Abstract
Activation of mitogen-activated protein (MAP) kinases is a common reaction of plant cells in defense-related signal transduction pathways. To gain insight into the mechanisms that determine specificity in response to a particular stimulus, a biochemical approach has been employed. Photoautotrophic suspension culture cells of tomato (Lycopersicon peruvianum) were used as experimental system to characterize MAP kinase activation by different stress-related stimuli. An elicitor preparation of the tomato-specific pathogen Fusarium oxysporum lycopersici was shown to result in the simultaneous induction of four kinase activities that could be separated by ion-exchange chromatography. The simultaneous activation of multiple MAP kinases was further substantiated by distinct pharmacological and immunological properties: a differential sensitivity toward various protein kinase inhibitors and a differential cross-reaction with isoform-specific MAP kinase antibodies. In contrast to the two fungal elicitors chitosan and the F. oxysporum lycopersici preparation, the plant-derived stimuli polygalacturonic acid and salicylic acid were shown to activate distinctly different subsets of MAP kinases. Application of a voltage pulse was introduced as a transient stress-related stimulus that does not persist in the culture. Voltage application activates a distinct set of MAP kinases, resembling those activated by salicylic acid treatment, and generates a refractory state for the salicylic acid response. The inhibitory effect of nifedipine indicates that current application may directly affect voltage-gated calcium channels, thus, providing a tool to study various calcium-dependent pathways.
During their whole life, plants need to cope with a variety of attacking pathogens. They developed appropriate defense responses that protect them against impairment by most of them. These defense responses against several different pathogens have been extensively studied, however, the components that determine the specificity of the defense gene activation remain to be elucidated. After a plant cell is challenged by an elicitor, one or more signal transduction pathways are invoked by a ligand-receptor interaction (Nürnberger, 1999) that lead to the activation of a set of defense-related genes appropriate for the defense against the attacking pathogen (Jabs et al., 1997).
The involvement of mitogen-activated protein (MAP) kinases in biotic and abiotic stress-mediated defense gene activation has been extensively studied and MAP kinases that respond to elicitors (Zhang et al., 1998), wounding (Stratmann and Ryan, 1997), cold and drought stress (Jonak et al., 1996), salinity (Munnik et al., 1999), and endogenous signals (Zhang and Klessig, 1997) have been described. The activities were assigned to MAP kinase genes by the observation of coordinated transcriptional up-regulation and the use of specific antibodies. In-depth analysis of MAP kinase activation in tobacco revealed that the MAP kinase salicylate-induced protein kinase is activated by salicylic acid (Zhang and Klessig, 1997), fungal elicitors (Zhang et al., 1998), wounding (Zhang and Klessig, 1998b), and tobacco mosaic virus infection (Zhang and Klessig, 1998a). Therefore activity in response to several stimuli was ascribed to only one MAP kinase. This raises the question of which factors determine the specific pattern of gene activation induced by defense-related stimuli. A specific response may arise from the activation of more than one MAP kinase pathway in response to a particular stimulus. It has been demonstrated by isoelectric focussing that after wounding, four MAP kinases were activated with three of them having the same Mr (Usami et al., 1995). Cardinale et al. (2000) recently showed that a yeast elicitor resulted in the activation of four different MAP kinases as revealed by immunoprecipitation with peptide-specific antibodies.
An involvement of calcium signaling upstream of MAP kinase pathways has been proposed based on the observation that MAP kinase activation was prevented by the addition of calcium channel blockers (Lebrun-Garcia et al., 1998). Calcium concentration is kept very low in the cytosol, enabling fast responses by the opening of calcium channels. Thus calcium is capable of fast transduction of stress and pathogen signals. Calcium increases were reported in response to several stress-related stimuli like touch, wind, and cold (Knight et al., 1991). The number of publications on the importance of calcium in defense signaling in response to pathogen-related signals (Kawano et al., 1998), pathogens (Xu and Heath, 1998), race-specific peptides (Gelli et al., 1997), peptide receptor interaction (Blume et al., 2000), or oligosaccharides (Mithoefer et al., 1999) is also increasing.
In the present study we investigated the response of photoautotrophic suspension culture cells of tomato (Lycopersicon peruvianum) to fungal elicitors, endogenous stress-related signals, and application of voltage. A combination of biochemical methods, the use of various protein kinase inhibitors, and immunocomplex kinase assays substantiates the activation of multiple MAP kinases by one particular stimulus. Comparison of the effects of different pathogen-related stimuli revealed that the activation of specific subsets of kinases results in different physiological responses. The activation of MAP kinases was shown to require calcium influx across the plasma membrane. Voltage application was introduced as a transient stimulus that does not persist in the culture medium. Based on the sensitivity toward the calcium channel inhibitor nifedipine we propose that voltage application acts directly on voltage-gated calcium channels providing a tool to study the role of calcium signatures in signal transduction pathways.
RESULTS
An Elicitor Preparation of the Wilt-Inducing Fungus Fusarium oxysporum lycopersici Leads to MAP Kinase Activation
To investigate the defense response of tomato against one of its naturally occurring pathogens, an elicitor preparation of the wilt-inducing fungus F. oxysporum lycopersici, referred to as E-FOL, was used to challenge photoautotrophically growing suspension culture cells of tomato.
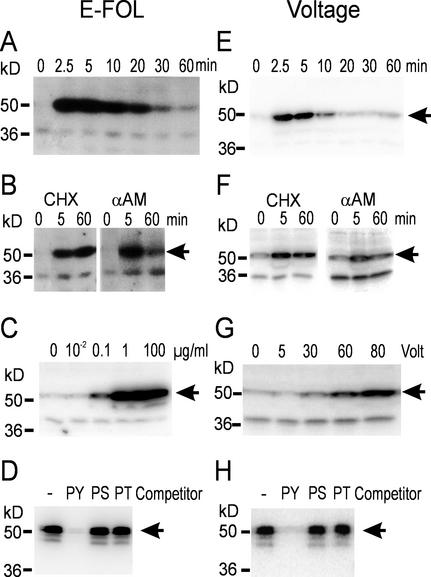
To assay for possible MAP kinase activation upon treatment with E-FOL, in-gel kinase assays with the model substrate myelin basic protein (MBP) were performed and revealed the fast and transient activation of a kinase of 52-kD molecular mass (Fig. 1A). Because previous reports on stress-activated MAP kinases demonstrated that the activation of those kinases occurs post-translationally (Zhang et al., 1998), we pretreated the cells with the RNA-polymerase II inhibitor α-amanitin or the protein biosynthesis inhibitor cycloheximide. The results shown in Figure 1B demonstrate that the activation of the 52-kD kinase only depends on post-translational modifications because it is not blocked by either inhibitor. Inactivation in contrast is dependent on transcription and translation of a negative regulator as is revealed by the sustained activity after treatment with both inhibitors.
Figure 1.
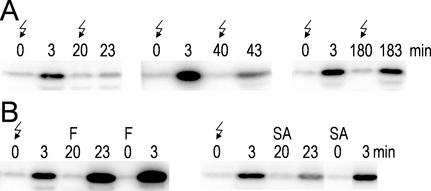
MAP kinase activation by treatment with E-FOL or voltage. Suspension cultures were treated with 100 μg mL−1 E-FOL (A and B) or 60 V (E and F) at time point 0. Samples were taken at the indicated time points and analyzed by in-gel kinase assay with MBP as substrate. B and F, Cells were pretreated with cycloheximide (CHX) or α-amanitin (αAM) 15 min before stimulation. C and G, Cells treated with the indicated concentrations of E-FOL (C) and voltage (G) were collected 5 min after stimulation and analyzed by in-gel kinase assay. D and H, Immunoprecipitation with phospho-Tyr-specific antibody 4G10. Crude extract of E-FOL- (D) or voltage- (H) treated cells was used for immunoprecipitation without competitor (−) or in the presence of 1 mm phospho-Tyr (PY), phospho-Ser (PS), or phospho-Thr (PT). The precipitate was analyzed by in-gel kinase assay.
To further characterize the activation of the 52-kD MBP-phosphorylating kinase activated by treatment with E-FOL, we conducted dose response experiments (Fig. 1C) that demonstrate the induction of maximal kinase activity by addition of 1 μg mL−1 E-FOL preparation.
A known feature of MAP kinases is their activation by phosphorylation on the conserved sequence motif TXY, both at Tyr and Thr (Canagarajah et al., 1997). To further support that the 52-kD MBP-phosphorylating kinase activated by E-FOL treatment belongs to the MAP kinase family, immunoprecipitation with the phospho-Tyr-specific antibody 4G10 and subsequent analysis with an in-gel kinase assay were performed. As shown in Figure 1D, the E-FOL-activated protein kinase is recognized by the 4G10 antibody. To ensure the specificity of the antibody, competition of the immunocomplex formation by the phospho-amino acids phospho-Tyr, phospho-Ser, and phospho-Thr was tested. The results demonstrate specific recognition of phospho-Tyr and thus support Tyr phosphorylation of the activated MAP kinase.
Application of a Voltage Pulse Leads to MAP Kinase Activation
To challenge the cells by a stress-related stimulus that does not persist in the culture we introduced a novel type of stress stimulus: A constant voltage was applied to the suspension culture for a limited time. Two concentric rings of platinum wires were submerged as electrodes into a 50-mL culture of tomato cells with a distance of 2.5 cm between the electrodes. With a direct current power supply, 60 V were applied for 10 s. As revealed by the in-gel kinase assay shown in Figure 1E, this treatment resulted in a fast and transient kinase activation. Analysis of the dose response reveals that the lowest inducing D.C. voltage was 30 V (Fig. 1G). Comparison of the effects of E-FOL and voltage treatment reveals a lower activity and a faster deactivation after voltage treatment as compared with E-FOL. This may reflect the duration of perception of the signal: Whereas the voltage treatment lasts only 10 s, the elicitor treatment is a persistent signal and therefore stimulates the cells longer.
Further characterization of the activation of the MBP-phosphorylating protein kinase reveals that both α-amanitin and cycloheximide do not interfere with the activation but inhibit the fast inactivation (Fig. 1F). Thus activation is independent of transcription and protein translation, whereas inactivation requires synthesis of a regulatory protein. Tyr phosphorylation was demonstrated by immunoprecipitation by the phospho-Tyr-specific antibody 4G10 (Fig. 1H). These data substantiate that voltage, as shown before for E-FOL, results in the activation of a protein kinase belonging to the MAP kinase family.
Calcium Influx Is Necessary for MAP Kinase Activation
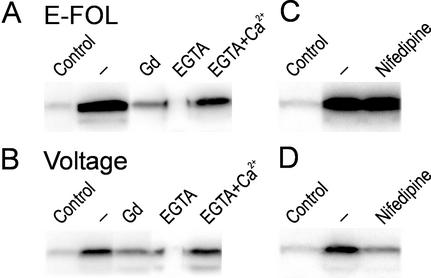
Calcium has been shown to be involved in the initiation of plant defense responses and was a prerequisite for MAP kinase activation in response to hypoosmotic shock and elicitor treatment (Takahashi et al., 1997). To test whether calcium is involved in the signal transduction leading to MAP kinase activation after treatment with E-FOL and voltage, we used the calcium channel inhibitor gadolinium.
Figure 2, A and B, shows that after 4 min of preincubation with 1 mm gadolinium, the response to E-FOL and voltage is reduced. The kinase activation is also reduced if the suspension medium is depleted of calcium by addition of 8 mm EGTA 2 min before stimulation. To demonstrate the specificity of the EGTA treatment we added 8 mm CaCl2 together with 8 mm EGTA. In combination with calcium, EGTA did not interfere with MAP kinase activation (Fig. 2, A and B). This demonstrates that the influx of calcium is responsible to invoke MAP kinase activation after treatment with E-FOL or voltage.
Figure 2.
Calcium is necessary for MAP kinase activation. Untreated cells (Control) or cells treated with E-FOL (A) or voltage (B) were analyzed by in-gel kinase assay. Cells were pretreated 4 min before stimulation with the calcium channel blockers gadolinium(III) chloride (Gd) or nifedipine. EGTA or EGTA and calcium chloride (EGTA+Ca2+) was added 2 min before stimulation to test for the involvement of extracellular calcium.
Voltage Treatment Acts on Voltage-Dependent Calcium Channels
To further substantiate the results of the general calcium channel blockers, we used the calcium channel inhibitor nifedipine, which specifically blocks voltage-gated calcium channels of the l-type in animal cells (Catterall and Striessnig, 1992) and has been used successfully to block voltage-gated channels in plants (Gelli and Blumwald, 1997). Addition of a final concentration of 20 μm nifedipine reduced MAP kinase activation in response to voltage treatment to about 60% (Fig. 2D). In contrast the E-FOL-induced MAP kinase activation is not inhibited by nifedipine (Fig. 2C), demonstrating the specificity of the inhibitor because calcium-mediated signal transduction is not blocked in general. This result indicates that voltage treatment acts specifically through voltage-gated calcium permeable channels and the applied voltage possibly directly opens these channels.
Characterization of Effects of Several Pathogen-Related Stimuli
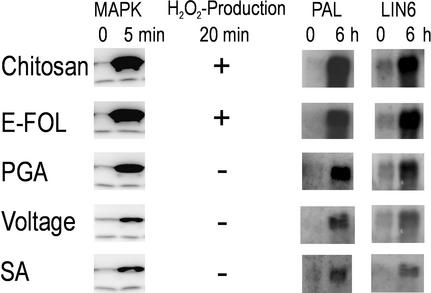
To further characterize the response to E-FOL and voltage treatment we tested the production of H2O2 as a common physiological response to pathogen infection. E-FOL treatment induced a fast increase of H2O2 concentration up to 5 μm in the media, whereas voltage treatment did not elicit H2O2 production.
To characterize the specificity in MBP-kinase activation in response to different signals we tested three further stress signals: chitosan, polygalacturonic acid (PGA), and salicylic acid. Chitosan is a known elicitor of plant defense responses and is part of fungal cell walls (Hadwiger and Beckman, 1980). PGA is a degradation product of plant cell walls and thus a host-derived elicitor (Ryan, 1987). Salicylic acid is an important signaling molecule involved in intra- and inter-cellular defense signaling (Reymond and Farmer, 1998).
In addition to E-FOL, only the other fungal elicitor chitosan, but none of the plant derived signals PGA and salicylic acid, led to H2O2 production (Fig. 3). The tomato suspension culture cells differentially responded toward pathogen-related signals that are endogenously produced by the plant in response to infection and stimuli derived from pathogens such as E-FOL or chitosan. Only upon contact with the latter signal H2O2 was produced.
Figure 3.
Downstream effects of defense-related signals. Cells treated with chitosan, E-FOL, PGA, voltage, or salicylic acid (SA) were assayed for MAP kinase activation, H2O2 production, and gene induction. Five-minute samples were subjected to in-gel kinase assay. In chitosan- and E-FOL-treated cultures, H2O2 concentration had increased to 5 μm above untreated cultures within 20 min. Gene regulation was determined by northern-blot analysis with probes for Pal and Lin6.
Figure 3 shows by in-gel kinase assay that all of the tested stimuli induce MAP kinase activity. Using histone or casein instead of MBP as substrate resulted in strongly reduced activity as is expected for MAP kinases (data not shown). E-FOL, chitosan, and PGA resulted in a higher MAP kinase activity compared with the other stimuli. This different magnitude of MAP kinase activation correlates to the effect on the regulation of defense-related genes (Fig. 3). As markers for defense gene activation we probed for Phe-ammonium-lyase (Pal) expression, which is the key enzyme of the phenylpropanoid pathway involved in the production of various defense-related products including salicylic acid (Coquoz et al., 1998). As a second marker gene we probed for Lin6, an extracellular invertase, which is up-regulated upon stress to increase sink strength and supply the cell with additional sugar as energy source during defense reactions (Ehness and Roitsch, 1997). Northern-blot analysis revealed that all stimuli lead to induction of the defense genes Pal and Lin6, whereas the differential level of mRNA induction correlates with the level of MAP kinase activity.
Several MAP Kinases Are Activated by One Specific Stimulus
The differences in H2O2 production and the levels of kinase activity and mRNA regulation indicated that differences in MAP kinase activation may reflect the different stimuli. We characterized the MBP-kinase activities in response to various signals by biochemical separation.
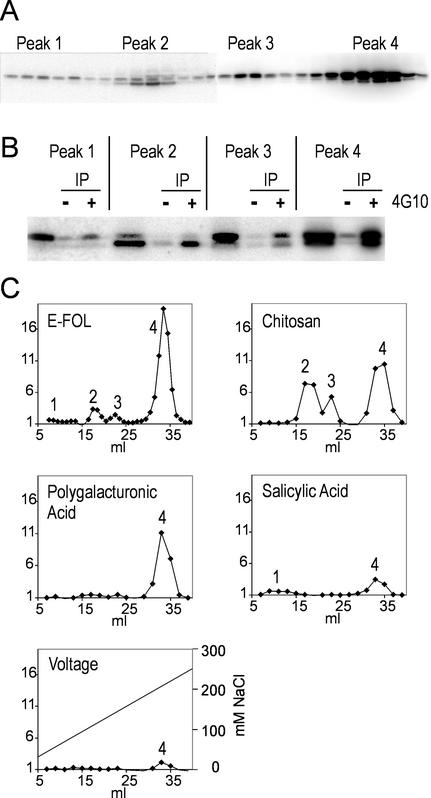
Anion-exchange chromatography was used to separate the protein extracts of control cells and of cells treated for 5 min with E-FOL. As shown in Figure 4A by an in-gel kinase assay, we were able to separate four peaks with MBP-phosphorylating activity after E-FOL treatment. The untreated cells had no comparable activity (data not shown). In-solution kinase assay as used for experiment of Figures 4C and 5A revealed a similar activity profile. This separation indicates that there is not only one but at least four kinases activated by treatment with E-FOL. There is no detectable elution of MBP-phosphorylating kinase activity at higher salt concentrations. Comparison with in-gel kinase assays with casein and histone as substrate revealed that MBP is the preferred substrate (data not shown), which is in accordance with the published substrate specificity for MAP kinases. To further support the identity as MAP kinases the peak fractions were immunoprecipitated with the phospho-Tyr-specific antibody 4G10. As shown by in-gel kinase assay in Figure 4B, each peak contains Tyr-phosphorylated protein kinases that are able to phosphorylate MBP, strongly supporting that the separated kinases belong to the MAP kinase family.
Figure 4.
Separation of cell extracts by anion-exchange chromatography reveals four kinase activity peaks. A, Chromatography fractions 10 to 37 of E-FOL-treated cells were analyzed by in-gel kinase assay. B, Peak fractions were immunoprecipitated with phospho-Tyr-specific antibody 4G10 and analyzed by in-gel kinase assay. Peak fraction and same fraction immunoprecipitated without antibody (−) and with 4G10 (+) are depicted. C, Extracts from cells treated with the indicated stimuli were separated and analyzed by in-solution kinase assay. Activity was quantified by phosphor imager and diagrammed as x-fold activity of untreated cells. The salt-gradient is exemplary depicted in the voltage diagram.
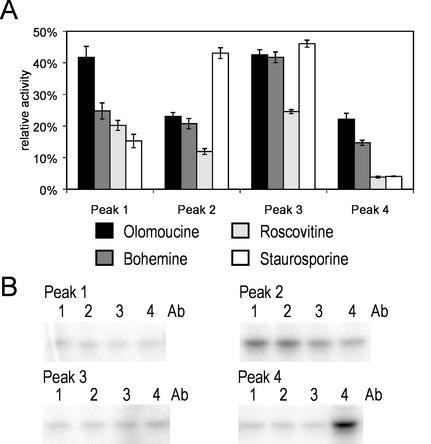
Figure 5.
Separated peaks of E-FOL-treated cells analyzed by inhibitor sensitivity and immunoprecipitation. A, Peak fractions were analyzed by in-solution kinase assay in the presence of the inhibitors olomoucine, bohemine, roscovitine, or staurosporine and without inhibitor. The activity was quantified and is depicted as percentage of the activity without addition of inhibitor. sd of three experiments is depicted. B, Peak fractions were used for immunoprecipitation with the antibodies against the alfalfa MAP kinases SIMK (1), MMK2 (2), MMK3 (3), or SAMK (4) and subsequent in-solution kinase assay.
The Different Peak MAP Kinase Activities Display Differential Inhibitor Sensitivity
To ensure that the different activity peaks eluting from the Resource Q column are due to different MAP kinases, we applied a set of inhibitors to determine their effects on the separated MPB-phosphorylating protein kinases. For these experiments, we used the purine analogs olomoucine, bohemine, and roscovitine. These inhibitors are used as potent inhibitors of cyclin-dependent kinases (De Azevedo et al., 1997) and were shown in one study to also inhibit an alfalfa MAP kinase (Binarová et al., 1998). Furthermore we included the kinase inhibitor staurosporine that was shown to discriminate between different MAP kinases (Romeis et al., 1999).
We tested the effect of 100 μm olomoucine, roscovitine, or bohemine, or 1 μm staurosporine on the four MAP kinase fractions in an in-solution kinase assay. Figure 5A displays that the four different inhibitors act differentially on the four different fractions. Each peak is characterized by a unique sensitivity profile with respect to the four different inhibitors. The differential effect of the purine analogs as well as staurosporine on the MBP-phosphorylating activity in the four fractions supports that the four separated peaks reflect different MAP kinases with distinct properties. Because there was only low activation in peak 1 we cannot exclude that the distinct inhibitor profile of peak 1 is due to other MBP-phosphorylating kinases that coelute.
Immunoprecipitation with Specific MAP Kinase Antibodies
The activation of at least four kinases by E-FOL, which is evident from the biochemical separation and the inhibitor studies described above, raises the question whether homologs of known MAP kinases are among those. Because for tomato no MAP kinase antibodies are available, we used previously characterized antibodies directed against MAP kinases from alfalfa. Isoform-specific antibodies directed against SIMK, Medicago MAP kinase (MMK)2, MMK3, and stress-activated MAP kinase (SAMK) were shown to recognize specific MAP kinases without cross-reacting against other MAP kinase homologs and are thus used to identify distinctly different MAP kinases involved in various physiological events (Cardinale et al., 2000). Because synthetic peptides of the poorly conserved C termini were used for generation of the four alfalfa MAP kinase antibodies, we searched expressed sequence tag databases for putative MAP kinase homologs in tomato which in particular are characterized by a high degree of homology at the C terminus with the alfalfa MAP kinases. The search revealed tomato MAP kinase homologs to SIMK (AW933300), MMK2 (TC52211), and SAMK (TC78176), which share a conserved C-terminal motive of 6, 5, and 6 amino acids of the corresponding 7, 11, and 6 amino acid peptide sequences used for antibody generation, respectively (Cardinale et al., 2000; tentative consensus numbers: The Institute for Genomic Research LeGI database). This indicates that these alfalfa antibodies may be a suitable tool to also precipitate MAP kinases of tomato. No tomato MAP kinase with high C-terminal homology to the C-terminal peptide sequence of MMK3 was found in the database.
Figure 5B shows that the MBP-phosphorylating kinase activity of peak 2 was precipitated by SIMK and MMK2 antibody. No activity was precipitated from peaks 1 and 3. The activity of peak 4 was solely precipitated by the SAMK antibody, suggesting the presence of a tomato MAP kinase with homology to SAMK. The MAP kinases present in peaks 1 and 3, which are distinguished by their inhibitor sensitivity, apparently represent tomato MAP kinases with homology too low to be precipitated by the applied alfalfa antibodies. The differential cross-reactions of the MAP kinase fractions with the four alfalfa MAP kinase antibodies further supports that a set of at least four MAP kinases are simultaneously activated by E-FOL and separated by ion-exchange chromatography.
Distinct Subsets of MAP Kinases Are Activated by Different Stimuli
After establishing an experimental system to separate and distinguish different MAP kinases activated after treatment with an individual stimulus, the fungal elicitor E-FOL, we investigated the effect of different stress-related stimuli on MAP kinase activation. We used the in-solution kinase test to compare the activation profiles after treatment with the different stimuli.
From the profiles depicted in Figure 4C it is obvious that there is activation of different subsets of kinases depending on the stimulus used; treatment with E-FOL, PGA, and chitosan resulted in strong activity in peak 4. But whereas also peak 2 and 3 had a high activity after E-FOL and chitosan treatment, PGA-treated cells displayed only little activity in these fractions. Both salicylic acid and voltage treatment resulted in a comparable low MAP kinase activity in peak 4. Thus treatment of the cell cultures with different pathogen-related stimuli leads to unique profiles of MAP kinase activation. The voltage treatment results in a profile resembling salicylic acid treatment.
Refractory Experiments Support the Activation of an Identical MAP Kinase Pathway by Salicylic Acid and Voltage
The result that both voltage treatment and salicylic acid induced activation of the same MAP kinase subsets and did not elicit H2O2 production indicated similarities between these two stimuli. To further substantiate this, we made use of the fact that a MAP kinase pathway, once activated, remains in a refractory state for some time (Bögre et al., 1997). A common feature of MAP kinase pathways is a fast deactivation even if the stimulus is not removed. As shown in Figure 1, B and F, the activation of MAP kinases by E-FOL and voltage can be preserved by inhibiting transcription or translation. This demonstrates that gene activation and protein synthesis is required for deactivation of the MAP kinase. This may be caused by induction of a specific phosphatase that inactivates the MAP kinase (Gupta et al., 1998). For a second treatment or other stimuli acting through the same pathway this results in a refractory time during which a stimulus does not invoke a response because dephosphorylation is competing phosphorylation. On whole plants a refractory time has been shown for repeated wounding of leaves (Bögre et al., 1997). In cell cultures, the difficulty arises that the stimuli usually applied to elicit defense responses cannot be removed from the system without applying additional stress. The newly established voltage stimulus is in particular suited for testing of a refractory state because it is applied only for a defined short time and thus not persistently present in the medium unlike fungal elicitors.
In an initial experiment addressing the refractory phase of the voltage-induced MAP kinase activation, we found that the refractory time period lasts at least 40 min after voltage treatment because during this time period a second stimulation induces only weak MAP kinase activation. After 3 h, the responsiveness of the signal transduction pathway is completely recovered (Fig. 6A).
Figure 6.
Voltage generates a refractory state for MAP kinase activation. Samples were analyzed by in-gel kinase assay. A, Cells were treated with voltage (↯) at 0 min. After 20, 40, or 180 min the culture was treated a second time. B, Cells were treated 20 min after voltage treatment with E-FOL (F) or salicylic acid (SA). To compare with non-refractory cells, a second culture was directly treated with E-FOL (F) or salicylic acid (SA).
We now used this experimental setup to investigate the connection of voltage-induced signaling with the pathways activated by E-FOL and salicylic acid. Reviewing the results described above, the voltage treatment mimics salicylic acid-treatment most; both stimuli lead to a comparable MAP kinase activation in in-gel kinase assays, the activity mainly elutes in peak 4 of anion-exchange chromatography, and both stimuli do not result in H2O2 production. In accordance to these results, voltage-treated cells were refractory for salicylic acid treatment (Fig. 6B). In contrast, E-FOL application after voltage treatment resulted in a normal activation of MAP kinases. Thus voltage treatment results in the activation of the same specific MAP kinase pathway as salicylic acid treatment presumably by imitating the calcium signature of salicylic acid signaling.
DISCUSSION
A pivotal role of MAP kinases in the initiation of defense response of higher plants has been demonstrated in several cases. In the present study we focus on the analysis of how signals are integrated and specified by the activation of MAP kinases during the initiation of a defense response. MAP kinases integrate signals transduced by various mechanisms, e.g. calcium, receptor Tyr kinases, or G-proteins (Widmann et al., 1999), and an increasing number of publications demonstrate the activation of MAP kinases by diverse stimuli in plants (Ligterink, 2000). However, the data elaborated so far yielded a limited number of MAP kinases that are positioned upstream of a far bigger number of physiological responses. This discrepancy might be due to other signal transduction pathways and so-far-unknown MAP kinases cross-reacting with the known MAP kinase pathways. Recently, analysis of alfalfa cells by immunokinase assays revealed the activation of four different MAP kinases upon treatment with yeast elicitor (Cardinale et al., 2000). Immunoprecipitation limits the results in general to the proteins against which antibodies exist. To overcome this limitation we used a biochemical approach. Photoautotrophic tomato suspension culture cells were treated with an elicitor preparation of the tomato pathogen F. oxysporum lycopersicon (E-FOL). The treatment resulted in a strong, post-translational activation of a MAP kinase as revealed by in-gel kinase assay with myelin basic protein as substrate and immunoprecipitation with a phospho-Tyr-specific antibody. By partial purification on an anion-exchange column, we were able to separate the activity into four distinct protein kinase activities. The in-gel kinase assay of the separated fractions revealed in two of the four peaks two MBP-phosphorylating kinases of different molecular weights. The additional bands might be due to degradation products or they might represent additional kinases. Therefore up to six proteins are activated in response to one stimulus. The finding that the separated kinases are Tyr phosphorylated supports their identity as MAP kinases.
The simultaneous activation of several kinases was substantiated by two further sets of experimental evidence to rule out that complex formation with other proteins or different phosphorylation states account for the separation. In a previous study bohemine, olomoucine, and roscovitine, inhibitors of cyclin-dependent kinases, have been shown to also inhibit the MMK1 MAP kinase from alfalfa (Binarová et al., 1998). Along with these, the general protein kinase inhibitor staurosporine was applied. A comparison of the sensitivity profiles of the separated protein kinases toward the four inhibitors reveals characteristic differences, thus demonstrating the presence of proteins with distinct biochemical properties, and thus most likely different MAP kinases. Further we used antibodies directed against the alfalfa MAP kinases SIMK, MMK2, MMK3, and SAMK (Munnik et al., 1999) to immunoprecipitate the separated protein kinases. The MAP kinase activity of peaks 2 and 4 are precipitated by different antibodies, whereas no activity can be detected after precipitation of peaks 1 and 3 (Fig. 5B). Therefore the separation of the MAP kinase activities by ion-exchange chromatography, the differential inhibitor sensitivity and the differential cross-reaction with the four alfalfa MAP kinase antibodies support that at least four different MAP kinases are activated by E-FOL stimulation.
After establishing a purification procedure suitable to distinguish four different MAP kinases activated after elicitation with E-FOL, we compared this profile with that induced by several other stress-related stimuli. Given the diversity of the stimuli applied in this study, we anticipated that they elicit distinguishable responses. A test for different physiological responses is provided by the measurement of H2O2 production after elicitation of the cells. These experiments demonstrated, that only the fungal elicitors E-FOL and chitosan elicited H2O2 production, whereas the endogenous effectors PGA and salicylic acid as well as voltage treatment did not. However, the mRNA for the defense-related genes Pal and Lin6 are induced by all of these stimuli. Further analysis of the elution profiles of those MAP kinases activated by PGA, voltage, and salicylic acid demonstrated that these only induce subsets of the kinases activated by E-FOL or chitosan treatment. The use of overlapping sets of MAP kinases seems to be an economical way of plants to cope with the big numbers of different stimuli they are exposed to while maintaining the capability to elicit specific responses with a limited number of proteins.
We used the described analysis of MAP kinase profiles to compare the downstream effects invoked by voltage with the effects of the physiological stimuli characterized before. This comparison elucidated a striking similarity of the effects invoked by voltage and salicylic acid treatment. To further substantiate that both stimuli actually induce the same pathway we tested the refractory properties of the MAP kinases activated. The activity of MAP kinases is kept transient by a concomitantly induced phosphatase which inactivates the MAP kinase module. Thus, a second stimulation of the same pathway during the time of presence of the phosphatase results only in a weak activation of the MAP kinase: the refractory state. In accordance with this, voltage induced a refractory time period during which a second voltage treatment resulted in a highly reduced activation. Although this treatment did not interfere with signaling of E-FOL, the salicylic acid-induced activity was also markedly reduced. This strongly indicates that voltage indeed results in a natural response activating a MAP kinase pathway that is normally activated after reception of salicylic acid.
Calcium has been reported to be involved in signal transduction invoked by a variety of stimuli. There has been a great progress in calcium signaling research by the establishment of methods to record calcium concentrations in living cells by calcium dyes and the aequorin system (Knight et al., 1991). Enabled by these techniques spatial differences in calcium concentration have been reported as well as temporal patterns of several peaks of defined amplitudes and repeated spikes (Rudd and Franklin-Tong, 1999). Yet the significance of these observations for specific signaling remains to be elucidated. As we have shown in this study, calcium is a prerequisite for full activation of MAP kinases of tomato in response to stimulation with E-FOL or voltage. Voltage-induced signaling is in contrast to the E-FOL response sensitive to nifedipine, a known inhibitor of voltage-dependent calcium channels. This indicates that the applied voltage might act directly on voltage-gated calcium channels. We conclude from the refractory experiments with voltage and salicylic acid that both treatments resulted in the activation of the same signaling pathway presumably because the chosen voltage treatment resulted in a calcium influx and signature that mimics the salicylic acid-induced calcium signature. Application of voltage pulses to elevate intracellular calcium would open a field of potential applications to study various calcium-dependent pathways. Increasing the cytosolic calcium concentration in a controlled way by voltage treatment would provide a tool to specifically generate calcium signatures. Using this unique experimental approach it would be possible to address the physiological relevance of a distinct calcium signature for the induction of a specific physiological response.
MATERIALS AND METHODS
Growth of Suspension Culture Cells
Photoautotrophic suspension culture cells of Lycopersicon peruvianum were established by Beimen et al. (1992) and were subcultured every 2 weeks in Murashige and Skoog medium and incubated shaking under continuous light conditions with an atmosphere containing 2% (w/v) CO2. Cells were used for the experiments during the second week after subculturing. To ensure same starting conditions for all cultures of an experiment, cultures were mixed and divided again 2 h before treatment.
Stimuli and inhibitors were applied at the following concentrations unless indicated differently: E-FOL, 1 μg dry hyphae mL−1 culture; chitosan, 100 μg mL−1; PGA, 100 μg mL−1; salicylic acid, 250 μm; cycloheximide, 5 μg mL−1; αα-amanitin, 5 μm; gadolinium(III) chloride, 1 mm; nifedipine, 20 μm; EGTA, 8 mm; and calcium chloride 8 mm.
One percent (w/v) PGA (Sigma, St. Louis) was dissolved in 0.1 n NaOH and dialyzed for 18 h in water (Ohto et al., 1992). One percent (w/v) chitosan (Roth, Karlsruhe, Germany) was dissolved in 1 m acetic acid and dialyzed for 18 h in water.
Voltage Treatment
To generate an electrical field in culture vessels, two platinum wires were submerged as electrodes in a cell culture of 50 mL. The electrodes were designed as a large and a small circle creating a distance in-between of 25 mm. Voltage was applied for 10 s by two Gene Power Supply GPS200/400 (Pharmacia, Piscataway, NJ). A current of 0.7 A was observed at 60 V.
Preparation of an Elicitor from Fusarium oxysporum lycopersici
The pathogenic fungus F. oxysporum Schlecht.: Fr.f.sp. lycopersici (Sacc.) was obtained from the Centraalbureau voor Schimmelcultures (Baarn, The Netherlands). The fungus was cultured in a medium containing 50 g L−1 Glc, 8 g L−1 casamino acids, 0.5 g L−1 yeast extract, 0.2 g L−1 each MgSO4 and FeSO4, 20 mg L−1 CaCl2, and 1.5 mg L−1 each MnSO4 and Na2MoO4, in 25 mm potassium phosphate at pH 7.5. After 4 d of shaking at 29°C the culture was autoclaved, washed three times with water, and lyophilized.
Extraction of mRNA and RNA Gel-Blot Analysis
For the isolation of RNA, cells were harvested by centrifugation, snap frozen in liquid nitrogen, and ground in the presence of liquid nitrogen. Total RNA was isolated according to the method described in Chomczynski and Sacchi (1987). Northern-blot analysis was carried out as described previously (Godt and Roitsch, 1997).
H2O2 Determination
For H2O2 determination, 100 μL of culture supernatant was mixed with 800 μL of 70 μm luminol (5-amino-2,3-dihydro-1,4-phthalazinedione) in 50 mm KHPO4, pH 7.9. Light emission was started by addition of 100 μL of 12.5 mm K3Fe(CN)6 in water and quantified with a FB12 luminometer (Berthold, Germany).
Preparation of Crude Extracts
Cells were harvested by centrifugation, snap frozen in liquid nitrogen, and ground in the presence of liquid nitrogen. Extracts from ground cells were prepared in the following buffer: 100 mm HEPES/KOH pH 7.5, 5 mm EDTA, 5 mm EGTA, 10 mm dithiothreitol (DTT), 10 mm Na3VO4, 10 mm NaF, 50 mm β-glycerophosphate, 10% (w/v) glycerol, 7.5% (w/v) polyvinylpolypyrrolidone, 1 μg mL−1 antipain, 0.1 mm phenylmethylsulfonyl fluoride, and 1 mm benzamidine.
In-Gel Kinase Assay
For the determination of kinase activity in polyacrylamide gels, crude extracts were centrifuged for 10 min at 20,000g. An aliquot of the supernatant containing 40 μg of total protein as determined by Bradford assay (Bradford, 1976) was loaded on a 10% (w/v) polyacrylamide gel embedded with 0.3 mg mL−1 MBP (Upstate Biotechnology, Lake Placid, NY) in the separating gels as substrate for the kinase. As control for substrate specificity MBP was substituted by the same amount of histone or caseine. After electrophoresis proteins were renatured and assayed for kinase activity as described by Zhang and Klessig (1997). Activities were visualized by autoradiography and by a phosphor imager (Cyclone, Phosphor Storage Systems, Madison, WI).
In-Solution Kinase Assay
For kinase determination in solution, 5 μL of sample was mixed with reaction buffer to give a final volume of 15 μL containing 25 mm Tris/HCl, pH 7.5, 5 mm MgCl2, 1 mm EGTA, 1 mm DTT, 0.5 mg mL−1 MBP, 25 μm ATP, and 1 μCi [γγ-32P]ATP. Incubation at room temperature was stopped after 20 min by addition of 10 μL of 5× SDS sample buffer. The unincorporated [γγ-32P]ATP was separated from the MBP by SDS-gel electrophoresis. Activities were visualized and quantified using a phosphor imager (Cyclone, Phosphor Storage Systems).
Inhibitor Studies
The inhibitors bohemine (6-benzylamino-2-[3-hydroxypropylamino]-9-isopropylpurine), olomoucine (6-benzylamino-2-[2-hydroxyethylamino]-9-methylpurine), and A.2.3.9R (6-[3-hydroxybenzylamino]-2-[R]-[1-{hydroxymethyl} propylamino]-9-isopropylpurine) were synthesized (Havlicek et al., 1997) and prepared as 100 mm stock solutions in pure dimethyl sulfoxide. A.2.3.9R is derivative of roscovitine with an additional hydroxy group on the N6 benzyl ring. It is referred to as roscovitine throughout this publication. A 100 mm solution in dimethyl sulfoxide was diluted in water to a final concentration of 100 μm in the in-solution kinase assay. Staurosporine (Boehringer Mannheim, Basel) was used at a final concentration of 1 μm.
Immunoprecipitation with MMK Antibodies
The antibodies specific for SIMK (M23), MMK2 (H140), MMK3 (H141), and SAMK (M24) were a generous gift from H. Hirt (University of Vienna). One microliter of serum, 0.1% (v/v) Nonidet P-40, and 75 mm NaCl were added to 60 μL of the chromatography fraction to be analyzed. After 2 h of shaking at 4°C 20 μL of protein A-Sepharose (50% suspension, Calbiochem, San Diego) was added. After another 3 h we proceeded with washings and kinase assay exactly as described by Munnik et al. (1999) to ensure the same specificity.
Immunoprecipitation with Phospho-Tyr- Specific Antibody
For the immunoprecipitation 200 μg of total protein in a crude extract was brought to 150 mm NaCl and 1% (v/v) Nonidet P40. After addition of 1 μg of the phospho-Tyr specific monoclonal antibody 4G10 (UBI, Lake Placid, NY) the assay was shaken at 4°C for 2 h and, after the subsequent addition of 15 μL of protein A-Sepharose (50% suspension, Calbiochem), for another 4 h. Competing phospho-amino acids phospho-Ser, phospho-Thr, and phospho-Tyr were included in the precipitation mixture in a concentration of 1 mm. The protein A-Sepharose with the bound antigen was pelleted at 20,000g for 2 min and washed with 500 μL of 100 mm HEPES/KOH, pH 7.5, 5 mm EDTA, 5 mm EGTA, 10 mm DTT, 10 mm Na3VO4, 10 mm NaF, 50 mm β-glycerophosphate, 10% (w/v) glycerol, 1 μg mL−1 antipain, 0.1 mm phenylmethylsulfonyl fluoride, 1 mm benzamidine, 150 mm NaCl, and 1% (v/v) Nonidet P40. The pellet was resuspended in 40 μL of SDS-loading buffer and incubated at 37°C for 15 min and at 65°C for another 15 min. After centrifugation, the supernatant was analyzed by in-gel kinase assay with MBP as substrate.
Chromatographic Separation
Ground cells of a 50 mL of culture were used to prepare the crude extract with 20 mL of buffer A (25 mm Tris/HCl, pH 7.5, 5 mm MgCl2, 5 mm EDTA, 1 mm DTT, 5% [w/v] glycerol, 20 mm β-glycerophosphate, and 10 mm NaF) containing 0.1 mm phenylmethylsulfonyl fluoride, 1 mm benzamidine, and 1 mm Na3VO4. After 40 min of centrifugation at 120,000g, supernatant containing 20 mg of total protein was loaded on the strong anion-exchange column Resource Q (6 mL, Pharmacia). The column was washed with 30 mL of buffer A and then eluted with a 80-mL linear gradient of 0 to 500 mm NaCl in buffer A. Fractions were analyzed by in-solution kinase assay or in-gel kinase assay and quantified by phosphor imager. Relative activities depicted in Figure 4C were calculated by dividing the measured activity by the basal activity of unstimulated cells processed in the same way.
ACKNOWLEDGMENTS
The authors thank M.R. Knight for sharing stimulating ideas on the mechanism of voltage action and H. Hirt for the generous gift of the MMK antibodies.
Footnotes
This work was supported by scholarships from the Verband der Chemischen Industrie e.V. (to V.L.L.), Studienstiftung des Deutschen Volkes (to M.G.H.), and Alexander von Humboldt foundation (to A.K.S.), respectively, and by the Deutsche Forschungsgemeinschaft (grant no. Ro 758/4–1 to T.R.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010569.
LITERATURE CITED
- Beimen A, Witte L, Barz W. Growth characteristics and elicitor-induced reactions of photosynthetically active and heterotrophic cell suspension culture of Lycopersicon peruvianum (Mill.) Bot Acta. 1992;105:152–160. [Google Scholar]
- Binarová P, Dolezel J, Draber P, Heberle-Bors E, Strnad M, Bögre L. Treatment of Vicia faba root tip cells with specific inhibitors to cyclin-dependent kinases leads to abnormal spindle formation. Plant J. 1998;16:697–707. doi: 10.1046/j.1365-313x.1998.00340.x. [DOI] [PubMed] [Google Scholar]
- Blume B, Nürnberger T, Nass N, Scheel D. Receptor-mediated increase in cytoplasmic free calcium required for activation of pathogen defense in parsley. Plant Cell. 2000;12:1425–1440. doi: 10.1105/tpc.12.8.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bögre L, Ligterink W, Meskiene I, Barker PJ, Heberle-Bors E, Huskisson NS, Hirt H. Wounding induces the rapid and transient activation of a specific MAP kinase pathway. Plant Cell. 1997;9:75–83. doi: 10.1105/tpc.9.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Canagarajah BJ, Khokhlatchev A, Cobb MH, Goldsmith EJ. Activation mechanism of the MAP kinase ERK2 by dual phosphorylation. Cell. 1997;90:859–869. doi: 10.1016/s0092-8674(00)80351-7. [DOI] [PubMed] [Google Scholar]
- Cardinale F, Jonak C, Ligterink W, Niehaus K, Boller T, Hirt H. Differential activation of four specific MAPK pathways by distinct elicitors. J Biol Chem. 2000;275:36734–36740. doi: 10.1074/jbc.M007418200. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Striessnig J. Receptor sites for Ca2+ channel antagonists. Trends Pharmacol Sci. 1992;13:256–262. doi: 10.1016/0165-6147(92)90079-l. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Coquoz JL, Buchala A, Metraux JP. The biosynthesis of salicylic acid in potato plants. Plant Physiol. 1998;117:1095–1101. doi: 10.1104/pp.117.3.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Azevedo WF, Leclerc S, Meijer L, Havlicek L, Strnad M, Kim SH. Inhibition of cyclin-dependent kinases by purine analogues: crystal structure of human cdk2 complexed with roscovitine. Eur J Biochem. 1997;243:518–526. doi: 10.1111/j.1432-1033.1997.0518a.x. [DOI] [PubMed] [Google Scholar]
- Ehness R, Roitsch T. Co-ordinated induction of mRNAs for extracellular invertase and a glucose transporter in Chenopodium rubrum by cytokinins. Plant J. 1997;11:539–548. doi: 10.1046/j.1365-313x.1997.11030539.x. [DOI] [PubMed] [Google Scholar]
- Gelli A, Blumwald E. Hyperpolarization-activated Ca2+-permeable channels in the plasma membrane of tomato cells. J Membr Biol. 1997;155:35–45. doi: 10.1007/s002329900156. [DOI] [PubMed] [Google Scholar]
- Gelli A, Higgins VJ, Blumwald E. Activation of plant plasma membrane Ca2+-permeable channels by race specific fungal elicitors. Plant Physiol. 1997;113:269–279. doi: 10.1104/pp.113.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt DE, Roitsch T. Regulation and tissue-specific distribution of mRNAs for three extracellular invertase isoenzymes of tomato suggests an important function in establishing and maintaining sink metabolism. Plant Physiol. 1997;115:273–282. doi: 10.1104/pp.115.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R, Huang Y, Kieber J, Luan S. Identification of a dual-specificity protein phosphatase that inactivates a MAP kinase from Arabidopsis. Plant J. 1998;16:581–589. doi: 10.1046/j.1365-313x.1998.00327.x. [DOI] [PubMed] [Google Scholar]
- Hadwiger LA, Beckman JM. Chitosan as a component of pea-Fusarium solani interactions. Plant Physiol. 1980;66:205–211. doi: 10.1104/pp.66.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havlicek L, Hanus J, Vesely J, Leclerc S, Meijer L, Shaw G, Strnad M. Cytokinin-derived cyclin-dependent kinase inhibitors: synthesis and cdc2 inhibitory activity of olomoucine and related compounds. J Med Chem. 1997;40:408–412. doi: 10.1021/jm960666x. [DOI] [PubMed] [Google Scholar]
- Jabs T, Tschoepe M, Colling C, Hahlbrock K, Scheel D. Elicitor-stimulated ion fluxes and O2− from the oxidative burst are essential components in triggering defense gene activation and phytoalexin synthesis in parsley. Proc Natl Acad Sci USA. 1997;94:4800–4805. doi: 10.1073/pnas.94.9.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonak C, Kiegerl S, Ligterink W, Barker PJ, Huskisson NS, Hirt H. Stress signaling in plants: A mitogen-activated protein kinase pathway is activated by cold and drought. Proc Natl Acad Sci USA. 1996;93:11274–11279. doi: 10.1073/pnas.93.20.11274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano T, Sahashi N, Takahashi K, Uozumi N, Muto S. Salicylic acid induces extracellular superoxide generation followed by an increase in cytosolic calcium ion in tobacco suspension culture: the earliest events in salicylic acid signal transduction. Plant Cell Physiol. 1998;39:721–730. [Google Scholar]
- Knight MR, Campbell AK, Smith SM, Trewavas AJ. Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature. 1991;352:524–526. doi: 10.1038/352524a0. [DOI] [PubMed] [Google Scholar]
- Lebrun-Garcia A, Ouaked F, Chiltz A, Pugin A. Activation of MAPK homologues by elicitors in tobacco cells. Plant J. 1998;15:773–781. doi: 10.1046/j.1365-313x.1998.00269.x. [DOI] [PubMed] [Google Scholar]
- Ligterink W. MAP kinases in plant signal transduction: how many, and what for? Results Probl Cell Differ. 2000;27:11–27. doi: 10.1007/978-3-540-49166-8_2. [DOI] [PubMed] [Google Scholar]
- Mithoefer A, Ebel J, Bhagwat AA, Boller T, Neuhaus-Url G. Transgenic aequorin monitors cytosolic calcium transients in soybean cells challenged with beta-glucan or chitin elicitors. planta. 1999;207:566–574. [Google Scholar]
- Munnik T, Ligterink W, Meskiene I, Calderini O, Beyerly J, Musgrave A, Hirt H. Distinct osmo-sensing protein kinase pathways are involved in signalling moderate and severe hyper-osmotic stress. Plant J. 1999;20:381–388. doi: 10.1046/j.1365-313x.1999.00610.x. [DOI] [PubMed] [Google Scholar]
- Nürnberger T. Signal perception in plant pathogen defense. Cell Mol Life Sci. 1999;55:167–182. doi: 10.1007/s000180050283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohto MA, Nakamura-Kito K, Nakamura K. Induction of expression of genes coding for sporamin and beta-amylase by polygalacturonic acid in leaf-petiole cuttings of sweet potato. Plant Physiol. 1992;99:422–427. doi: 10.1104/pp.99.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond P, Farmer EE. Jasmonate and salicylate as global signals for defense gene expression. Curr Opin Plant Biol. 1998;1:404–411. doi: 10.1016/s1369-5266(98)80264-1. [DOI] [PubMed] [Google Scholar]
- Romeis T, Piedras P, Zhang S, Klessig DF, Hirt H, Jones JD. Rapid Avr9- and Cf-9-dependent activation of MAP kinases in tobacco cell cultures and leaves: convergence of resistance gene, elicitor, wound, and salicylate responses. Plant Cell. 1999;11:273–287. doi: 10.1105/tpc.11.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd JJ, Franklin-Tong VE. Calcium signaling in plants. Cell Mol Life Sci. 1999;55:214–232. doi: 10.1007/s000180050286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan CA. Oligosaccharide signalling in plants. Annu Rev Cell Biol. 1987;3:295–317. doi: 10.1146/annurev.cb.03.110187.001455. [DOI] [PubMed] [Google Scholar]
- Stratmann JW, Ryan CA. Myelin basic protein kinase activity in tomato leaves is induced systemically by wounding and increases in response to systemin and oligosaccharide elicitors. Proc Natl Acad Sci USA. 1997;94:11085–11089. doi: 10.1073/pnas.94.20.11085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Isobe M, Muto S. An increase in cytosolic calcium ion concentration precedes hypoosmotic shock-induced activation of protein kinases in tobacco suspension culture cells. FEBS Lett. 1997;401:202–206. doi: 10.1016/s0014-5793(96)01472-x. [DOI] [PubMed] [Google Scholar]
- Usami S, Banno H, Nishihama R, Machida Y. Cutting activates a 46-kilodalton protein kinase in plants. Proc Natl Acad Sci USA. 1995;92:8660–8664. doi: 10.1073/pnas.92.19.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- Xu H, Heath MC. Role of calcium in signal transduction during the hypersensitive response caused by basidiospore-derived infection of the cowpea rust fungus. Plant Cell. 1998;10:585–598. doi: 10.1105/tpc.10.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Du H, Klessig DF. Activation of the tobacco SIP kinase by both a cell wall-derived carbohydrate elicitor and purified proteinaceous elicitins from Phytophthora spp. Plant Cell. 1998;10:435–450. doi: 10.1105/tpc.10.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Klessig DF. Salicylic acid activates a 48-kD MAP kinase in tobacco. Plant Cell. 1997;9:809–824. doi: 10.1105/tpc.9.5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Klessig DF. Resistance gene N-mediated de novo synthesis and activation of a tobacco mitogen-activated protein kinase by tobacco mosaic virus infection. Proc Natl Acad Sci USA. 1998a;95:7433–7438. doi: 10.1073/pnas.95.13.7433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Klessig DF. The tobacco wounding-activated mitogen-activated protein kinase is encoded by SIPK. Proc Natl Acad Sci USA. 1998b;95:7225–7230. doi: 10.1073/pnas.95.12.7225. [DOI] [PMC free article] [PubMed] [Google Scholar]