Abstract
Signaling through heterotrimeric G proteins is conserved in diverse eukaryotes. Compared to vertebrates, the simpler repertoire of G-protein complex and accessory components in Arabidopsis (Arabidopsis thaliana) offers a unique advantage over all other multicellular, genetic-model systems for dissecting the mechanism of G-protein signal transduction. One of several biological processes that the G-protein complex regulates in Arabidopsis is cell division. We determined cell production rate in the primary root and the formation of lateral roots in Arabidopsis to define individually the types of modulatory roles of the respective G-protein α- and β-subunits, as well as the heterotrimer in cell division. The growth rate of the root is in part a consequence of cell cycle maintenance in the root apical meristem (RAM), while lateral root production requires meristem formation by founder pericycle cells. Thus, a comparison of these two parameters in various genetic backgrounds enabled dissection of the role of the G-protein subunits in modulation of cell division, both in maintenance and initiation. Cell production rates were determined for the RAM and lateral root formation in gpa1 (Arabidopsis G-protein α-subunit) and agb1 (Arabidopsis G-protein β-subunit) single and double mutants, and in transgenic lines overexpressing GPA1 or AGB1 in agb1 or gpa1 mutant backgrounds, respectively. We found in the RAM that the heterotrimeric complex acts as an attenuator of cell proliferation, whereas the GTP-bound form of the Gα-subunit's role is a positive modulator. In contrast, for the formation of lateral roots, the Gβγ-dimer acts largely independently of the Gα-subunit to attenuate cell division. These results suggest that Arabidopsis heterotrimeric G-protein subunits have differential and opposing roles in the modulation of cell division in roots.
Heterotrimeric GTP-binding proteins (G proteins) are critical molecular switches, regulating diverse signaling pathways in eukaryotic cells (Gilman, 1987; Hamm, 1998; Neubig and Siderovski, 2002; Pierce et al., 2002). Recently, a role for the heterotrimeric G-protein complex in asymmetrical cell division and differentiation in Drosophila was added to this diversity (e.g. Schwabe et al., 2005; Wang et al., 2005). The Arabidopsis (Arabidopsis thaliana) genome contains genes encoding only one canonical G-protein α-subunit (Gα), one β-subunit (Gβ), two γ-subunits (Gγ), one regulator of G-protein-signaling (RGS) protein, and few putative G-protein-coupled receptors (GPCR; Jones and Assmann, 2004). In contrast, humans have 23 α-, six β-, and 12 γ-subunits of the heterotrimeric G-protein complexes, and 37 RGS proteins and as many as 800 GPCRs (Jones and Assmann, 2004). The small repertoire of the heterotrimeric G-protein elements in plants offers a unique advantage over its mammalian counterparts for dissecting their role in signal transduction pathways. Studies using Arabidopsis and rice (Oryza sativa) G-protein mutants and transgenic lines suggest that G proteins are involved in diverse developmental processes (i.e. seed germination; leaf, flower, and fruit development; and stress responses; Perfus-Barbeoch et al., 2004). The underlying mechanism for many of these developmental processes lies at the level of regulation of cell proliferation in plants (Ullah et al., 2001, 2003; Chen et al., 2003). In particular, null alleles of Arabidopsis Gα (gpa1) have a reduced number of lateral root primordia, whereas null alleles of Arabidopsis Gβ (agb1) have enhanced cell division in roots, resulting in excessive lateral roots (Ullah et al., 2003). Null alleles of the single RGS gene (RGS1), as well as expression of a constitutively active Gα, confer increased cell division in the root apical meristem (RAM; Chen et al., 2003), indicating that the GTP-bound form of GPA1 plays a positive role in cell proliferation.
The root is ideal to quantitate cell division in situ. The root system originates from a root primordium that forms during embryogenesis. Stem cells of the RAM generate all of the cell types through stereotypic divisions, followed by cell elongation and differentiation (Scheres et al., 2001). Subsequently, the primary root produces lateral roots that are initiated from the pericycle cells located adjacent to protoxylem poles at some distance from the primary root meristem (Dubrovsky et al., 2000, 2001). One viewpoint is that, while most pericycle cells are arrested at G1, the distally located founder pericycle cells positioned at the protoxylem poles commit to a lateral root fate by re-entry to the cell cycle from the G2 phase and that formation of a lateral root primordium is the direct consequence of a G2-to-M transition (Doerner et al., 1996; Beeckman et al., 2001; Himanen et al., 2004). It has been suggested that cell cycle blocks are occurring to arrest pericycle cells in both G1 and G2 (founder) stages (Malamy, 2005). The G1 arrest is likely maintained by Kip-related protein2 (Himanen et al., 2002). Himanen et al. (2004) examined the gene expression profiles of roots over time after auxin application that induced prolific and ectopic cell division throughout the pericycle. Gene profiles suggest that pericycle cells can re-enter the cell cycle from G1 but does not address cell cycle control under normal conditions.
Many genes are involved in various aspects of root development, ranging from distal patterning, radial patterning, epidermal patterning, and cell division to cell expansion (Helariutta et al., 2000; Sabatini et al., 2003; Blilou et al., 2005; Wildwater et al., 2005). The lateral roots are essentially identical to the primary roots in structure and formation (Malamy and Benfey, 1997). However, primary and lateral roots have different responses to some stimuli. The growth and development of primary and lateral roots are regulated by both intrinsic and environmental stimuli (for review, see Beeckman et al., 2001; Casimiro et al., 2003; Malamy, 2005). For example, exogenously applied auxin promotes lateral root formation, whereas it inhibits primary root growth. Constitutive overexpression of the protein Ser-Thr kinase PINOID (PID) leads to the loss of meristem initials followed by terminal differentiation in primary roots but not in lateral roots (Christensen et al., 2000; Benjamins et al., 2001; Friml et al., 2004), and is preceded by a reduction in expression from auxin-responsive promoters, such as DR5∷β-glucuronidase, and free indole-3-acetic acid concentration (Friml et al., 2004). In lateral roots, the free indole-3-acetic acid concentration and DR5∷β-glucuronidase expression in wild-type and 35S∷PID plants did not differ significantly. Because a PID-dependent binary switch controls auxin efflux carrier PIN polarity and mediates changes in auxin flow (Friml et al., 2004), it has been suggested that the primary and lateral roots use different auxin transport mechanisms and sample different auxin streams (Rashotte et al., 2000, 2001). Not only are root growth and behavior dependent on control of cell division, but also the establishment and maintenance of its complex anatomy lies at the heart of cell division control (Vernoux and Benfey, 2005). The meristem is comprised of a niche of stem cells containing at its core a group of quiescent center cells that are mitotically dormant but are essential for stem cell maintenance. Thus, the root stem cell population involves complex control of mitosis. The partial spatial overlap in expression of two sets of transcription factors establishes the zones of cell division activity (Vernoux and Benfey, 2005). The cell cycle arrest of the quiescent center cells is mediated by the plant homolog of the retinoblastoma protein, operating analogously to the G1→S block that the retinoblastoma protein serves in animal cells (Wildwater et al., 2005).
Analysis of Arabidopsis and rice G-protein mutants and transgenic lines revealed two crucial concepts of G-protein action in plants (Perfus-Barbeoch et al., 2004). First, Gα and Gβγ each predominantly mediate certain physiological responses. Second, G-protein subunits act in a cell type-specific and developmentally regulated manner. Although the existence of a heterotrimeric form of the G-protein complex in plants has been proven by both molecular modeling and biochemical assays (Mason and Botella, 2000, 2001; Ullah et al., 2003; Kato et al., 2004), a functional requirement for the heterotrimer in any developmental process has not been addressed. This is a critical point because no GPCR has been unequivocally identified in plants, leaving the possibility that the heterotrimeric state may not represent the basal state.
Here we use the Arabidopsis root to dissect the role of the heterotrimeric G-protein complex and the released subunits in root development. Previously, we proposed that AGB1, presumably acting as a dimer with the Arabidopsis G-protein γ-subunit AGG1 or AGG2, is a negative regulator for lateral root formation (Ullah et al., 2003). Our previous work was based on null alleles of GPA1 and AGB1 and transgenic plants overexpressing GPA1 or AGB1 in wild-type backgrounds. However, because Gα sequesters Gβγ, the phenotypes reported from loss of function of Gα could also be due to the release of free Gβγ. Similarly, the phenotypes observed in Gβ loss-of-function mutations could also be due primarily to the loss of action of Gα, if, for example, as in animals, Gβ is required for recruitment to a GPCR. Similarly, the heterotrimer itself may have a signaling role, although unusual. Therefore, to distinguish between these different modes of action, we generated double mutants using loss-of-function alleles of gpa1 and agb1. Furthermore, we overexpressed Gα (GPA1) and Gβ (AGB1) in loss-of-function Gβ (agb1) and Gα (gpa1) mutant backgrounds, respectively, to dissect the modulatory role of individual subunits in root cell division. Overexpression of Gα in the absence of Gβ would allow a direct test of the role of Gα because Gβ is no longer available for recruitment by Gα to form the heterotrimer, whereas overexpression of Gβ in the absence of Gα would allow a direct test of the role of the Gβ because the sequestration of Gβ by Gα is eliminated. A comparison of the phenotypes in these different lines having known proportions of the G-protein subunits would enable us to determine the respective prominence in signaling, which subunits collaborate in signaling, a possible role for the heterotrimer, and aspects of cell proliferation independently modulated by a specific subunit. Such an approach was first successfully utilized in determining the specific roles of the G-protein-complex subunits in controlling asymmetrical division in Drosophila neuroblasts (Schaefer et al., 2001).
Here we provide genetic evidence that both Gα- and Gβ-subunits, as well as the heterotrimer itself, distinctly modulate the rate of cell proliferation differently and with different efficacy. These results support our earlier findings that cell proliferation in plants is regulated by heterotrimeric G-protein subunits (Ullah et al., 2001, 2003; Chen et al., 2003), and further extend those findings by demonstrating the differential regulatory roles of individual G-protein subunits in root cell proliferation. To our knowledge, this is the first example of a division of labor among the G-protein subunits and heterotrimer in control of proliferation for a eukaryotic cell in a multicellular organism.
RESULTS
GPA1 and AGB1 Are Expressed in Roots
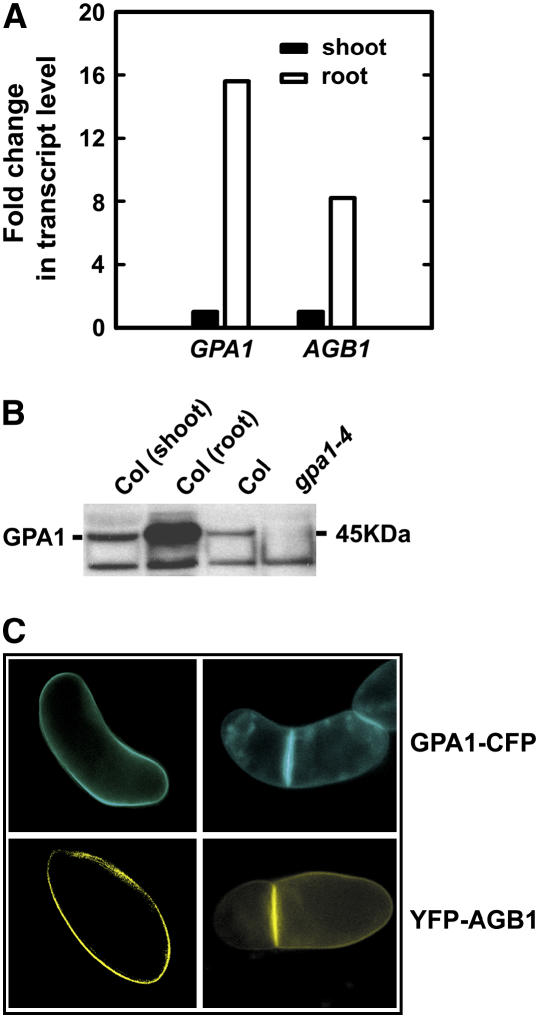
The results of quantitative real-time PCR and immunoblot analyses indicated that both GPA1 and AGB1 are more strongly expressed in roots than in shoots in young seedlings (Fig. 1, A and B). Using Arabidopsis suspension cells expressing GPA1-cyan fluorescent protein (CFP) or yellow fluorescent protein (YFP)-AGB1, we found that the fusion proteins are preferentially distributed at the cell plate in newly divided cells (Fig. 1C), suggesting a role in cytokinesis. Recently, studies in both invertebrates and vertebrates have revealed an essential function of the heterotrimeric G proteins in positioning of the mitotic spindle and attaching microtubules to the cell cortex, which is distinct from their well-studied role in signal transduction downstream of seven-transmembrane (7TM) receptors (Afshar et al., 2004; Couwenbergs et al., 2004; Du and Macara, 2004; Hampoelz and Knoblich, 2004; Hess et al., 2004; Martin-McCaffrey et al., 2004). To address the molecular mechanism by which the heterotrimeric G-protein complex regulates plant cell proliferation, we chose to examine cell division in roots, an organ where this growth parameter can be dissected both spatially and temporally within a multicellular context (i.e. not in yeast or in a cell suspension culture).
Figure 1.
GPA1 and AGB1 expression. A, Both GPA1 and AGB1 transcripts are expressed at higher levels in roots than in shoots. Transcripts were analyzed in 7-d-old, light-grown seedlings. B, GPA1 protein level (45 kD) is higher in the root than that in the shoot. GPA1 protein level was analyzed in 7-d-old, light-grown seedlings. The lower band is a nonspecific band recognized by the anti-GPA1 peptide antibody, which often shows up in gpa1 mutant background. C, GPA1 and AGB1 cellular localization. Both GPA1-CFP and YFP-AGB1 localize at the plasma membrane. Both GPA1-CFP and YFP-AGB1 accumulate at the nascent cell plates in dividing Arabidopsis cells. Cells were taken from a population of suspension cells transformed with 35S:GPA1-CFP or 35S:YFP-AGB1 binary vector. No CFP or YFP fluorescence was detected in untransformed cells. 35S:GPA1-CFP and 35S:YFP-AGB1 constructs rescued gpa1-4 and agb1-2 mutants, respectively (data not shown), indicating that the fusion proteins are functional.
G-Protein Mutants Have Defects in Both Primary and Lateral Root Development
The morphological differences observed between wild-type and gpa1 and agb1 plants were ascribed to differences in cell proliferation rate and not histogenesis (Ullah et al., 2001, 2003). We show that root growth rate in agb1-2 mutants was greater than Columbia (Col)-0 wild type, whereas gpa1-3 and gpa1-4 mutants have wild-type root growth rate (Supplemental Fig. 1). Root growth rate combines the rate at which the RAM produces cell derivatives that are recruited into the distal root and the rate at which these derivatives subsequently elongate. However, it is possible to indirectly calculate both parameters (described in “Materials and Methods”). agb1-2 and gpa1 (gpa1-3 and gpa1-4) mutants had more and fewer lateral roots, respectively (Supplemental Fig. 1). The formation of a lateral root requires an initiation of division of one pericycle cell, arguably at the G2/M transition (Doerner et al., 1996; Beeckman et al., 2001; Himanen et al., 2004). Therefore, the number of lateral roots indicates the number of cell cycle entry events (Malamy and Benfey, 1997). We determined both the rate of cell production in the primary RAM and the number of lateral roots to pinpoint the role of G-protein subunits in cell division in two different root cell types and compared and contrasted them.
For clarity, data on only one allele of the gpa1 (gpa1-4) and agb1 (agb1-2) single mutants and one allele of the gpa1 agb1 double mutant (gpa1-4 agb1-2) are presented. These same single mutant alleles were used to generate the double and triple mutant combinations with gcr1 (Chen et al., 2004) or with Atrgs1 (Fig. 4). Data on the second allele of the gpa1 single mutant, gpa1-3, and the second allele of the gpa1 agb1 double mutant, gpa1-3 agb1-2, are identical to those of gpa1-4 and gpa1-4 agb1-2, respectively (Supplemental Figs. 1 and 2). The agb1-1 allele (Lease et al., 2001) is a point mutation mutant that behaved differently from agb1-2 mutant in the primary root growth assay (i.e. segregating phenotype), though it behaved similarly to agb1-2 in the lateral root formation assay (Supplemental Fig. 2), implying that it is not a null allele or that additional mutations are present. Consistent with this, we detected a larger AGB1 transcript in agb1-1 mutants (data not shown). Because agb1-2 was shown to be transcript null (Ullah et al., 2003) and because a 35S:AGB1 construct completely rescued the agb1-2 mutant phenotype (Tables I and II), only the agb1-2 mutant was used for this study.
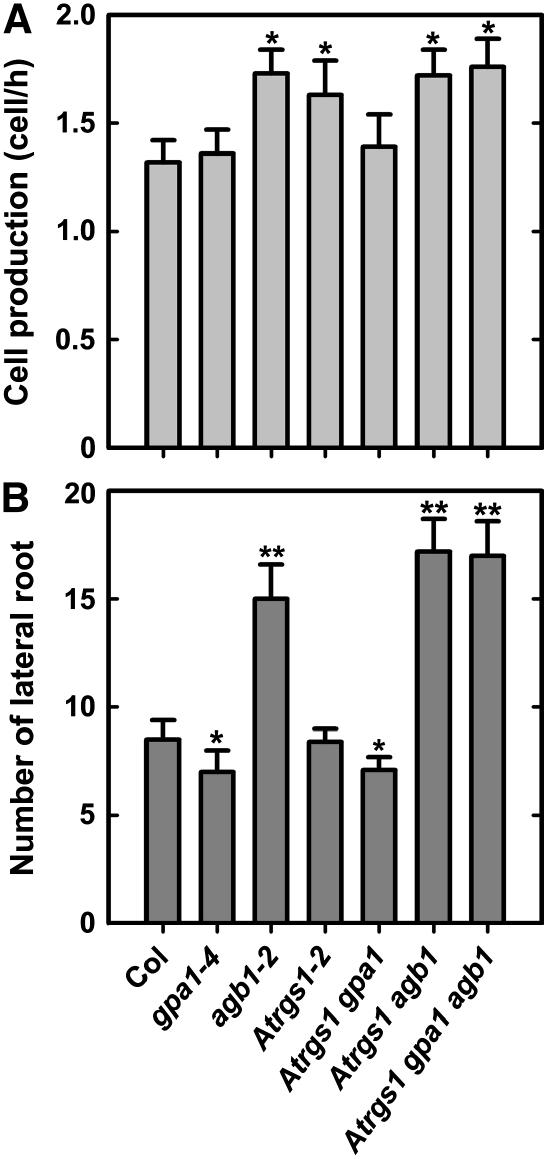
Figure 4.
Analyses of cell production in the primary root and lateral root formation of double and triple mutants among Atrgs1, gpa1, and agb1 mutants. A, Rate of cell production in the primary root. Primary root elongation rate and cortical root cell length were collected from at least 10 seedlings. At least 20 cortical cells at the mature root region were measured in each seedling. The rate of cell production was calculated as the rate of primary root elongation divided by the cortical cell length. Shown are means ± sd of at least 10 seedlings. B, The number of lateral roots. The numbers of lateral roots were counted on 9-d-old roots. Shown are the average numbers of lateral roots from at least 10 seedlings ± sd. Pairwise Student's t test was used to compare values to the wild type (Col). *, Significant (P = 0.05); **, highly significant (P = 0.01).
Table I.
Rates of cell production in the primary root of gpa1 and agb1 mutants and transgenic lines overexpressing GPA1 or AGB1
Root elongation rate and cortical root cell length were collected from at least 10 seedlings. se is indicated. Pairwise Student's t test was used to compare values to the control (1). *, Significant (P = 0.05); **, highly significant (P = 0.01).
| Genotype | Root Elongation Rate | Cortex Cell Length | Cell Production Rate |
|---|---|---|---|
| μm/h | μm/cell | cell/h | |
| No transgene | |||
| (1) Col | 176.67 ± 12.11 | 175.43 ± 18.34 | 1.01 ± 0.05 |
| (2) gpa1-4 | 180.00 ± 14.15 | 171.66 ± 20.22 | 1.05 ± 0.06 |
| (3) agb1-2 | 290.83 ± 16.68 | 176.53 ± 19.48 | 1.65 ± 0.10** |
| (4) gpa1-4 agb1-2 | 309.67 ± 21.52 | 180.50 ± 21.13 | 1.72 ± 0.12** |
| Overexpression of GPA1: | |||
| (5) in Col | 128.62 ± 10.20 | 151.24 ± 18.13 | 0.85 ± 0.04* |
| (6) in gpa1-4 | 180.02 ± 13.36 | 166.13 ± 21.12 | 1.08 ± 0.08 |
| (7) in agb1-2 | 300.00 ± 17.62 | 181.66 ± 20.22 | 1.65 ± 0.11** |
| (8) in gpa1-4 agb1-2 | 303.86 ± 22.64 | 177.46 ± 20.54 | 1.71 ± 0.10** |
| Overexpression of AGB1: | |||
| (9) in Col | 132.50 ± 9.48 | 166.59 ± 19.72 | 0.80 ± 0.05** |
| (10) in gpa1-4 | 186.67 ± 16.62 | 169.46 ± 19.22 | 1.10 ± 0.09 |
| (11) in agb1-2 | 162.50 ± 15.48 | 175.87 ± 21.04 | 0.92 ± 0.08 |
| (12) in gpa1-4 agb1-2 | 187.50 ± 16.58 | 177.03 ± 19.68 | 1.06 ± 0.08 |
Table II.
Lateral root numbers of gpa1 and agb1 mutants and transgenic lines overexpressing GPA1 or AGB1
Lateral roots were counted from 9-d-old, light-grown seedlings. Shown are the average numbers of lateral roots from at least 10 seedlings with associated se. Pairwise Student's t test was used to compare values to the control (1). *, Significant (P = 0.05); **, highly significant (P = 0.01).
| Genotype | Lateral Root No. | t Value |
|---|---|---|
| No transgene | ||
| (1) Col | 5.2 ± 0.58 | – |
| (2) gpa1-4 | 3.4 ± 0.42 | 2.51* |
| (3) agb1-2 | 14.6 ± 1.30 | 6.61** |
| (4) gpa1-4 agb1-2 | 14.2 ± 1.26 | 6.49** |
| Overexpression of GPA1: | ||
| (5) in Col | 7.7 ± 0.80 | 2.26* |
| (6) in gpa1-4 | 5.8 ± 0.60 | 0.72 |
| (7) in agb1-2 | 15.1 ± 1.26 | 7.14** |
| (8) in gpa1-4 agb1-2 | 14.7 ± 1.32 | 6.59** |
| Overexpression of AGB1: | ||
| (9) in Col | 3.3 ± 0.52 | 2.44* |
| (10) in gpa1-4 | 3.0 ± 0.44 | 3.02** |
| (11) in agb1-2 | 4.0 ± 0.45 | 1.63 |
| (12) in gpa1-4 agb1-2 | 3.5 ± 0.48 | 2.26* |
agb1-2 Is Epistatic to gpa1-4
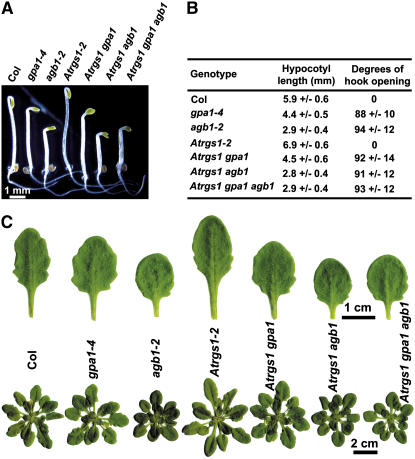
Combination of the two loss-of-function alleles, agb1-2 and gpa1-4, conferred longer primary roots and more lateral root phenotypes similar to the agb1-2 mutant allele acting alone (Supplemental Fig. 1). This genetic relationship was also the case for auxin-induced adventitious root formation in hypocotyls (Supplemental Fig. 2). While the focus of this study is on cell division in the root, for completeness sake we examined non-root phenotypes of G-protein mutants and found that, for all scorable traits, the agb1-2 mutant allele was epistatic to the gpa1-4 allele (Fig. 2).
Figure 2.
Phenotype of gpa1 agb1 double mutant. A, gpa1 mutants are protein null. B, RT-PCR of gpa1 agb1 double mutants. ACTIN2 primers were added together with GPA1 or AGB1 primers in each PCR reaction. C, Phenotype of 2-d-old, dark-grown seedlings. D, Phenotype of 10-d-old, light-grown seedlings. Arrows indicate that both agb1 and gpa1 agb1 double mutant have large and round cotyledons. E, Lengths of hypocotyls and degrees of hook opening. The lengths of hypocotyls were measured from 2-d-old, dark-grown and 10-d-old, light-grown seedlings, respectively. The degrees of hook opening were measured from 2-d-old, dark-grown seedlings. Shown are the average lengths of hypocotyls from at least 20 seedlings ± sd. F, Phenotype of 43-d-old plants. The plants were grown at 8 h (light)/16 h (dark) short-day conditions. Shown below are the tenth rosette leaves. G, Phenotype of rosette leaves of mature plants. The whole rosette leaves were taken from plants upon flowering. The numbers of rosette leaves are indicated, and the flower buds are asterisked. H, The phenotype of flower. I, The phenotype of the stigma of silique.
AGB1 Modulation of Cell Proliferation in the Primary Root May Require a Functional GPA1
If G-protein-coupled signaling in Arabidopsis follows the mechanism of action established in animal systems, a comparison of the phenotypes of these single and double mutants permits prediction of which subunit, namely, the activated Gα, the Gβγ-dimer, and/or the heterotrimeric complex, facilitates the primary signal transduction leading to root growth and lateral root formation. For example, because activation of the Gα-subunit leads to release of the Gβγ-dimer, opposite phenotypes, such as lateral root formation for gpa1 and agb1 null mutations, are generally interpreted to mean that the Gβγ-subunit is the predominant form regulating pericycle cell division. Because the Gβγ-dimer is required for proper coupling of Gα to its receptor in animal cells, phenotypes that are similar in gpa1 and agb1 mutants are generally interpreted to mean that either the Gα-subunit is the predominant active form or that the heterotrimeric complex is signaling. Because GPA1 and AGB1 form a molecular complex, mechanistic interpretations of epistasis relationships are precarious. Therefore, to address these issues, we overexpressed GPA1 or AGB1 in agb1 or gpa1 mutant backgrounds, respectively. This enabled us to dissect the individual roles of α and β/γ G-protein subunits as well as the heterotrimer in root cell proliferation. For example, we reasoned that a phenotype from overexpression of AGB1 in the absence of GPA1 precludes the interpretation of GPA1 sequestration. Similarly, overexpression of GPA1 in the absence of AGB1 enables a direct test of an individual GPA1 role in root cell proliferation because AGB1 is no longer available for recruitment by GPA1 to form the heterotrimer.
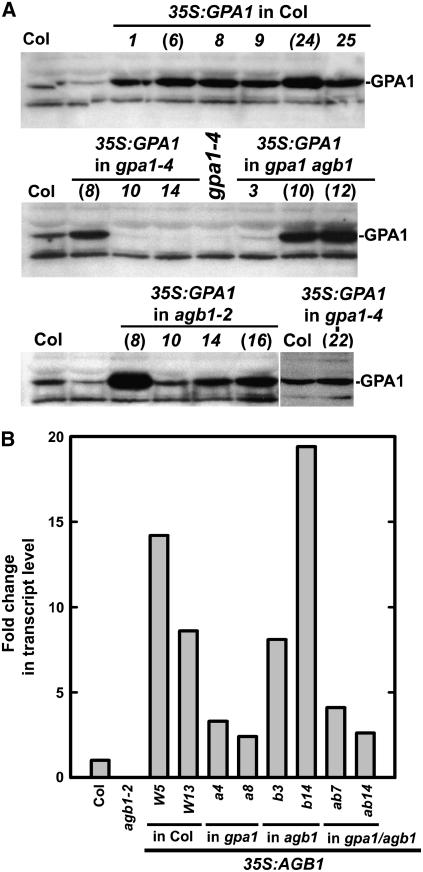
The expression levels of GPA1 protein in 35S∷GPA1 transgenic lines were examined by immunoblot analysis using antibodies directed against the C terminus of GPA1. Because an exhausted attempt to make specific antibodies to the Arabidopsis Gβ-subunit failed, AGB1 transcript levels were measured by quantitative real-time PCR as an indirect assessment of AGB1 levels in overexpressing lines (Fig. 3). Based on the GPA1 protein levels in transgenic lines overexpressing GPA1 and the AGB1 transcript levels in transgenic lines overexpressing AGB1, two independent transgenic lines were chosen from each transformation for subsequent analyses of root cell division. These independent transgenic lines are designated by parentheses in Figure 3A or labeled with line numbers in Figure 3B. Subsequently, the data collected from these two independent transgenic lines were pooled and presented in Tables I and II, with corresponding statistical tests.
Figure 3.
Overexpression of GPA1 and AGB1 in gpa1 and agb1 single and double mutant backgrounds. A, Immunoblot analyses of GPA1 protein levels in 35S:GPA1 transgenic lines. Those independent transgenic lines used in subsequent studies are designated by parentheses. B, Quantitative real-time PCR analysis of AGB1 transcript levels in 35S:AGB1 transgenic lines. The transcript levels of AGB1 in independent transgenic lines harboring 35S:AGB1 in wild-type Col-0 (lines W5 and W13), gpa1-4 (lines a4 and a8), agb1-2 (lines b3 and b14), and gpa1-4 agb1-2 (lines ab7 and ab14) backgrounds were examined.
The increased root growth in both agb1-2 single and gpa1-4 agb1-2 double mutants was due to an increased cell production in the RAM (Table I, 1–4). Moreover, overexpression of AGB1 confers decreased cell production in the RAM (Table I, compare 1 and 9), demonstrating that the cell production rate in wild-type RAMs is not at basal level. The capacity for a lower cell production rate than for the control is a critical point as it enabled us to assign meaning to a “no change in rate” phenotype observed in the other genotypes overexpressing individual subunits. Combining both loss- and gain-of-function results in the wild-type background indicated that AGB1 is an attenuator of cell division in the primary root.
We tested if the attenuation of root cell division by AGB1 requires a functional GPA1. We examined overexpression of AGB1 in the absence of GPA1 and determined if cell division in the RAM is altered. Overexpression of AGB1 in the agb1 mutant complemented the primary root phenotype (Table I), indicating that the transgene is functional. When AGB1 was overexpressed in gpa1 or gpa1 agb1 mutant backgrounds, no effect on primary root growth was observed (Table I, compare 9, 10, 11 and 12), indicating that AGB1 action requires a functional GPA1. This suggests that either Gα acts downstream of AGB1 or that the intact heterotrimeric complex itself acts to modulate cell division in the RAM.
To distinguish between these two possibilities, the reciprocal experiment was performed. We examined overexpression of GPA1 in the absence of AGB1 and determined if cell division in the RAM is altered. We found that ectopic expression of GPA1 in a background containing AGB1 reduced cell proliferation (Table I, compare 1–5). However, this decrease in cell proliferation by additional GPA1 required a functional AGB1 (Table I, compare 5–8). These results are consistent with the conclusion that the heterotrimeric state of the G-protein complex is required to negatively modulate cell proliferation in the RAM. A signaling role for the intact heterotrimeric complex is rare but not unprecedented (Peleg et al., 2002), although this is the first report, to our knowledge, for a role by the heterotrimer in cell division.
AtRGS1 Works Together with the Heterotrimeric G Proteins to Modulate Cell Division in the Primary Root
We previously identified a 7TM protein, RGS1, as the sole regulator of G-signaling protein in Arabidopsis (Chen et al., 2003). RGS1 contains an RGS domain at its C terminus, preferentially binds to the activated form (GTP bound) of GPA1, and negatively regulates G-protein signaling by accelerating the GTPase activity of Gα-subunits (GAP activity). Loss of function of rgs1 resulted in increased cell production in the primary root, whereas it had no significant effect on lateral root formation (Chen et al., 2003). With a single RGS gene in Arabidopsis, it was possible to increase the pool of the activated (GTP-bound) form of GPA1 by disruption of RGS1 (rgs1-2; Chen et al., 2003). As shown in Figure 4A, an increase in activated Gα conferred a statistically significant increase in cell production in the primary root in the presence of a functional Gβ-subunit.
We further generated double and triple mutants among gpa1-4, agb1-2, and rgs1-2 loss-of-function mutants to test the genetic interaction between RGS1 and the heterotrimeric G-protein-complex genes in the regulation of root cell division by measuring the cell production rate in the primary root and lateral root formation in these double and triple mutants. We found that the stimulatory effect in the rgs1-2 mutant was abrogated in the absence of GPA1 (Fig. 4A), suggesting that RGS1 acts through GPA1 to regulate cell division in the primary root. Both rgs1 and agb1 mutants had increased cell production in the primary root, but no additive or synergistic effects were observed in rgs1 agb1 and rgs1 gpa1 agb1 double and triple mutants (Fig. 4A), indicating that RGS1 acts in the same pathway with the heterotrimeric G-protein-complex genes. Again, while the focus here is on root cell proliferation, for completeness sake we extended our investigation to aerial phenotypes. For all other scorable traits, the gpa1-4 and agb1-2 mutants were epistatic to the rgs1-2 allele (Fig. 5).
Figure 5.
Phenotypic analyses of double and triple mutants among Atrgs1, gpa1, and agb1 mutants. A, Phenotypes of 2-d-old, dark-grown seedlings. B, Lengths of hypocotyls and degrees of hook opening of 2-d-old, dark-grown seedlings. Shown are means ± sd of at least 20 seedlings. C, Phenotype of 43-d-old plants. The plants were grown at 8 h (light)/16 h (dark) short-day conditions. Shown on top are the tenth rosette leaves. In the dark, Atrgs1 mutants had longer hypocotyl and closed hook, whereas gpa1 and agb1 mutants had shorter hypocotyl and partially opened hook. Atrgs1 gpa1 double mutant phenocopied the gpa1 single mutant, and Atrgs1 agb1 and Atrgs1 gpa1 agb1 double and triple mutants phenocopied the agb1 single mutant. Of light-grown plants, Atrgs1 gpa1 and Atrgs1 agb1 double mutants phenocopied the gpa1 and agb1 single mutants, respectively, in terms of shape of rosette leaves and size of the rosette. Atrgs1 gpa1 agb1 triple mutant phenocopied the agb1 single mutant.
AGB1 Inhibits Lateral Root Formation
Previously, we proposed a model in which AGB1 acts downstream of GPA1 and negatively regulates lateral root formation (Ullah et al., 2003). Here we analyzed gpa1 agb1 double mutant and transgenic lines overexpressing GPA1 or AGB1 in agb1 or gpa1 mutant backgrounds, respectively, to test this model. The opposite phenotypes of gpa1 and agb1 single mutants in lateral root formation permitted a robust epistasis analysis. The gpa1 agb1 double mutant phenocopied the agb1 single mutant lateral root phenotype (Table II, compare 1–4), indicating that the agb1-2 allele is epistatic to gpa1-4.
gpa1 mutants produced fewer lateral roots than the wild type. Overexpression of GPA1 in the gpa1-4 mutant restored the number of lateral roots in the mutant to the wild-type level (Table II, 6), indicating that the GPA1 transgene was functional. We observed an increased number of lateral roots when GPA1 was overexpressed in the wild-type background (Table II, compare 1 and 5). However, overexpression of GPA1 in the agb1 or gpa1 agb1 mutant backgrounds did not further increase the number of lateral roots of these mutants (Table II, 7 and 8), indicating that GPA1 acts through AGB1. The most likely explanation of this observation is that overexpression of GPA1 sequesters AGB1 into the heterotrimeric complex.
Overexpression of AGB1 complemented the agb1 mutant phenotype of excessive lateral root formation (Table II, 11), indicating that the AGB1 transgene was functional. When AGB1 was overexpressed, a decrease in lateral root formation was observed compared to the no-transgene controls, regardless of the presence or absence of GPA1 (Table II, 10 and 12). Because loss of function of AGB1 promotes lateral root formation while overexpression of AGB1 inhibits it, we conclude that AGB1 is a negative modulator of lateral root formation. These results also support the notion that AGB1 acts downstream of GPA1 and that AGB1 can function independently of GPA1 in regulating lateral root formation.
Moreover, an increase in the activated form of GPA1 through a loss-of-function allele of rgs1 did not affect the lateral root formation, either in the wild-type background or in the gpa1 and agb1 single or double mutant backgrounds (Fig. 4B), indicating that the interaction of RGS1 and the heterotrimeric G-protein complex may not be required for the regulation of lateral root formation.
Taken together, the data of gpa1 and agb1 single and double mutants and of transgenic lines overexpressing GPA1 and AGB1 in different mutant backgrounds support a testable model in which AGB1 acts downstream of GPA1 to inhibit lateral root formation.
DISCUSSION
Based on cell proliferation and lateral root formation in roots of gpa1, agb1, and rgs1 single, double, and triple mutants and of transgenic lines overexpressing GPA1 or AGB1 in agb1 or gpa1 mutant backgrounds, we propose the following working model for the heterotrimeric G-protein complex in root cell division (Fig. 6). First, the heterotrimeric complex itself attenuates cell division in the primary roots. In lieu of structural data for the heterotrimeric complex, the term heterotrimer is used in a genetic sense here, but the most likely interpretation is indeed that the inactive state of Gα within its heterotrimeric physical state is the attenuating structural form. The question of whether or not complete dissociation of an activated Gα from the heterotrimeric complex occurs has recently been raised (Frank et al., 2005) but does not constrain our present interpretation. Second, the GTP-bound form of GPA1 accelerates cell division in the RAM. Third, the Gβγ-dimer inhibits cell division in the pericycle founder cells. However, the exact position at which each signaling element influences the cell cycle is not known at present (discussed below).
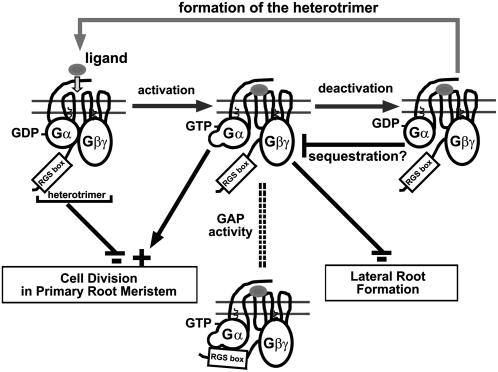
Figure 6.
Working model for the heterotrimeric G-protein modes of action in root cell division. Shown here is the classical heterotrimeric G-protein activation-deactivation cycle. Ligand binding (gray ovals) to its cognate 7TM cell surface receptor activates receptor-mediated GDP/GTP exchange on the α-subunit (Gα), causing dissociation of Gα from the βγ-dimer (Gβγ). Both activated Gα-subunit and Gβγ-dimer bind to downstream target proteins, which results in the relevant cellular responses. Intrinsic GTPase activity of Gα hydrolyzes GTP to GDP, thereby allowing Gα to reassociate with the Gβγ-dimer. RGS proteins accelerate the intrinsic GTPase activity of the Gα-subunit, thus returning the heterotrimer to its basal GDP-bound state. Gα, GPA1; Gβ, AGB1; Gγ, AGG1/AGG2. RGS1 is a 7TM protein with a functional RGS box shown to accelerate the intrinsic GTPase activity of GPA1. Shown here are the resting (left and right) and activated states (middle) states of the G-protein complex with RGS1. Arrows depict acceleration and bars indicate attenuation of cell division. It is proposed here that RGS1 controls the G-protein state through its GTPase-accelerating function (GAP activity). The effect of RGS1 on the heterotrimer is depicted by a dashed double line. GPA1 is a positive modulator of cell production in RAM, whereas the heterotrimer is a negative modulator. The heterotrimer may not have a modulatory role in lateral root formation. AGB1 is the primary subunit that regulates lateral root formation. GPA1 inhibits AGB1 action presumably by the sequestration of AGB1 to reform the heterotrimer.
The model shown in Figure 6 is unique in that both the active and inactive states of GPA1 have opposite modulatory functions. Another aspect of this model is the potential role for RGS1 in regulating the GPA1 state. As described above, RGS1 is unusual in that it contains both a 7TM domain and a functional RGS domain. RGS1 could serve on its own as the membrane scaffold, the guanine nucleotide-exchange factor, and/or the GAP protein. This membrane signaling platform could serve to integrate signals that modulate cell proliferation. One signal known to influence cell proliferation is d-Glc. We (J.P. Taylor and A.M. Jones, unpublished data) have shown that applied d-Glc causes the wild-type cells at the root tip to stop dividing and expand dramatically, whereas cells lacking the Gα-subunit are less responsive to d-Glc. RGS1 may be a sugar-regulated GAP on GPA1, consistent with the proposed model. This model is consistent with our published data that overexpression of a constitutively active form of GPA1, GPA1Q222L, and loss of rgs1 function have no effect on lateral root formation (Chen et al., 2003; Ullah et al., 2003; Fig. 4).
Mutations that alter root development can be divided into three classes: mutations that affect (1) both primary and lateral roots; (2) the primary root but not the lateral root; and (3) the lateral root but not the primary root. For example, the alf4-1 allele prevents initiation of lateral roots but does not affect the primary root (Celenza et al., 1995). ALF4 encodes a plant-specific, nuclear-localized protein (DiDonato et al., 2004). Although auxin has been shown to be a critical stimulus for the initiation of the developmental program for lateral root formation, it was found that ALF4 functions independently of auxin signaling. Instead, ALF4 maintains the pericycle in the mitotically competent state needed for lateral root formation. We show here that mutations in G-protein-signaling components spread all three root mutant classes. For example, agb1-2 mutants have a longer primary root and more lateral roots (class 1), rgs1 mutants have a longer primary root but the lateral roots are wild type (class 2), and gpa1 mutants have fewer lateral roots but the primary root is near wild type (class 3). Null mutants of the putative GPCR in Arabidopsis, gcr1 (Chen et al., 2004; Pandey and Assmann, 2004), did not show any defects in primary and lateral root development under normal growth and development conditions (Pandey et al., 2006). The downstream effectors for GPA1 or AGB1 in regulating primary and lateral root development remain unknown. It is not clear if mutations in GPA1-interacting proteins, AtPirin1 (Lapik and Kaufman, 2003) and PLDα1 (Zhao and Wang, 2004), have any defects in root development. It would also be informative to test if loss of function of AGG1 or AGG2 (not yet available) affects root development.
Cell elongation also is required for root development. Root growth and architecture involves a balance between cell production in the apical and lateral root meristems and the subsequent elongation of those cells. This raises an interesting complexity if these two processes involve cross-regulation through G proteins. We do not rule out possible roles of GPA1 and AGB1 in cell elongation in both primary and lateral root development. For example, the length of cortex cells was reduced in roots of transgenic lines overexpressing GPA1 (Table I), implying that GPA1 may inhibit root cell elongation. It is unclear if this altered cell elongation is due to an indirect effect of altered cell division because cell division and cell elongation are often found to compensate for each other (Jones et al., 1998).
The precise nuclear stage in the cell cycle for the modulatory target in the RAM and pericycle by the heterotrimeric G-protein complex and Gα is unclear. However, overexpressing GPA1 in synchronized tobacco (Nicotiana tabacum) BY-2 suspension cells shortened the G1 phase of the cell cycle, suggesting its cell-cycle-accelerating function targets this stage (Ullah et al., 2001). Recent studies support the G2 re-entry hypothesis for lateral root initiation, at least for founder cells quite distal to the RAM (for review, see Beeckman et al., 2001; Casimiro et al., 2003; Malamy, 2005). Because AGB1 acts downstream of GPA1 to negatively regulate lateral root formation, AGB1 may specifically inhibit G2 re-entry of pericycle founder cells.
In conclusion, this work, to our knowledge, represents the first in planta study of the role of a heterotrimeric G protein in modulation of cell proliferation. It does so within the context of root growth and architecture. The multicellular root provides the cellular heterogeneity to analyze integrative signaling, but this work should be combined with future studies using synchronizable cells in culture with altered G-protein elements so that the precise phases of the cell cycle that are modulated differentially by G-protein subunit may be determined.
MATERIALS AND METHODS
gpa1 and agb1 Single and Double Mutants
All mutants Arabidopsis (Arabidopsis thaliana) are in the Col background (Col-0). T-DNA insertion mutant alleles of GPA1, gpa1-3 and gpa1-4, were used as described by Jones et al. (2003). The T-DNA insertion mutant allele of AGB1, agb1-2, has been described by Ullah et al. (2003). gpa1 agb1 double mutants were generated by crossing gpa1-3 or gpa1-4 to agb1-2, and plants homozygous for both gpa1-3 or gpa1-4 and agb1-2 loci were identified from the F2 progeny by PCR genotyping using gene-specific primers flanking the T-DNA insertion sites and a T-DNA left-border primer (5′-GGCAATCAGCTGTTGCCCGTCTCACTGGTG-3′). The gpa1 agb1 double mutants were confirmed by reverse transcription (RT)-PCR analysis. We made all mutant genotypes publicly available through the Arabidopsis Biological Resource Center stock center.
gpa1, agb1, and rgs1 Double and Triple Mutants
The null allele of RGS1, rgs1-2, is described by Chen et al. (2003). Double or triple mutants among rgs1-2, gpa1-4, and agb1-2 were isolated from the F2 progeny from a cross between rgs1-2 mutant and gpa1-4 agb1-2 double mutant by PCR genotyping using gene-specific primers flanking the T-DNA insertion sites and a T-DNA left-border primer (5′-GGCAATCAGCTGTTGCCCGTCTCACTGGTG-3′). For clarity, double or triple mutants rgs1 gpa1, rgs1 agb1, and rgs1 gpa1 agb1 refer to rgs1-2 gpa1-4, rgs1-2 agb1-2, and rgs1-2 gpa1-4 agb1-2, respectively.
Quantitative Real-Time PCR
GPA1 and AGB1 transcript levels in the shoots and roots of 7-d-old, light-grown wild-type seedlings or in the whole seedlings of 35S:AGB1 transgenic lines were determined by quantitative real-time PCR. GPA1 transcripts were amplified using primers GPA1 RT-FW (5′-AGAAGTTTGAGGAGTTATATTACCAG-3′) and GPA1 RT-RV (5′-AAGGCCAGCCTCCAGTAA-3′). AGB1 transcripts were amplified using primers AGB1 RT-FW (5′-CTGCTGATGTACTAAGCGTCTCA-3′) and AGB1 RT-RV (5′-CTGCATGTTCCATCGTCTGA-3′). The GPA1 and AGB1 transcript levels were normalized against ACTIN2 transcripts, which were amplified using primers Actin2 RT-FW (5′-CCAGAAGGATGCATATGTTGGTGA-3′) and Actin2 RT-RV (5′-GAGGAGCCTCGGTAAGAAGA-3′). The real-time PCR was performed using the MJ MiniOpticon real-time PCR system (Bio-Rad Laboratories) and IQ SYBR Green Supermix (Bio-Rad Laboratories).
Generation of Transgenic Lines
The entire open-reading frames of GPA1 (At2g26300) and AGB1 (At4g34460) were amplified by PCR from a cDNA library made from seedlings grown in light for 10 d, cloned into the pENTR/D-TOPO vector (Invitrogen), and then subcloned into Gateway plant transformation destination binary vector pB2GW7 (Karimi et al., 2002) by LR recombination reactions. The construction of GPA1-CFP fusion has been described previously (Chen et al., 2003). The coding region of the enhanced CFP (CLONTECH) was inserted in the first loop (between amino acids 97 and 98) of GPA1 and moved into the plant destination binary vector pGWB2 (Research Institute of Molecular Genetics). For the construction of the AGB1-YFP fusion, AGB1 was moved into the Gateway plant destination binary vector pGWB42 (Research Institute of Molecular Genetics). In these constructs, expression of GPA1, AGB1, GPA1-CFP, and YFP-AGB1 was driven by the 35S promoter of the Cauliflower mosaic virus. All constructs were transformed into Arabidopsis plants (Col-0) or Arabidopsis suspension cells by Agrobacterium-mediated transformation (Bechtold and Pelletier, 1998; Ferrando et al., 2000). Both 35S:GPA1 and 35S:AGB1 constructs were also transformed into gpa1-4, agb1-2, and gpa1-4 agb1-2 mutant backgrounds.
Relative expression of GPA1 was quantitated by immunoblot analysis. Briefly, approximately 20 10-d-old, light-grown seedlings were ground into powder under liquid nitrogen. Total protein was isolated by incubating the tissues with 100 μL of freshly made lysis buffer (50 mm Tris, 50 mm NaCl, 5 mm EGTA, 2 mm dithiothreitol, 1% Triton X-100, and 1× protease inhibitor cocktail [Sigma], pH 7.5) at 4°C for 30 min, followed by rocking at 4°C for another 30 min. Total proteins in the supernatant were collected by centrifuging at 14,000 rpm for 15 min at 4°C. Protein samples (30 μg per well) were separated by SDS-PAGE, electroblotted onto polyvinylidene difluoride membrane, and immunoblotted with 1:2,000 anti-GPA1 peptide antibodies (serum no. 9572, rabbit polyclonal antiserum directed against a peptide representing the last 15 amino acids of GPA1). AGB1 transcript levels in 35S:AGB1 transgenic lines were determined by quantitative real-time PCR described above.
Plant Growth Conditions
For petri-dish-based phenotypic analyses, wild-type and mutant seeds were sterilized, sown in petri dishes containing one-half-strength Murashige and Skoog basal medium with Gamborg's vitamins (ICN Biomedicals), 1% Suc, 0.5% phytoagar (Research Products International), adjusted to pH 5.7, and treated at 4°C in the dark for 3 d, then moved to a growth chamber with 23°C and light intensity of approximately 100 μmol m−2 s−1. For the phenotypic analysis of 2-d-old, dark-grown seedlings, the petri dishes were wrapped in aluminum foil and placed in the darkness at 23°C.
For soil-based phenotypic analysis, wild-type and mutant plants were either grown in an Arabidopsis growth chamber under short-day conditions (8 h [light]/16 h [dark]) for the observation of leaf phenotype, or grown in the greenhouse (12 h [light]/12 h [dark]) for the observation of flower and silique phenotype.
Root Assays
Seeds from wild type, mutants, and transgenic lines sown in petri dishes were grown vertically under constant light conditions (100 μmol m−2 s−1), and the positions of the root tips were recorded daily. Rates of primary root growth were calculated over 3-d periods from day 3 to day 6. Seedlings were sampled at day 6, fixed, and cleared in chloral hydrate solution (chloral hydrate:glycerol:water = 8:2:1). The lengths of about 20 cortex cells in the differentiation zone of each root were measured using a Zeiss Axiovert 200M DIC microscope (Carl Zeiss) equipped with a digital image acquisition and processing system (AxioVision Release 4.2). Cell production was calculated as the rate of root growth divided by the average cortex cell length. In separate experiments, the seedlings were grown for an additional 3 d, and the numbers of lateral root primordia and lateral roots were measured from each plant under a dissecting microscope.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers NC_003071, NC_003075, and NC_003074.
Supplementary Material
This work was supported by grants from the National Institutes of Health (grant no. GM65989–01) and the National Science Foundation (grant no. MCB–0209711) to A.M.J. Work in J.-G.C.'s lab is supported by the Natural Sciences and Engineering Research Council of Canada, the Canada Foundation for Innovation, the British Columbia Ministry of Advanced Education, and the University of British Columbia. Y.G. is supported by a scholarship from the China Scholarship Council.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Alan M. Jones (alan_jones@unc.edu).
The online version of this article contains Web-only data.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.106.079202.
References
- Afshar K, Willard FS, Colombo K, Johnston CA, McCudden CR, Siderovski DP, Gonczy P (2004) RIC-8 is required for GPR-1/2-dependent Galpha function during asymmetric division of C. elegans embryos. Cell 119: 219–230 [DOI] [PubMed] [Google Scholar]
- Bechtold N, Pelletier G (1998) In planta Agrobacterium-mediated transformation of adult Arabidopsis thaliana plants by vacuum infiltration. Methods Mol Biol 82: 259–266 [DOI] [PubMed] [Google Scholar]
- Beeckman T, Burssens S, Inzé D (2001) The peri-cell-cycle in Arabidopsis. J Exp Bot (Special Issue) 52: 403–411 [DOI] [PubMed] [Google Scholar]
- Benjamins R, Quint A, Weijers D, Hooykaas P, Offringa R (2001) The PINOID protein kinase regulates organ development in Arabidopsis by enhancing polar auxin transport. Development 128: 4057–4067 [DOI] [PubMed] [Google Scholar]
- Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aida M, Palme K, Scheres B (2005) The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433: 39–44 [DOI] [PubMed] [Google Scholar]
- Casimiro I, Beeckman T, Graham N, Bhalerao R, Zhang H, Casero P, Sandberg G, Bennett MJ (2003) Dissecting Arabidopsis lateral root development. Trends Plant Sci 8: 165–171 [DOI] [PubMed] [Google Scholar]
- Celenza JL Jr, Grisafi PL, Fink GR (1995) A pathway for lateral root formation in Arabidopsis thaliana. Genes Dev 9: 2131–2142 [DOI] [PubMed] [Google Scholar]
- Chen JG, Pandey S, Huang J, Alonso JM, Ecker JR, Assmann SM, Jones AM (2004) GCR1 can act independently of heterotrimeric G-protein in response to brassinosteroids and gibberellins in Arabidopsis seed germination. Plant Physiol 135: 907–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JG, Willard FS, Huang J, Liang J, Chasse SA, Jones AM, Siderovski DP (2003) A seven-transmembrane RGS protein that modulates plant cell proliferation. Science 301: 1728–1731 [DOI] [PubMed] [Google Scholar]
- Christensen SK, Dagenais N, Chory J, Weigel D (2000) Regulation of auxin response by the protein kinase PINOID. Cell 100: 469–478 [DOI] [PubMed] [Google Scholar]
- Couwenbergs C, Spilker AC, Gotta M (2004) Control of embryonic spindle positioning and Galpha activity by C. elegans RIC-8. Curr Biol 14: 1871–1876 [DOI] [PubMed] [Google Scholar]
- DiDonato RJ, Arbuckle E, Buker S, Sheets J, Tobar J, Totong R, Grisafi P, Fink GR, Celenza JL (2004) Arabidopsis ALF4 encodes a nuclear-localized protein required for lateral root formation. Plant J 37: 340–353 [DOI] [PubMed] [Google Scholar]
- Doerner P, Jorgensen JE, You R, Steppuhn J, Lamb C (1996) Control of root growth and development by cyclin expression. Nature 380: 520–523 [DOI] [PubMed] [Google Scholar]
- Du Q, Macara IG (2004) Mammalian Pins is a conformational switch that links NuMA to heterotrimeric G proteins. Cell 119: 503–516 [DOI] [PubMed] [Google Scholar]
- Dubrovsky JG, Doerner PW, Colon-Carmona A, Rost TL (2000) Pericycle cell proliferation and lateral root initiation in Arabidopsis. Plant Physiol 124: 1648–1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovsky JG, Rost TL, Colon-Carmona A, Doerner P (2001) Early primordium morphogenesis during lateral root initiation in Arabidopsis thaliana. Planta 214: 30–36 [DOI] [PubMed] [Google Scholar]
- Ferrando A, Farras R, Jasik J, Schell J, Koncz C (2000) Intron-tagged epitope: a tool for facile detection and purification of proteins expressed in Agrobacterium-transformed plant cells. Plant J 22: 553–560 [DOI] [PubMed] [Google Scholar]
- Frank M, Thumer L, Lohse MJ, Bunemann M (2005) G Protein activation without subunit dissociation depends on a Gαi-specific region. J Biol Chem 280: 24584–24590 [DOI] [PubMed] [Google Scholar]
- Friml J, Yang X, Michniewicz M, Weijers D, Quint A, Tietz O, Benjamins R, Ouwerkerk PB, Ljung K, Sandberg G, et al (2004) A PINOID-dependent binary switch in apical-basal PIN polar targeting directs auxin efflux. Science 306: 862–865 [DOI] [PubMed] [Google Scholar]
- Gilman AG (1987) G proteins: transducers of receptor-generated signals. Annu Rev Biochem 56: 615–649 [DOI] [PubMed] [Google Scholar]
- Hamm HE (1998) The many faces of G protein signaling. J Biol Chem 273: 669–672 [DOI] [PubMed] [Google Scholar]
- Hampoelz B, Knoblich JA (2004) Heterotrimeric G proteins: new tricks for an old dog. Cell 119: 453–456 [DOI] [PubMed] [Google Scholar]
- Helariutta Y, Fukaki H, Wysocka-Diller J, Nakajima K, Jung J, Sena G, Hauser MT, Benfey PN (2000) The SHORT-ROOT gene controls radial patterning of the Arabidopsis root through radial signaling. Cell 101: 555–567 [DOI] [PubMed] [Google Scholar]
- Hess HA, Roper JC, Grill SW, Koelle MR (2004) RGS-7 completes a receptor-independent heterotrimeric G protein cycle to asymmetrically regulate mitotic spindle positioning in C. elegans. Cell 119: 209–218 [DOI] [PubMed] [Google Scholar]
- Himanen K, Boucheron E, Vanneste S, de Almeida Engler J, Inzé D, Beeckman T (2002) Auxin-mediated cell cycle activation during early lateral root initiation. Plant Cell 14: 2339–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himanen K, Vuylsteke M, Vanneste S, Vercruysse S, Boucheron E, Alard P, Chriqui D, Van Montagu M, Inzé D, Beeckman T (2004) Transcript profiling of early lateral root initiation. Proc Natl Acad Sci USA 101: 5146–5151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AM, Assmann SM (2004) Plants: the latest model system for G-protein research. EMBO Rep 5: 572–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AM, Ecker JR, Chen JG (2003) A reevaluation of the role of the heterotrimeric G protein in coupling light responses in Arabidopsis. Plant Physiol 131: 1623–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AM, Im KH, Savka MA, Wu MJ, DeWitt NG, Shillito R, Binns AN (1998) Auxin-dependent cell expansion mediated by overexpressed auxin-binding protein 1. Science 282: 1114–1117 [DOI] [PubMed] [Google Scholar]
- Karimi M, Inzé D, Depicker A (2002) GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci 7: 193–195 [DOI] [PubMed] [Google Scholar]
- Kato C, Mizutani T, Tamaki H, Kumagai H, Kamiya T, Hirobe A, Fujisawa Y, Kato H, Iwasaki Y (2004) Characterization of heterotrimeric G protein complexes in rice plasma membrane. Plant J 38: 320–331 [DOI] [PubMed] [Google Scholar]
- Lapik YR, Kaufman LS (2003) The Arabidopsis cupin domain protein AtPirin1 interacts with the G protein alpha-subunit GPA1 and regulates seed germination and early seedling development. Plant Cell 15: 1578–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lease KA, Wen J, Li J, Doke JT, Liscum E, Walker JC (2001) A mutant Arabidopsis heterotrimeric G-protein β subunit affects leaf, flower, and fruit development. Plant Cell 13: 2631–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy JE (2005) Intrinsic and environmental response pathways that regulate root system architecture. Plant Cell Environ 28: 67–77 [DOI] [PubMed] [Google Scholar]
- Malamy JE, Benfey PN (1997) Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124: 33–44 [DOI] [PubMed] [Google Scholar]
- Martin-McCaffrey L, Willard FS, Oliveira-dos-Santos AJ, Natale DR, Snow BE, Kimple RJ, Pajak A, Watson AJ, Dagnino L, Penninger JM, et al (2004) RGS14 is a mitotic spindle protein essential from the first division of the mammalian zygote. Dev Cell 7: 763–769 [DOI] [PubMed] [Google Scholar]
- Mason MG, Botella JR (2000) Completing the heterotrimer: isolation and characterization of an Arabidopsis thaliana G protein γ-subunit cDNA. Proc Natl Acad Sci USA 97: 14784–14788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MG, Botella JR (2001) Isolation of a novel G-protein γ-subunit from Arabidopsis thaliana and its interaction with Gβ. Biochim Biophys Acta 1520: 147–153 [DOI] [PubMed] [Google Scholar]
- Neubig RR, Siderovski DP (2002) Regulators of G-protein signalling as new central nervous system drug targets. Nat Rev Drug Discov 1: 187–197 [DOI] [PubMed] [Google Scholar]
- Pandey S, Assmann SM (2004) The Arabidopsis putative G-protein-coupled receptor GCR1 interacts with the G protein α subunit GPA1 and regulates abscisic acid signaling. Plant Cell 16: 1616–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey S, Chen JG, Jones AM, Assmann SM (2006) G-protein complex mutants are hypersensitive to abscisic acid regulation of germination and postgermination development. Plant Physiol 141: 243–256 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Peleg S, Varon D, Ivanina T, Dessauer CW, Dascal N (2002) Gαi controls the gating of the G protein-activated K+ channel, GIRK. Neuron 33: 87–99 [DOI] [PubMed] [Google Scholar]
- Perfus-Barbeoch L, Jones AM, Assmann SM (2004) Plant heterotrimeric G protein function: insights from Arabidopsis and rice mutants. Curr Opin Plant Biol 7: 719–731 [DOI] [PubMed] [Google Scholar]
- Pierce KL, Premont RT, Lefkowitz RJ (2002) Seven-transmembrane receptors. Nat Rev Mol Cell Biol 3: 639–650 [DOI] [PubMed] [Google Scholar]
- Rashotte AM, Brady SR, Reed RC, Ante SJ, Muday GK (2000) Basipetal auxin transport is required for gravitropism in roots of Arabidopsis. Plant Physiol 122: 481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashotte AM, DeLong A, Muday GK (2001) Genetic and chemical reductions in protein phosphatase activity alter auxin transport, gravity response, and lateral root growth. Plant Cell 13: 1683–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini S, Heidstra R, Wildwater M, Scheres B (2003) SCARECROW is involved in positioning the stem cell niche in the Arabidopsis root meristem. Genes Dev 17: 354–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer M, Petronczki M, Dorner D, Forte M, Knoblich JA (2001) Heterotrimeric G proteins direct two modes of asymmetric cell division in the Drosophila nervous system. Cell 107: 183–194 [DOI] [PubMed] [Google Scholar]
- Scheres B, Benfey P, Dolan L (2001) Root development. In CR Somerville, EM Meyerowitz, eds, The Arabidopsis Book. American Society of Plant Biologists, Rockville, MD, doi/10.1199/tab.0101, www.aspb.org/publications/arabidopsis
- Schwabe T, Baintoin RJ, Fetter RD, Heberlein U, Gaul U (2005) GPCR signaling is required for blood-brain barrier formation in Drosophila. Cell 123: 133–144 [DOI] [PubMed] [Google Scholar]
- Ullah H, Chen JG, Temple B, Boyes DC, Alonso JM, Davis KR, Ecker JR, Jones AM (2003) The β-subunit of the Arabidopsis G protein negatively regulates auxin-induced cell division and affects multiple developmental processes. Plant Cell 15: 393–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah H, Chen JG, Young JC, Im KH, Sussman MR, Jones AM (2001) Modulation of cell proliferation by heterotrimeric G protein in Arabidopsis. Science 292: 2066–2069 [DOI] [PubMed] [Google Scholar]
- Vernoux T, Benfey PN (2005) Signals that regulate stem cell activity during plant development. Curr Opin Genet Dev 15: 388–394 [DOI] [PubMed] [Google Scholar]
- Wang H, Ng KH, Qian H, Siderovski DP, Chia W, Yu F (2005) Ric-8 controls Drosophila neural progenitor asymmetric division by regulating heterotrimeric G proteins. Nat Cell Biol 7: 1091–1098 [DOI] [PubMed] [Google Scholar]
- Wildwater M, Campilho A, Perez-Perez JM, Heidstra R, Blilou I, Korthout H, Chatterjee J, Mariconti L, Gruissem W, Scheres B (2005) The RETINOBLASTOMA-RELATED gene regulates stem cell maintenance in Arabidopsis roots. Cell 123: 1337–1349 [DOI] [PubMed] [Google Scholar]
- Zhao J, Wang X (2004) Arabidopsis phospholipase Dα1 interacts with the heterotrimeric G-protein α-subunit through a motif analogous to the DRY motif in G-protein-coupled receptors. J Biol Chem 279: 1794–1800 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.