Iron (Fe), manganese (Mn), magnesium (Mg), and copper (Cu) are essential cofactors for the operation of the oxygenic photosynthetic electron transfer apparatus. The overall metal quota required by photosynthetic organisms because of these processes is much larger than the requirement of nonphotosynthetic organisms. To ensure an adequate supply of metals, photosynthetic organisms from cyanobacteria to vascular plants have developed efficient strategies for metal uptake and accumulation. However, the photosynthetic apparatus presents unique challenges to metal homeostasis. Whereas metals play a key role as cofactors in oxygenic photosynthesis, they pose at the same time a major oxidative risk factor due to their deleterious interaction with oxygen. The extreme redox chemistry performed by the two photosystems provides multiple sites at which reactive oxygen species (ROS) can be generated. Therefore, metal transport and storage need to be tightly regulated to ensure adequate supply and to protect against oxidative damage. The proliferation of all photosynthetic organisms depends on this delicate balance between the metal requirements and oxidative damage. This Update focuses on metal biology from a photosynthetic perspective, detailing strategies for metal uptake, accumulation, and cofactor assembly, as well as the inherent risks of metal homeostasis in cyanobacteria and chloroplasts.
Oxygenic photosynthesis exerts unique stresses on photosynthetic organisms. The photosynthetic apparatus is composed of a number of membrane-embedded protein supercomplexes that contain many cofactors (Fig. 1). Among them are Fe cofactors such as ironsulfur (Fe-S) clusters, cytochromes, and nonheme Fe, the Mn cluster of PSII, Cu in plastocyanin, as well as Mg in the center of each chlorophyll (chl) ring. As a result, the demand for these metals far exceeds that of other, nonphotosynthetic organisms. The cellular Mn quota of the oxygenic photosynthetic cyanobacterium Synechocystis sp. PCC 6803 is 2 orders of magnitude higher than that of the nonoxygenic purple bacterium Rhodobacter capsulatus (Keren et al., 2002). The Fe quota of Synechocystis cells is 1 order of magnitude higher than that of the nonphotosynthetic bacterium Escherichia coli (Keren et al., 2004). Furthermore, more than 25% of the Fe quota in Synechocystis cells is in PSI alone (Keren et al., 2004). In plant leaves, 60% to 80% of cellular Fe content is in chloroplasts (Terry and Low, 1982).
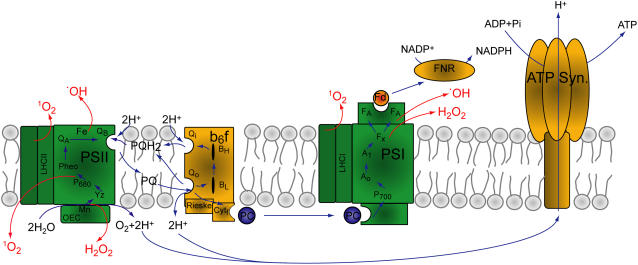
Figure 1.
Photosynthetic electron transport, ROS generation, and metal requirements. The photosynthetic electron transport chain is composed of the two photosystems (PSI and PSII) and cytochrome b6f. Extraction of electrons from water is catalyzed on the donor side of PSII by a cluster of four Mn ions. In addition, PSII contains two Fe atoms, a nonheme Fe on the acceptor side, and cytochrome b559. Cyanobacteria contain an extra cytochrome (cytochrome c550) attached to the donor side of the complex. The reaction center contains 36 chl molecules. Additional LHCII chls can increase the effective absorption cross section of the photosystem. Electrons are transferred from PSII to cytochrome b6f via plastoquinol molecules. Cytochrome b6f contains four b-type cytochromes (bH, bL, b6, and heme x), one c-type cytochrome f, and one 2Fe-2S cluster. The function of a single chl in the cytochrome structure is still unknown. Cytochrome b6f is the major proton pump that creates the potential gradient that is utilized by ATP synthase to produce ATP. Plastocyanin (PC), a Cu protein, transfers electrons from cytochrome b6f to PSI. In PSI, three 4Fe-4S clusters (FX, FA, and FB) participate in the electron transfer chain. Electrons from PSI are transferred to ferredoxin (Fd), a 4Fe-4S soluble protein. The reaction center core of PSI contains 96 chls with additional LHCI chls that contribute to the absorption cross section. Electron transfer reactions are indicated by blue arrows and ROS-generating reactions are indicated by red arrows.
It is important to note that metal homeostasis processes are dynamic in nature and the direction of transport changes in response to availability, annual cycles, and growth phase of photosynthetic organisms. In the surface water of marine environments, the concentrations of Fe, Cu, and Mn are often limiting (Morel and Price, 2003). On land, Fe limits plant growth in arid and semiarid regions (Guerinot, 2001). Photosynthetic organisms have developed strategies that compensate for metal limitation. Under Cu limitation, several algae and cyanobacteria species induce the production of cytochrome c6, which replaces plastocyanin in the photosynthetic electron transfer pathway (Eriksson et al., 2004). In vascular plant chloroplasts, where cytochrome c6 is absent, Cu limitation results in a decrease in copper-zinc (Cu-Zn) superoxide dismutase (SOD) levels (Seigneurin-Berny et al., 2005). Fe-limited cyanobacteria produce a photosynthetic antenna complex that facilitates excitation transfer to PSI (Kouril et al., 2005), in effect compensating for limiting Fe with abundant Mg.
Metal transport and cofactor biogenesis often take place on a large scale in a concerted manner, as in the case of greening. Etiolated seedlings that germinate in the dark synthesize the entire photosynthetic apparatus within hours of transfer to the light. Etiolated unicellular algal cells can accumulate 2 × 10−6 μg chl/cell within 8 h of transfer to the light, which corresponds to a rate of approximately 4 × 104 chl/s per cell (Ohad et al., 1967). Taking into account a photosynthetic unit size of approximately 500 chls in these cells, the corresponding rates of incorporation of Fe and Mn into photosynthetic cofactors are in the 1 × 103 and 3 × 102 atoms/s per cell range, respectively. These truly amazing rates have to be supported by mobilization of metals from internal pools as well as by transport from the environment.
Considering the limited bioavailability of metals in the environment, it is no surprise that plants remobilize metals from senescing tissues to young developing tissues. This is indeed the case for Fe and Cu in senescing Arabidopsis (Arabidopsis thaliana) leaves (Himelblau and Amasino, 2001). Similar processes take place on a much larger scale in deciduous trees where nutrients are remobilized from leaves before they are shed during autumn.
As in the case of cofactor biogenesis, degradation of photosynthetic cofactors in general and chl in particular is performed in an organized fashion to avoid ROS production during the process. Anthocyanins, which are responsible for the beautiful autumn colors of deciduous trees, provide protection against photooxidative reactions during the latter stages of this process (Keskitalo et al., 2005).
THE INHERENT DANGERS OF PHOTOSYNTHETIC CATALYSIS
Transporting, accumulating, and assembling transition metals into functional cofactors are particularly dangerous tasks when the photosynthetic apparatus is concerned. The photosynthetic apparatus performs some of the most extreme enzymatic reactions in energetic terms (Hillier and Babcock, 2001). Consequently, in the course of photosynthetic catalysis, ROS can be produced (Fig. 1). A number of metal-binding sites within the photosynthetic apparatus are especially prone to harmful interactions.
Chl, Mg, and Singlet Oxygen
Unlike most molecules in nature, O2 is a ground-state triplet (i.e. it contains two paired electrons with parallel spins). As a result, 3O2 reacts slowly with molecules in the singlet ground state. Oxygen can be converted to the reactive excited singlet state by absorption of energy. To do so, O2 needs to interact with an excited triplet (Krieger-Liszkay, 2005).
Chls, which contain a Mg porphyrin, participate in excitation and electron transfer reactions in the photosynthetic apparatus (Fig. 1). As a rule, after absorbing light energy, chl converts to the singlet excited state. However, triplet chl can be formed by a small percentage of excited chls (Krieger-Liszkay, 2005). Whereas carotene molecules in photosynthetic complexes protect against harmful oxidative interactions, the rate of singlet oxygen (1O2) production increases substantially when chl molecules are free. Unassembled chl can accumulate following the break down of antenna and reaction center proteins, which results in extensive light-induced oxidative damage (Hundal et al., 1995).
An additional source of triplet chl is the PSII reaction center core. Following absorption of light energy, the reaction center chls, P680, undergo charge separation and donate an electron to secondary electron carriers. Under light intensities subsaturating for growth, the electron will reduce the plastoquinol molecule in the QB pocket and proceed along the path of the electron transfer chain (Fig. 1). However, under supersaturating high light intensities, intermediary electron carrier pools are completely reduced and electrons flow backward from the QA site and recombine with the oxidized P680+. Recombining electrons can either regenerate the original excited singlet state or an excited triplet that can interact with oxygen and damage the reaction center. This process, termed acceptor-side photoinhibition, is a major source of light-induced damage in plants (Adir et al., 2003).
Hydrogen Peroxide Production by Impaired PSII Mn Clusters
The Mn-calcium (Ca)-chloride (Cl) cluster on the donor side of PSII catalyzes the extraction of electrons from water and production of O2. This highly reactive ion cluster is protected from the aqueous environment by a number of extrinsic proteins bound to the lumenal side of PSII. However, when the rate of proton pumping through the thylakoid membrane exceeds the rate of H+-ATPase activity and the pH in the lumen of the thylakoid drops, the protection that the extrinsic proteins provide to the Mn cluster can be breached. As a result, water-splitting activity is impaired and PSII electron carriers remain oxidized, a situation referred to as donor-side photoinhibition (Adir et al., 2003). One consequence of impaired Mn cluster activity is the production of hydrogen peroxide (H2O2) instead of O2 (Krieger and Rutherford, 1998).
Generation of Hydroxyl Radicals by the Nonheme Fe of PSII
Fe cations are considered among the most reactive transition metal ions. In PSII, a nonheme Fe cation is positioned between the QA and QB quinone molecules and it is assumed that its role is to facilitate electron transfer between the two (Fig. 1). Whereas under subsaturating light intensities electrons are transferred from the acceptor side of PSII to the plastoquinol pool, under high excitation pressures electrons can be transferred to O2 as well. Superoxide (O2−) and nonheme Fe can interact and generate a chain reaction producing hydroxyl radicals (.OH) through Fenton chemistry (Pospisil et al., 2004).
Generation of ROS by the Fe-S Clusters of PSI
Electrons are transferred along the photosynthetic electron transfer pathway from PSI through ferredoxin to the ferredoxin-NADP+ oxidoreductase (Fig. 1). However, when the ferredoxin pool is reduced, electrons can flow from the PSI FX center directly to O2, producing O2−. Superoxide, through the activity of membrane-bound SOD, is disproportionate to H2O2 and O2. SOD enzymes can contain Fe, Cu-Zn, or Mn cofactors, depending on the plant or cyanobacterium species. Ascorbate peroxidases reduce H2O2 to water. In this enzymatic pathway, every O2 molecule produced ends up as water again and therefore it is called the water-water cycle (Asada, 2000).
Under standard growth conditions, these reactions are utilized as a safety valve for releasing excess electrons flowing through the photosynthetic apparatus. However, under chilling temperatures in the light, the Fe-S clusters of PSI can be damaged by interaction with oxygen. The damage is most likely a consequence of interactions between H2O2 and reduced FA and FB Fe-S clusters resulting in the generation of .OH (Sonoike, 1996). As in the case of hydroxyl radical production by the PSII nonheme Fe, a chain reaction of ROS generation can be catalyzed by the Fe-S clusters through Fenton chemistry (Sonoike, 1996).
TRANSITION METAL TRANSPORT PATHWAYS IN PHOTOSYNTHETIC ORGANISMS
When considering the dangers of radical chemistry between photosynthetic cofactors and oxygen, the need for tightly regulated transport, accumulation, and cofactor assembly in the vicinity of the photosynthetic apparatus becomes clear. Free transition metal species can enhance the rate of ROS generation and cause extensive damage. Over the course of the evolution of oxygenic photosynthetic organisms, efficient mechanisms have evolved to ensure safe transition metal handling.
The first step in transport pathways of chloroplasts and cyanobacteria is through the outer envelope membrane. Outer membranes were initially viewed as nonselective size-based barriers. However, recent investigations suggest that selective ion transport systems function in these membranes (Vothknecht and Soll, 2005). Subsequent transport through the inner/ plasma and thylakoid membranes completes the transport of metals to storage compounds or biosynthetic pathways (Fig. 2).
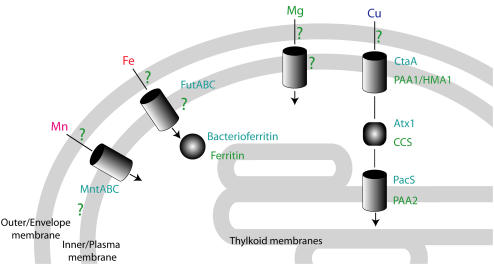
Figure 2.
Metal transport pathway in chloroplasts and cyanobacteria. Schematic representation of transport pathways for Mn (pink), Fe (red), Mg (green), and Cu (blue) through the three membrane systems of chloroplasts and cyanobacteria (inner, outer, and thylakoid membranes in plants; envelope, plasma, and thylakoid membranes in cyanobacteria). Known transporters are colored blue green for cyanobacteria and green for chloroplasts. Transport steps for which the carriers are still unknown are marked with a question mark. Mn is transported by MntABC in cyanobacteria (Bartsevich and Pakrasi, 1995). Additional components of the Mn transport pathway are still unknown. Fe is transported by FutABC in cyanobacteria (Katoh et al., 2001), stored in ferritin family complexes (Curie and Briat, 2003; Keren et al., 2004), or utilized for Fe cofactor biosynthesis. The pathways for heme and Fe-S cluster biosynthesis are not represented in this scheme. Mg transport pathways have not been elucidated yet. Cu transport is carried out by the CtaA and PacS P-type ATPases in cyanobacteria (Tottey et al., 2005) and by PAA1, HMA1, and PAA2 in plants (Abdel-Ghany et al., 2005; Seigneurin-Berny et al., 2005). Intracellular Cu transport is carried out by the Atx1 in cyanobacteria and by the Cu chaperone for SOD (CCS) in plants (Chu et al., 2005; Tottey et al., 2005).
Cu Transport Pathway
The best-characterized metal transport pathway in photosynthetic cells is that for Cu. Cu is required as a cofactor in the thylakoid lumen electron transport protein plastocyanin. In cyanobacteria, Cu is transported through the plasma membrane by a P-type ATPase. A second P-type ATPase is required for the transport of Cu across the thylakoid membrane. Inside the cyanobacterial cell, Cu is chaperoned by a small, soluble protein that contains the signature CXXC Cu-binding motif (Tottey et al., 2005; Fig. 2). In Arabidopsis chloroplasts, two P-type ATPases function in the inner membrane and an additional P-type ATPase functions in the thylakoid membrane (Abdel-Ghany et al., 2005; Seigneurin-Berny et al., 2005). One chloroplast copper chaperone of the CCS family has been identified (Chu et al., 2005; Fig. 2). The detailed view of Cu transport that emerges from these studies indicates that the operational mode of transport has been conserved through the evolution of cyanobacteria and higher plants.
Fe Transport Pathway
Fe is required as a cofactor for all three photosynthetic electron transfer chain supercomplexes. However, PSI with its three 4Fe-4S clusters contains more Fe than the other supercomplexes together. Transport of Fe presents a unique problem. Whereas Fe is abundant in the earth's crust, the bioavailability of Fe in the current oxidative terrestrial environment is limited. This is due to the fact that, in the presence of dioxygen at neutral pH, Fe precipitates as Fe oxides.
In the cyanobacterium Synechocystis sp. PCC 6803, the Fe ATP-binding cassette transporter FutABC was identified as a major contributor to ferric Fe transport through the plasma membrane (Katoh et al., 2001; Fig. 2). In plants, a number of root transport systems have been identified (Curie and Briat, 2003). These include ferric Fe transporters and Fe siderophore transporters. In chloroplasts, active Fe(II) transport through the inner membrane was measured (Shingles et al., 2001). However, it is still not known whether an ABC transporter, similar to that found in cyanobacteria, is involved in this process.
Mn Transport Pathway
Mn is required specifically for PSII function. The Mn cluster in the donor side of PSII catalyzes the water-splitting reaction (Rutherford and Boussac, 2004). Under limiting conditions, the high-affinity MntABC transport system is induced in cyanobacteria (Bartsevich and Pakrasi, 1995; Fig. 2). Inactivation of this transport system results in a loss of PSII activity. However, it is clear that additional low-affinity Mn transport systems exist. Homologs of the MntABC transporter were not found in chloroplasts and, while a number of transporters were implicated in Mn transport into plant cells and vacuoles (Pittman, 2005), the details of chloroplast transport pathways still remain to be discovered.
Mg Transport Pathway
Mg is ligated at the center of the chl ring and is by far the most abundant metal in the thylakoid membrane. Unlike Mn, Cu, and Fe, which are required in trace amounts, Mg is a plant macronutrient. A staggering 133 Mg atoms are required per photosynthetic electron transfer chain in core complexes alone (Fig. 1). When considering the light-harvesting complex (LHC) I and II proteins, this number can grow several-fold. In addition, Mg ions play a role in the buildup of the electrochemical gradient across thylakoid membranes (Krause, 1977). However, the identity of components of the chloroplast Mg transport pathway is unknown.
METAL PROCESSING IN CHLOROPLASTS AND IN CYANOBACTERIA
Metal Storage
Once inside the chloroplast or the cyanobacterial cell, metals can be stored or utilized for the assembly of active photosynthetic cofactors. Fe is stored in the center of ferritins or bacterioferritin complexes. Transport of Fe through membranes takes place in the soluble, but redox active Fe(II) form. Ferritins and bacterioferritins oxidize Fe(II) to insoluble Fe(III) prior to its deposition in the core of the protein complex (Curie and Briat, 2003). In cyanobacteria, Fe storage in bacterioferritins can account for up to 50% of the cellular Fe quota (Keren et al., 2004) and in pea (Pisum sativum) seedlings, ferritin Fe accounts for up to 90% of the cellular Fe quota (Marentes and Grusak, 1998). Mn is efficiently leached from the environment by cyanobacteria and is accumulated in the envelope layer of the cells (Keren et al., 2002). In both cases, metal is stored in a secluded compartment, removed from the site of photosynthetic catalysis.
Cofactor Biogenesis
Whereas stored metals can be protected from ROS-generating reactions, processes of cofactor assembly run the risk of oxidative damage from partially assembled photosynthetic complexes. Therefore, the assembly process takes place in concert with the biosynthesis of photosynthetic complex proteins to avoid accumulation of unassembled cofactors. In addition, during the process, partially assembled cofactors are protected from the environment by chaperones or scaffolding proteins.
The assembly of the Mn cluster of PSII is one example of a concerted metal cofactor biogenesis process. The PSII core D1 protein is transcribed as a precursor containing a C-terminal extension. As long as the extension exists, Mn and Mn cluster-stabilizing proteins cannot bind. Only after assembly of the PSII core, the extension is cleaved by a specific protease, Mn ions and the stabilizing proteins bind, and electron transfer activity is initiated (Roose and Pakrasi, 2004).
Similar concerted pathways are found in Fe-S cluster biogenesis processes. Fe-S centers are constructed on scaffold proteins before transfer to their final destination. Multiple assembly pathways were found in cyanobacteria and chloroplasts, including the well-characterized prokaryotic Suf pathway, as well as pathways involving proteins specific to photosynthetic organisms like HCF101 or NFU (Balk and Lobreaux, 2005). Interestingly, deletion mutants in these proteins result in specific inactivation of an Fe-S center containing photosynthetic protein complexes, linking assembly pathways with a single or limited number of targets (Balk and Lobreaux, 2005). A similar pathway-target link can be found between the chloroplast cytochrome c assembly pathway and cytochrome b6f biogenesis (Page et al., 2004).
CONCLUDING REMARKS
Although the importance of metal homeostasis to the photosynthetic apparatus is clear, our understanding of metal transport pathways in chloroplasts and cyanobacteria is limited. Metal transport in plants involves a series of approximately 15 consecutive steps between uptake from the soil and the chloroplast sink in the leaves (Hell and Stephan, 2003). The gaps in our knowledge of photosynthetically relevant metal transport pathways present an intriguing scientific question that is at the same time of great significance from both ecological and agricultural standpoints.
In addition, metals are required for balanced human nutrition. Fe deficiency constitutes a major public health risk (Theil, 2004). Attempts to fortify crops with Fe have only been partially successful. Rice (Oryza sativa) plants overproducing a soybean (Glycine max) chloroplast ferritin were able to accumulate excess Fe (Goto et al., 1999). However, the level of Fe accumulation in these plants did not parallel the level of ferritin accumulation, indicating that limitations in the Fe transport pathway prevent the loading of ferritins with Fe (Qu et al., 2005). Thorough understanding of chloroplast metal homeostasis processes will provide opportunities for improving the bioengineering of fortified crop plants in the future.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers mntA sll1599, mntB sll1600, mntC sll1589, futA1 slr1295, futA2 slr0513, futB slr0327, futC sll1878, ctaA slr1590, PacS sll1920, atx1 ssr2857, PAA1 At4g33520, PAA2 At5g21930, AtCCS At1g12520, HCF101 At3g24430, NFU At1g51390, and HMA1 At4g37270.
This work was supported by the Avron Even-Ari Minerva Center.
The author responsible for the distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Nir Keren (nirkeren@vms.huji.ac.il).
References
- Abdel-Ghany SE, Muller-Moule P, Niyogi KK, Pilon M, Shikanai T (2005) Two P-type ATPases are required for copper delivery in Arabidopsis thaliana chloroplasts. Plant Cell 17: 1233–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adir N, Zer H, Shochat S, Ohad I (2003) Photoinhibition—a historical perspective. Photosynth Res 76: 343–370 [DOI] [PubMed] [Google Scholar]
- Asada K (2000) The water-water cycle as alternative photon and electron sinks. Philos Trans R Soc Lond B Biol Sci 355: 1419–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balk J, Lobreaux S (2005) Biogenesis of iron-sulfur proteins in plants. Trends Plant Sci 10: 324–331 [DOI] [PubMed] [Google Scholar]
- Bartsevich VV, Pakrasi HB (1995) Molecular identification of an ABC transporter complex for manganese: analysis of a cyanobacterial mutant strain impaired in the photosynthetic oxygen evolution process. EMBO J 14: 1845–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CC, Lee WC, Guo WY, Pan SM, Chen LJ, Li HM, Jinn TL (2005) A copper chaperone for superoxide dismutase that confers three types of copper/zinc superoxide dismutase activity in Arabidopsis. Plant Physiol 139: 425–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curie C, Briat JF (2003) Iron transport and signaling in plants. Annu Rev Plant Biol 54: 183–206 [DOI] [PubMed] [Google Scholar]
- Eriksson M, Moseley JL, Tottey S, Del Campo JA, Quinn J, Kim Y, Merchant S (2004) Genetic dissection of nutritional copper signaling in Chlamydomonas distinguishes regulatory and target genes. Genetics 168: 795–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto F, Yoshihara T, Shigemoto N, Toki S, Takaiwa F (1999) Iron fortification of rice seed by the soybean ferritin gene. Nat Biotechnol 17: 282–286 [DOI] [PubMed] [Google Scholar]
- Guerinot ML (2001) Improving rice yields—ironing out the details. Nat Biotechnol 19: 417–418 [DOI] [PubMed] [Google Scholar]
- Hell R, Stephan UW (2003) Iron uptake, trafficking and homeostasis in plants. Planta 216: 541–551 [DOI] [PubMed] [Google Scholar]
- Hillier W, Babcock GT (2001) Photosynthetic reaction centers. Plant Physiol 125: 33–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himelblau E, Amasino RM (2001) Nutrients mobilized from leaves of Arabidopsis thaliana during leaf senescence. J Plant Physiol 158: 1317–1323 [Google Scholar]
- Hundal T, Forsmark-Andree P, Ernster L, Andersson B (1995) Antioxidant activity of reduced plastoquinone in chloroplast thylakoid membranes. Arch Biochem Biophys 324: 117–122 [DOI] [PubMed] [Google Scholar]
- Katoh H, Hagino N, Grossman AR, Ogawa T (2001) Genes essential to iron transport in the cyanobacterium Synechocystis sp. strain PCC 6803. J Bacteriol 183: 2779–2784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren N, Aurora R, Pakrasi HB (2004) Critical roles of bacterioferritins in iron storage and proliferation of cyanobacteria. Plant Physiol 135: 1666–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren N, Kidd MJ, Penner-Hahn JE, Pakrasi HB (2002) A light-dependent mechanism for massive accumulation of manganese in the photosynthetic bacterium Synechocystis sp. PCC 6803. Biochemistry 41: 15085–15092 [DOI] [PubMed] [Google Scholar]
- Keskitalo J, Bergquist G, Gardestrom P, Jansson S (2005) A cellular timetable of autumn senescence. Plant Physiol 139: 1635–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouril R, Arteni AA, Lax J, Yeremenko N, D'Haene S, Rogner M, Matthijs HC, Dekker JP, Boekema EJ (2005) Structure and functional role of supercomplexes of IsiA and photosystem I in cyanobacterial photosynthesis. FEBS Lett 579: 3253–3257 [DOI] [PubMed] [Google Scholar]
- Krause GH (1977) Light-induced movement of magnesium ions in intact chloroplasts: spectroscopic determination with Eriochrome blue SE. Biochim Biophys Acta 460: 500–510 [DOI] [PubMed] [Google Scholar]
- Krieger A, Rutherford AW (1998) The involvement of H2O2 produced by photosystem II in photoinhibition. In G Garab, ed, Photosynthesis: Mechanisms and Effects, Vol 3. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 2135–2213
- Krieger-Liszkay A (2005) Singlet oxygen production in photosynthesis. J Exp Bot 56: 337–346 [DOI] [PubMed] [Google Scholar]
- Marentes E, Grusak MA (1998) Iron transport and storage within the seed coat and embryo of developing seeds of pea (Pisum sativum L.). Seed Sci Res 8: 367–375 [Google Scholar]
- Morel FMM, Price NM (2003) The biogeochemical cycles of trace metals in the oceans. Science 300: 944–947 [DOI] [PubMed] [Google Scholar]
- Ohad I, Siekevitz P, Palade GE (1967) Biogenesis of chloroplast membranes. II. Plastid differentiation during greening of a dark-grown algal mutant (Chlamydomonas reinhardtii). J Cell Biol 35: 553–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page ML, Hamel PP, Gabilly ST, Zegzouti H, Perea JV, Alonso JM, Ecker JR, Theg SM, Christensen SK, Merchant S (2004) A homolog of prokaryotic thiol disulfide transporter CcdA is required for the assembly of the cytochrome b6f complex in Arabidopsis chloroplasts. J Biol Chem 279: 32474–32482 [DOI] [PubMed] [Google Scholar]
- Pittman JK (2005) Managing the manganese: molecular mechanisms of manganese transport and homeostasis. New Phytol 167: 733–742 [DOI] [PubMed] [Google Scholar]
- Pospisil P, Arato A, Krieger-Liszkay A, Rutherford AW (2004) Hydroxyl radical generation by photosystem II. Biochemistry 43: 6783–6792 [DOI] [PubMed] [Google Scholar]
- Qu LQ, Yoshihara T, Ooyama A, Goto F, Takaiwa F (2005) Iron accumulation does not parallel the high expression level of ferritin in transgenic rice seeds. Planta 222: 225–233 [DOI] [PubMed] [Google Scholar]
- Roose JL, Pakrasi HB (2004) Evidence that D1 processing is required for manganese binding and extrinsic protein assembly into photosystem II. J Biol Chem 279: 45417–45422 [DOI] [PubMed] [Google Scholar]
- Rutherford AW, Boussac A (2004) Water photolysis in biology. Science 303: 1782–1784 [DOI] [PubMed] [Google Scholar]
- Seigneurin-Berny D, Gravot A, Auroy P, Mazard C, Kraut A, Finazzi G, Grunwald D, Rappaport F, Vavasseur A, Joyard J, et al (2005) HMA1, a new Cu-ATPase of the chloroplast envelope, is essential for growth under adverse light conditions. J Biol Chem 281: 2882–2892 [DOI] [PubMed] [Google Scholar]
- Shingles R, North M, McCarty RE (2001) Direct measurement of ferrous ion transport across membranes using a sensitive fluorometric assay. Anal Biochem 296: 106–113 [DOI] [PubMed] [Google Scholar]
- Sonoike K (1996) Degradation of psaB gene product, the reaction center subunit of photosystem I, is caused during photoinhibition of photosystem I: possible involvement of active oxygen species. Plant Sci 115: 157–164 [Google Scholar]
- Terry N, Low G (1982) Leaf chlorophyll content and its relation to the intracellular location of iron. J Plant Nutr 5: 301–310 [Google Scholar]
- Theil EC (2004) Iron, ferritin, and nutrition. Annu Rev Nutr 24: 327–343 [DOI] [PubMed] [Google Scholar]
- Tottey S, Harvie DR, Robinson NJ (2005) Understanding how cells allocate metals using metal sensors and metallochaperones. Acc Chem Res 38: 775–783 [DOI] [PubMed] [Google Scholar]
- Vothknecht UC, Soll J (2005) Chloroplast membrane transport: interplay of prokaryotic and eukaryotic traits. Gene 354: 99–109 [DOI] [PubMed] [Google Scholar]