh serum, heat-inactivated serum or IMDM alone for 30 min at 37°C. The heat inactivation was done at 56°C for 30 min. The monolayer was then washed vigorously with ice-cold phosphate-buffered saline to remove bound but uningested neutrophils and was fixed in 2% glutaraldehyde. After washing with distilled water, the cells on the plates were stained for myeloperoxidase (MPO) at room temperature for 45 min, as a marker of the ingested neutrophils, using a DAB substrate kit (Roche Diagnostics GmbH, Mannheim, Germany) (24). After washing the plates, the cells on the plates were stained with Giemsa stain. The phagocytosis of apoptotic neutrophils was examined by means of ×400 phase-contrast microscopy by counting 200 macrophages per well. The percentage of AM that phagocytosed apoptotic neutrophils was determined as the percent phagocytosis in three different wells.
Pretreatment of AM with antibiotics or DEX.
In preliminary experiments, treatment of HMDM with ERY at 0.1 to 10 μg/ml for 72 h, but not 24 h, was required for the development of an increased capacity for the phagocytosis of apoptotic neutrophils, while treatment of HMDM with DEX at 10−7 M for 24 h was sufficient for the acquisition of this capacity (19). AM were, therefore, treated with each antibiotic at 0.1 to 10 μg/ml for 72 h, DEX at 10−7 M for 24 h, or RPMI 1640 containing 0.1% DMSO for 72 h in the presence of 10% heat-inactivated autologous serum.
Enzyme-linked immunosorbent assay.
The human IL-8 protein levels in the cell culture supernatants were determined by an enzyme-linked immunosorbent assay as previously described (28). The human tumor necrosis factor alpha (TNF-α) protein levels were determined using a commercially available kit (Biosource International, Inc. Camarillo, Calif.).
Inhibition of phagocytosis of apoptotic neutrophils by AM.
AM were preincubated with each inhibitor at 37°C for 30 min followed by washing, and exposure to autologous apoptotic neutrophils. The inhibitors included PS liposomes (containing 50: 50 molar ratio of PS to phosphatidylcholine, PC) or PC liposome at 0.1 mM as the control for PS liposomes (9, 43), a mouse monoclonal antibody (MAb) 217 (immunoglobulin M [IgM] kappa chain; Cascade Bioscience, Winchester, Mass.) or a control IgM MAb (7) at a final concentration of 100 μg/ml as the control for MAb 217, tetrapeptide RGDS or RGES (Sigma Chemical Co.) at a final concentration of 2 mM as the control for RGDS (36). The MAb 217 identifies a surface receptor expressed on transforming growth factor β (TGF-β)- and β-glucan-stimulated HMDM that recognizes PS on apoptotic cells (7).
Cytotoxic assay.
AM were incubated with each antibiotic for 72 h or with DEX for 24 h at 1.2 × 106 cells per ml in a 96-well culture plate. After incubation, the cells were washed with IMDM twice, 20 μl of alamarBlue solution (Biosource International, Camarillo, Calif.) was added to the wells containing 180 μl of RPMI 1640, followed by incubation for 4 h at 37°C (8). The fluorescence (excitation, 560 nm; detection, 590 nm) of each well was measured using a Fluoroskan II instrument (Labsystems Japan, Tokyo, Japan). The results are expressed as the percentage of viable cell.
Statistical analysis.
The percent phagocytosis of apoptotic neutrophils, the levels of cytokine production by AM or the percentage of viable cell in the cytotoxic assay were compared by one-way analysis of variance and multiple comparison methods by Bonferroni-Dunn's test. The difference in the percent phagocytosis between fresh serum and heat-inactivated serum was analyzed by the paired Student t test. Data were considered statistically significant when P values were less than 0.05.
RESULTS
Effects of macrolide or DEX on the cell viability of AM.
No significant effects on cell viability of AM as the result of treatment with each antibiotic, including 14-member macrolides at a concentration of 10 μg/ml for 72 h or DEX for 24 h, were found, compared with control media containing 0.1% DMSO (data not shown).
Phagocytosis of apoptotic neutrophils by AM.
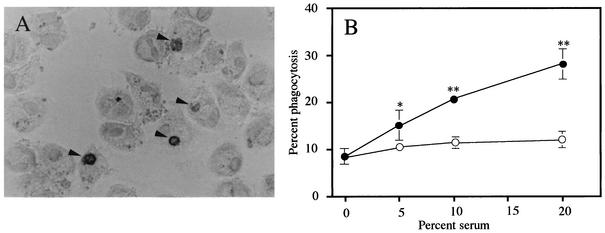
A photomicrograph of apoptotic neutrophils stained for MPO that have been phagocytosed by AM is shown in Fig. 1A. Fresh human serum in the range of 5 to 20% led to significant increases in phagocytosis by AM cultured for 24 h in a concentration-dependent manner (P < 0.05 for 5% serum, P < 0.01 for 10 or 20% serum, Fig. 1B). A significant reduction in phagocytosis by heat-inactivation of 10 and 20% fresh serum was found and is indicative of the complement-dependent phagocytosis of apoptotic neutrophils (P < 0.01) (22). No difference in phagocytosis was found for 10% heat-inactivated serum vis-a-vis IMDM alone.
FIG. 1.
(A) Photomicrograph of human AM that contain phagocytosed apoptotic neutrophils. The arrowhead indicates the intracellular apoptotic neutrophils which are stained for MPO within the MPO-negative AM. (B) Complement-dependent phagocytosis of apoptotic neutrophils by AM cultured for 24 h. Autologous apoptotic neutrophils were exposed to AM in the presence of fresh human serum (closed circles) or heat-inactivated serum (open circles). Each data point represents the mean ± standard deviation (error bars) of three determinations. Statistical significance: *, P < 0.05(versus no serum); **, P < 0.01(versus no serum and heat-inactivated serum with the same concentration).
Effect of macrolide antibiotic or DEX on phagocytosis of apoptotic neutrophils by AM.
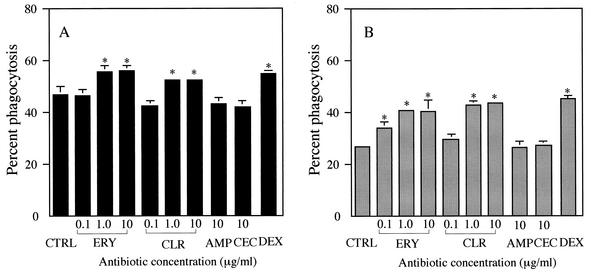
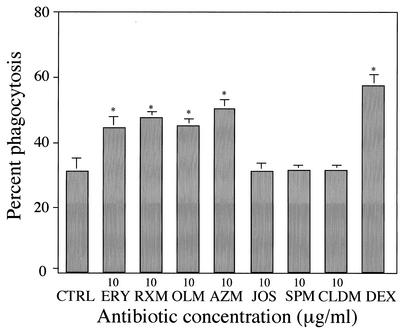
Treatment with DEX, a positive control, for 24 h significantly increased phagocytosis in the presence of 10% fresh human serum or medium alone, compared with control treatment (Fig. 2). These data are consistent with a previous report on the effect of DEX on the phagocytosis of apoptotic neutrophils by HMDM (19). Treatment of AM with ERY or CLR at concentrations of 1 and 10 μg/ml, but not 0.1 μg/ml, for 72 h also significantly increased phagocytosis in the presence of 10% fresh human serum (Fig. 2A). Similar enhancing effects as the result of treatment of AM with ERY or CLR occurred at 0.1 to 10 μg/ml in the presence of IMDM alone (Fig. 2B). Serum factors were not essential for the promoting effects on the phagocytosis of apoptotic neutrophils by these 14-member macrolide antibiotics, since these effects were observed in the absence as well as presence of fresh human serum during phagocytosis. RXM, OLM, or AZM at 10 μg/ml similarly enhanced phagocytosis in the presence of IMDM alone (Fig. 3). In contrast, treatment with a 16-member macrolide, such as JOS or SPM, CLDM, or a β-lactam antibiotic, involving AMP and CEC, had no effect on the phagocytosis of AM in the presence of 10% fresh human serum or medium alone (Fig. 2 and 3). The promoting effects of antibiotics on the phagocytosis were, therefore, specific for 14- and 15-member macrolide antibiotics.
FIG. 2.
Effects of 14-member macrolides on the phagocytosis of apoptotic neutrophils by human alveolar macrophages (AM) in the presence of 10% fresh homologous serum (A) or medium alone (B). AM were treated with ERY or CLR at concentrations of 0.1 to 10 μg/ml, with JOS, AMP, or CEC at a concentration of 10 μg/ml for 72 h. Treatment of AM with DEX (10−7 M) was done for 24 h. The control (CTRL) represents the treatment of AM with medium containing 0.1% DMSO. Each value represents the mean ± standard deviation (error bars) of three determinations. *, P < 0.01 (versus CTRL).
FIG. 3.
Effects of 14-member and 15-member macrolides on the phagocytosis of apoptotic neutrophils by human AM in the presence of medium alone. AM was treated with ERY, RXM, OLM, AZM, JOS, SPM, or CLDM at concentrations of 10 μg/ml for 72 h. Treatment of AM with DEX (10−7 M) was done for 24 h. The control (CTRL) represents the treatment of AM with medium containing 0.1% DMSO. Each value represents the mean ± SD of three determinations. *, P < 0.01 (versus CTRL).
Effects of ERY or DEX on IL-8 or TNF-α production by AM with or without the phagocytosis of apoptotic neutrophils.
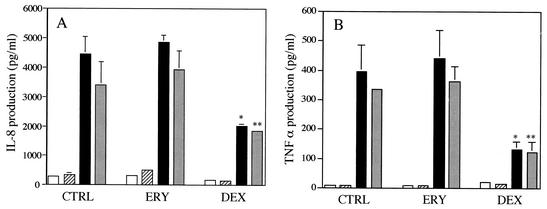
Treatment with DEX alone significantly suppressed IL-8 or TNF-α production by LPS-stimulated AM irrespective of the phagocytosis of apoptotic neutrophils, while ERY had no effects on the levels of IL-8 or TNF-α by unstimulated or LPS-stimulated AM irrespective of the phagocytosis of apoptotic neutrophils (Fig. 4). Furthermore, these drugs, which enhanced the phagotcyotosis of apoptotic neutrophils, did not alter the levels of IL-8 or TNF-α production by unstimulated or LPS-stimulated AM after the phagocytosis. These data suggest that ERY as well as DEX promote the nonphlogistic phagocytosis of apoptotic neutrophils by AM (19).
FIG. 4.
Effects of ERY or DEX on IL-8 production (A) or TNF-α (B) by unstimulated or LPS-stimulated human AM after the phagocytosis of apoptotic neutrophils. AM were treated with ERY at a concentration of 10 μg/ml, DEX at a concentration of 10−7 M or medium containing 0.1% DMSO (CTRL) for 72 h. AM were, then, incubated with LPS (10 ng/ml) or IMDM alone for 3 h, followed by exposure to apoptotic neutrophils or IMDM alone for 30 min. After removing unphagocytosed neutrophils, AM were cultured for 18 h. After incubation, the IL-8 or TNF-α levels in the cell culture supernatants were determined. Each bar denotes no phagocytosis without LPS (open bars), phagocytosis without LPS (slash bars), no phagocytosis with LPS (solid bars), and phagocytosis with LPS (gray bars), respectively. Each value represents the mean ± standard deviation (error bars) of three determinations. Statistical significance: *, P < 0.001 (versus LPS-stimulated AM without phagocytosis in CTRL treatment); **, P < 0.001 (versus LPS-stimulated AM with phagocytosis in CTRL treatment).
Receptors responsible for the phagocytosis of apoptotic neutrophils by AM.
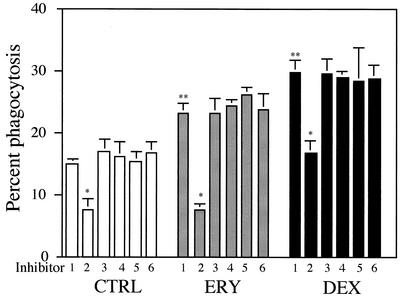
To determine which receptors are responsible for the promoting effects of 14-member macrolide antibiotics on phagocytosis, we next developed a receptor blocking assay in the presence of medium alone during phagocytosis. PS liposomes significantly suppressed the phagocytosis of untreated AM, compared with PC liposomes (Fig. 5). This suggests an involvement of the PS receptor in the phagocytosis. More importantly, PS liposomes significantly suppressed the ERY-induced enhanced phagocytosis to levels comparable to that observed for PS liposomes by untreated AM. In the case of DEX treatment, PS liposomes significantly inhibited phagocytosis, but did not reach the levels observed for PS liposomes by untreated AM. MAb 217 was used to block the MAb 217-reactive PS receptor, which is expressed at high levels on the surface of stimulated HMDM by TGF-β and β-glucan (7), and the tetrapeptide RGDS was used to block the αvβ3/CD36/thrombospondin system (36). Neither MAb 217 nor RGDS altered the levels of phagocytosis by untreated, ERY-treated or DEX-treated AM.
FIG. 5.
PS receptors are predominantly responsible for the ERY-induced phagocytosis of apoptotic neutrophils by human AM. AM were treated with ERY at a concentration of 10 μg/ml, DEX at a concentration of 10−7M or medium containing 0.1% DMSO (CTRL) for 72 h. AM were preincubated with each inhibitor at 37°C for 30 min, and then exposed to apoptotic neutrophils after washing the macrophages with medium. The inhibitors were as follows: 1, phosphatidylcholine (PC) liposomes at 0.1 mM; 2, PS liposomes at 0.1 mM; 3, MAb 217 at 100 μg/ml; 4, control IgM MAb at 100 μg/ml; 5, RGDS at 2 mM; 6, RGES at 2 mM. Each value represents the means ± standard deviations (error bars) of three determinations. Statistical significance: *, P < 0.01 (versus PC liposomes in each treatment); **, P < 0.01 (versus PC liposomes in CTRL treatment).
DISCUSSION
We demonstrate here that human AM involved the PS receptor (Fig. 5), but not the αvβ3/CD36/thrombospondin system which has been reported to be the pathway used for unstimulated HMDM, for the phagocytosis of apoptotic neutrophils (10, 31, 36). AM may involve receptors other than the PS receptor during this phagocytosis, because PS liposomes were found to partially inhibit the phagocytosis of apoptotic neutrophils by AM in this study. The clearance of apoptotic neutrophils by macrophages could be regulated by several known factors (23, 31, 33). It has been reported that glucocorticoids, such as methylpredonisolone, DEX, and hydrocortisone, but not nonglucocorticoid steroids, similarly potentiate the phagocytosis of apoptotic neutrophils by HMDM (19). The enhancing effect of glucocorticoids on phagocytosis also required only several hours of treatment, and did not involve the up-regulation of the αvβ3/CD36/thrombospondin recognition system or CD14.
In the present study, we found an increased capacity of phagocytosis of apoptotic neutrophils by DEX-treated AM in the presence or absence of fresh serum (Fig. 2). More interestingly, we found a novel action on the phagocytosis of apoptotic neutrophils by AM, which was specific for 14- and 15-member macrolide antibiotics at clinically acceptable levels (26). Long-term therapy with a low dose of a 14- or 15-member macrolide antibiotic, therefore, may lead to a suppression of neutrophil accumulation in the airways of patients with DPB, in part, through a mechanism involving an increased clearance of apoptotic neutrophils by AM without the liberation of neutrophil serine proteinases (13, 42).
Since PS liposomes completely suppressed the ERY-induced increase in phagocytosis by AM, the enhanced capacity of ERY on phagocytosis by these macrophages appears to be PS receptor-dependent (Fig. 5). An enhanced capacity of ERY was observed at 72 h postincubation, but not at 24 h postincubation. We, therefore, speculate that a 72 h incubation may be required for the induction of PS receptors on cultured AM. We also demonstrated that PS receptors were, in part, involved in the DEX-induced up-regulation of phagocytosis. These findings support the hypothesis that the treatment of AM with ERY or DEX may enhance PS receptor expression on their cell surfaces. A similar alteration of receptor use for apoptotic neutrophils has been reported for unstimulated vis-a-vis glucan-stimulated HMDM (10). Another study has also demonstrated a difference in receptor use between peritoneal macrophages and monocyte-derived macrophages (9). In addition, MAb 217 was found to have no effect on the phagocytosis of apoptotic neutrophils by ERY- or DEX-treated AM as well as untreated AM. We similarly found that MAb 217 had no effects on the phagocytosis of apoptotic neutrophils by ERY- or DEX-treated HMDM (data not shown). These data suggest a lack of expression of the MAb 217-reactive PS receptor on ERY- or DEX-treated, or untreated AM.
Fadok et al. demonstrated that the phagocytosis of apoptotic neutrophils actively inhibited the production CXC chemokines and other proinflammatory cytokines by HMDM or mouse macrophages, and increased the production of TGF-β (6, 21). The above investigators suggested that a feedback mechanism by endogenous TGF-β largely contributed to this active inhibition of CXC chemokines and proinflammatory cytokines. On the other hand, two other studies have recently reported that the phagocytosis of apoptotic neutrophils by HMDM does not trigger the release of IL-8, TNF-α, or MCP-1 (19, 32). These investigators proposed the nonphlogistic phagocytosis of apoptotic neutrophils by HMDM. In the present study, our findings are also consistent with the nonphlogistic phagocytosis of apoptosis because no up-regulation of IL-8 or TNF-α production by AM was found after the phagocytosis (Fig. 4). We also found an inhibitory effect of DEX, but not ERY, on IL-8 or TNF-α production by LPS-stimulated AM, although the inhibitory effects of 14-member macrolides on IL-8 production have also been reported for 1α,25-dihydroxyvitamin D3-treated human monocytic cell line cells after LPS stimulation (11). A difference in the inhibitory effect on IL-8 production may be present between AM and monocyte-derived macrophages since an inhibitory effect of ERY on IL-8 or TNF-α production in LPS-stimulated HMDM was found (data not shown). The inhibitory effects of DEX on IL-8 or TNF-α production by LPS-stimulated AM are consistent with its action (Fig. 4), in that it appears to interfere with the binding of NF-κB to its cognate cis-element, since NF-κB has been shown to regulate the gene transcription of IL-8 and TNF-α (2, 25). The increased capacity of DEX to promote the nonphlogistic phagocytosis of apoptotic neutrophils by AM and to inhibit IL-8 or TNF-α production by activated AM strongly suggests that they possess anti-inflammatory properties. The long-term oral administration of DEX, however, may impair pulmonary defense via the suppression of iNOS and TNF-α which are critical for combating bacterial infections (34).
In conclusion, we report on the novel in vitro action of 14- and 15-member macrolides on the improved phagocytosis of apoptotic neutrophils by AM. The mechanism of enhanced phagocytosis by ERY was found to be PS receptor-dependent, and was not associated with the additional production of IL-8 or TNF-α by AM. An enhanced nonphlogistic clearance of apoptotic neutrophils by AM, induced by 14- or 15-member macrolide, may provide partial support for the clinical efficacy of low-dose, long-term macrolide therapy for patients with DPB.
Acknowledgments
We are grateful to Y. Terai and M. Yanase for excellent technical support and to Y. Nakanishi for his critical comments on this manuscript.
This study was supported, in part, by a grant 11670583 from the Ministry of Education, Science, and Culture of Japan.
REFERENCES
- 1.Abe, S., H. Nakamura, S. Inoue, H. Takeda, H. Saito, S. Kato, N. Mukaida, K. Matsushima, and H. Tomoike. 2001. Interleukin-8 gene repression by clarithromycin is mediated by the activator protein-1 binding sites in human bronchial epithelial cells. Am. J. Respir. Cell. Mol. Biol. 22:51-60. [DOI] [PubMed] [Google Scholar]
- 2.Blackwell, T. S., and J. W. Christman. 1997. The role of nuclear factor-κB in cytokine gene regulation. Am. J. Respir. Cell. Mol. Biol. 17:3-9. [DOI] [PubMed] [Google Scholar]
- 3.Desaki, M., H. Takizawa, T. Ohtoshi, T. Kasama, K. Kobayashi, T. Sunazuka, Omura, K. Yamamoto, and K. Ito. 2000. Erythromycin suppresses nuclear factor-κB and activator protein-1 activation in human bronchial epithelial cells. Biochem. Biophys. Res. Commun. 267:124-128. [DOI] [PubMed] [Google Scholar]
- 4.Devitt, A., O. D. Moffatt, C. Raykundalia, J. D. Capra, D. L. Simmonda, and C. D. Gregory. 1998. Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature 392:505-509. [DOI] [PubMed] [Google Scholar]
- 5.Duvall, E., A. H. Wyllie, and R. G. Morris. 1985. Macrophage recognition of cells undergoing programmed cell death (apoptosis). Immunology 56:351-358. [PMC free article] [PubMed] [Google Scholar]
- 6.Fadok, V. A., D. L. Bratton, A. Konowal, P. W. Freed, J. Y. Westcott, and P. M. Henson. 1998. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J. Clin. Investig. 101:890-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fadok, V. A., D. L. Bratton, D. M. Rose, A. Pearson, R. A. B. Ezekewitz, and P. M. Henson. 2000. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature 405:85-90. [DOI] [PubMed] [Google Scholar]
- 8.Fadok, V. A., D. R. Voelker, P. A. Cambell, J. J. Cohen, D. L. Bratton, and P. M. Henson. 1992. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 148:2207-2216. [PubMed] [Google Scholar]
- 9.Fadok, V. A., J. S. C. Haslett, D. L. Bratton, D. E. Doherty, P. A. Cambell, and P. M. Henson. 1992. Different populations of macrophages use either the vitronectin receptor or the phosphatidylserine receptor to recognize and remove apoptotic cells. J. Immunol. 149:4029-4035. [PubMed] [Google Scholar]
- 10.Fadok, V. A., M. L. Warner, D. L. Bratton, and P. M. Henson. 1998. CD36 is required for phagocytosis of apoptotic cells by human macrophages that use either a phosphatidylserine receptor or the vitronection receptor (αvβ3). J. Immunol. 161:6250-6257. [PubMed] [Google Scholar]
- 11.Fujii, T., J. Kadota, T. Morikawa, Y. Matsubara, K. Kawakami, K. Iida, R. Shirai, H. Taniguchi, M. Kaseda, S. Kawamoto, and S. Kohno. 1996. Inhibitory effect of erythromycin on interleukin-8 production by 1α,25-dihydroxyvitamin D3-stimulated THP-1 cells. Antimicrob. Agents Chemother. 40:1548-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goswami, S. K., S. Kivity, and Z. Marom. 1990. Erythromycin inhibits respiratory glycoconjugate secretion from human airways in vitro. Am. Rev. Respir. Dis. 141:72-78. [DOI] [PubMed] [Google Scholar]
- 13.Haslett, C. 1999. Granulocyte apoptosis and its role in the resolution and control of lung inflammation. Am. J. Respir. Crit. Care Med. 160:S5-S11. [DOI] [PubMed] [Google Scholar]
- 14.Kadota, J., O. Sakito, S. Kohno, H. Sawa, H. Mukae, H. Oda, K. Kawakami, K. Fukushima, K. Hiratani, and K. Hara. 1993. A mechanism of erythromycin treatment in patients with diffuse panbronchiolitis. Am. Rev. Respir. Dis. 147:153-159. [DOI] [PubMed] [Google Scholar]
- 15.Kadota, J., O. Sakito, S. Khono, K. Abe, R. Shirai, K. Kawakami, K. Iida, T. Morikawa, S. Kusano, and K. Hara. 1994. Roxythromycin treatment in patients with chronic lower respiratory tract disease-its clinical efficacy and effect on cytokine. J. Jpn. Assoc. Infect. Dis. 68:27-33. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi, H., H. Takeda, S. Sakayori, Y. Kawakami, Y. Otsuka, M. Tamura, K. Konishi, S. Tanimoto, M. Fukakusa, and Shimada, K. 1995. Study on azithromycin in treatment of diffuse panbronchiolitis. J. Jpn. Assoc. Infect. Dis. 69:711-722. [DOI] [PubMed] [Google Scholar]
- 17.Kudoh, S., A. Azuma, M. Yamamoto, T. Izumi, and M. Ando. 1998. Improvement of survival in patients with diffuse panbronchiolitis treated with low-dose erythromycin. Am. J. Respir. Crit. Care Med. 157:1829-1832. [DOI] [PubMed] [Google Scholar]
- 18.Kudoh, S., T. Uetake, K. Hagiwara, L. H. Hus, H. Limura, and Y. Sugiyama. 1987. Clinical effect of low-dose, long-term erythromycin chemotherapy on diffuse panbronchiolitis. Jpn. J. Thorac. Dis. 25:632-642. [PubMed] [Google Scholar]
- 19.Liu, Y., J. M. Cousin, J. Hughes, J. V. Damme, J. R. Seckle, C. Haslette, I. Dransfield, J. Savill, and A. G. Rossi. 1999. Glucocorticoids promote nonphlogistic phagocytosis of apoptotic leukocytes. J. Immunol. 162:3639-3646. [PubMed] [Google Scholar]
- 20.Matute-Bello, G., W. C. Lilies, F. Radella, I. I., K. P. Steinber, J. P. Ruzinski, M. Jonas, R. Y. Chi, L. D. Hudson, and T. R. Martin. 1997. Neutrophil apoptosis in the acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 156:1969-1977. [DOI] [PubMed] [Google Scholar]
- 21.McDonald, P. P., V. A. Fadok, D. Bratton, and P. M. Henson. 1999. Transcriptional and translational regulation of inflammatory mediator production by endogenous TGF-β in macrophages that have ingested apoptotic cells. J. Immunol. 163:6164-6172. [PubMed] [Google Scholar]
- 22.Mevorach, D., J. O. Mascarenhas, D. Gershov, and K. B. Elkon. 1998. Complement-dependent clearance of apoptotic cells by human macrophages. J. Exp. Med. 188:2313-2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitchell, G. C., K. Harvey, N. A. Petasis, N. Hogg, and H. R. Brady. 2000. Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J. Immunol. 164:1663-1667. [DOI] [PubMed] [Google Scholar]
- 24.Morimoto, K., H. Amano, F. Sonoda, M. Baba, M. Senba, H. Yoshimine, H. Yamamoto, T. Ii, K. Oishi, and T. Nagatake. 2001. Alveolar macrophages that phagocytose apoptotic neutrophils produce hepatocyte growth factor during bacterial pneumonia in mice. Am. J. Respir. Cell. Mol. Biol. 24:608-615. [DOI] [PubMed] [Google Scholar]
- 25.Mukaida, N., M. Morita, Y. Ishikawa, N. Rice, S. Okamoto, T. Kasahara, and K. Matsushima. 1994. Novel mechanism of glucocorticoid-mediated gene repression. Nuclear factor-κB is target for glucocorticoid-mediated interleukin-8 gene repression. J. Biol. Chem. 269:13289-13295. [PubMed] [Google Scholar]
- 26.Nagai, H., H. Shishido, R. Yoneda, E. Yamaguchi, A. Tamura, and K. Kurashima. 1991. Long-term low-dose administration of erythromycin to patients with diffuse panbronchiolitis. Respiration 58:145-149. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura, H., K. Yoshimura, N. G. McElvaney, and R. G. Crystal. 1992. Neutrophil elastase in respiratory epithelial lining fluid of individuals with cystic fibrosis induces interleukin-8 gene expression in human bronchial epithelial cell lines. J. Clin. Investig. 89:1478-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oishi, K., B. Sarr, A. Wada, Y. Hidaka, S. Matsumoto, H. Amano, F. Sonoda, S. Kobayashi, T. Hirayama, T. Nagatatake, and K. Matsushima. 1997. Nitrite reductase from Pseudomonas aeruginosa induces inflammatory cytokines in cultured respiratory cells. Infect. Immun. 65:2648-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oishi, K., F. Sonoda, S. Kobayashi, A. Iwagaki, T. Nagatake, K. Matsushima, and K. Matsumoto. 1994. Role of interleukin-8 (IL-8) and an inhibitory effect of erythromycin on IL-8 release in the airways of patients with chronic airway diseases. Infect. Immun. 62:4145-4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Platt, N., H. Suzuki, Y. Kurihara, T. Kodama, and S. Gordon. 1996. Role of the class A macrophage scavenger receptor in the phagocytosis of apoptotic thymocytes in vitro. Proc. Natl. Acad. Sci. USA 93:12456-12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ren, Y., and J. Savill. 1995. Proinflammatory cytokines potentiate thrombospondin-mediated phagocytosis of neutrophils undergoing apoptosis. J. Immunol. 154:2366-2374. [PubMed] [Google Scholar]
- 32.Ren, Y., L. Stuart, F. P. Lindberg, A. R. Rosenkranz, Y. Chen, Y. N. Mayandas, and J. Savill. 2001. Nonphlogistic clearance of late apoptotic neutrophils by macrophages; efficient phagocytosis independent of β2 integrins. J. Immunol. 166:4743-4750. [DOI] [PubMed] [Google Scholar]
- 33.Rossi, A. G., J. C. McCutcheon, N. Roy, E. R. Chilvers, C. Haslett, and I. Dransfield. 1998. Regulation of macrophage phagocytosis of apoptotic cells by cAMP. J. Immunol. 160:3562-3568. [PubMed] [Google Scholar]
- 34.Satoh, S., K. Oishi, A. Iwagaki, M. Senba, A. Akaike, M. Akiyama, N. Mukaida, K. Matsushima, and T. Nagatake. 2001. Dexamethasone impairs pulmonary defense against Pseudomonas aeruginosa through suppressing iNOS gene expression and peroxynitrite production in mice. Clin. Exp. Immunol. 123:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Savill, J., I. Dransfield, N. Hogg, and C. Haslett. 1990. Vitronectin-mediated phagocytosis of cells undergoing apoptosis. Nature 343:170-173. [DOI] [PubMed] [Google Scholar]
- 36.Savill, J., N. Hogg, Y. Ren, and C. Haslett. 1992. Thrombospondin cooperates with CD36 and the vitronection receptor in macrophage recognition of neutrophils undergoing apoptosis. J. Clin. Investig. 90:1513-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takeda, H., H. Miura, M. Kawahira, H. Kobayashi, S. Otomo, and S. Nakaike. 1989. Long-term administration study on TE-031 (A-56268) in the treatment of diffuse panbronchiolitis. J. Jpn. Assoc. Infect. Dis. 63:71-78. [DOI] [PubMed] [Google Scholar]
- 38.Takizawa, H., M. Desaki, T. Ohtoshi, S. Kawasaki, T. Kohyama, M. Sato, M. Tanaka, T. Kasama, K. Kobayashi, J. Nakajima, and K. Ito. 1997. Erythromycin modulates IL-8 expression in normal and inflamed human bronchial epithelial cells. Am. J. Respir. Crit. Care. Med. 156:266-271. [DOI] [PubMed] [Google Scholar]
- 39.Tamaoki, J., K. Isono, N. Sakai, T. Kanemura, and K. Konno. 1992. Erythromycin inhibit Cl secretion across canine tracheal epithelial cells. Eur. Respir. J. 5:234-238. [PubMed] [Google Scholar]
- 40.Tateda, K., C. Tachel, P. Jean-Claude, K. Tilo, K. Yamaguchi, and V. D. Christian. 2001. Azithromycin inhibits quorum-sensing and reduces virulence factor production by Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 45:1930-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsuchihashi, Y., K. Oishi, H. Yoshimine, S. Suzuki, A. Kumatori, T. Sunazuka, S. Omura, K. Matshushima, and T. Nagatake. 2002. Fourteen-member macrolides suppress interleukin-8 production but do not promote apoptosis of activated neutrophils. Antimicrob. Agents Chemother. 46:1101-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vandivier, R. W., V. A. Fadok, P. R. Hoffmann, D. L. Bratton, C. Penvari. K. K. Brown, J. D. Brain, F. J. Accursa, and P. M. Henson. 2002. Elastase-mediated phosphatidylserine receptor cleavage impairs apoptotic cell clearance in cystic fibrosis and bronchiectasis. J. Clin. Investig. 109:661-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang, A., A. Wada, K. Yahiro, T. Nomura, Y. Fujii, K. Okamoto, Y. Mizuta, S. Kohno, J. Moss, and T. Hirayama. 1999. Identification and characterization of the Aeromonas sobria hemolysin glycoprotein receptor on intestine 407 cells. Microb. Pathog. 27:215-221. [DOI] [PubMed] [Google Scholar]
- 44.Ward, C., E. R. Chilver, M. F. Lawson, J. P. Pryde, S. Fujihara, S. N. Farrow, C. Haslette, and A. G. Rossi. 1999. NF-κB activation is critical regulator of human granulocytes apoptosis in vitro. J. Biol. Chem. 274:4309-4317. [DOI] [PubMed] [Google Scholar]
- 45.Watson, R. W. G., O. D. Rotstein, A. B. Nathen, J. Parodo, and J. C. Marshall. 1997. Neutrophil apoptosis is mediated by endothelial transmigration and adhesion molecule engagement. J. Immunol. 158:945-953. [PubMed] [Google Scholar]