Abstract
9-Chloro and 9-amino-2-methoxyacridines bearing different substituents in position 7, as well as their corresponding unsubstituted dimeric and tetrameric complexes, were investigated for in vitro antiproliferative properties against Leishmania infantum compared to toxicity towards human monocytes. The results clearly confirmed that several compounds of the 2-methoxyacridine series, together with their corresponding dimeric and tetrameric derivatives, had strong in vitro antiparasitic properties. Antileishmanial activity was shown to depend on the nature of both 7- and 9-substituted groups in monoacridines, while it varied according to the nature of the 9-substituted group and the length of the linker among bis- and tetra-acridines. The effects of acridine derivatives on DNA synthesis raised the hypothesis that DNA metabolism constituted their main target in Leishmania promastigotes; however, secondary effects on other biochemical pathways, including protein and lipid metabolism, were observed, suggesting that acridine compounds could be considered multitarget drugs.
Parasitic infections due to protozoan parasites remain a major public health concern affecting the lives of billions of people worldwide (16, 33). Among these diseases, leishmaniases, the vector-borne parasitic diseases resulting from infection of macrophages by obligate intracellular parasites of the genus Leishmania, continue to threaten millions of people worldwide (17). Clinical manifestations and evolution of the illness have been shown to depend greatly on the etiological agent. Nevertheless, three symptomatic forms, i.e., cutaneous, mucocutaneous, and visceral leishmaniases, have been described (17). A wide variety of treatment modalities have been used for all forms of leishmaniasis (7, 17, 22): pentavalent antimonial agents (widely employed since the 1940s), amphotericin B in its liposomal formulation, and, more recently, the oral antineoplastic agent miltefosine at present represent the best solutions (7, 11). However, none of these alternatives has been shown to be efficient and safe enough to be used as the first-line drug in all epidemiological scenarios (7, 27, 28).
Because of the many similarities between tumor cells and trypanosomatidae protozoa, and mainly because of their rapid replication, the urgent need for more selective and less toxic antileishmanial drugs has led to the hypothesis that anticancer agents could constitute possible antileishmanial candidates (8, 20). On this basis, various compounds of the acridine family have been investigated for their possible antiprotozoan action and demonstrated potent toxicity towards Leishmania parasites (12, 21, 30). Among these compounds, various members of the 9-amino-6-chloro-2-methoxyacridine series and their corresponding unsubstituted bisacridine derivatives were shown to display in vitro antitrypanosomal, antileishmanial, and antimalarial properties, and it was demonstrated that acridine moieties joined by a side chain could be more efficient than monoacridines (13, 14). In order to complete these data, we synthesized two series of 7-substituted 2-methoxyacridines bearing a 9-amino or a 9-chloro side chain together with corresponding unsubstituted bis- and tetra-acridine derivatives, and we assessed their in vitro activities against Leishmania infantum compared to their cytotoxicities to human cells. We then investigated their possible mechanisms of action on several parasite targets, such as the cell cycle, protein and lipid metabolism, membrane integrity, and mitochondrial activity.
MATERIALS AND METHODS
Strains and reagents.
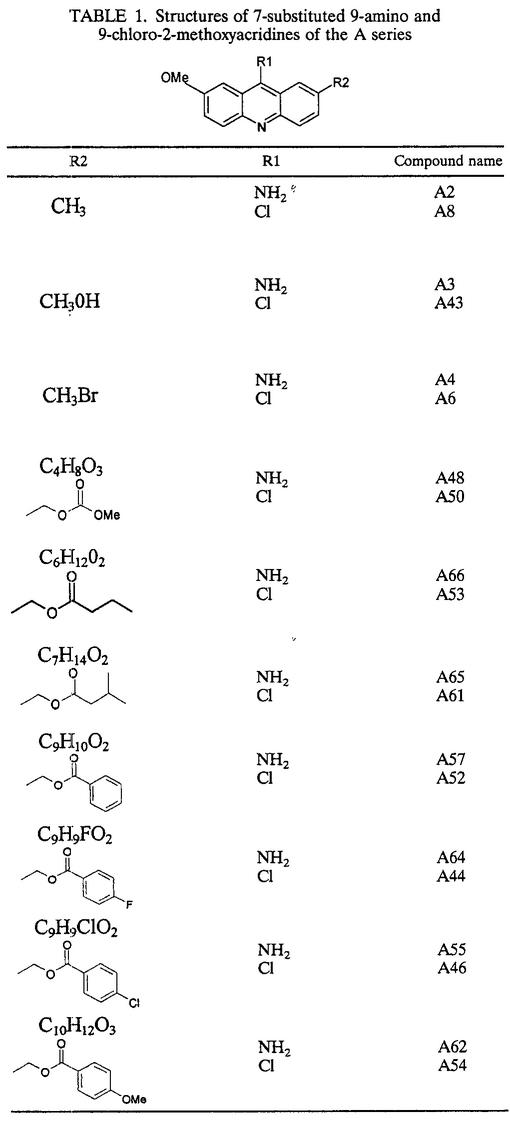
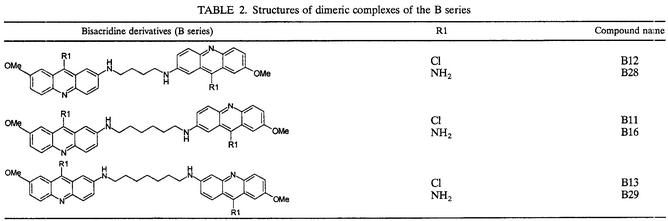
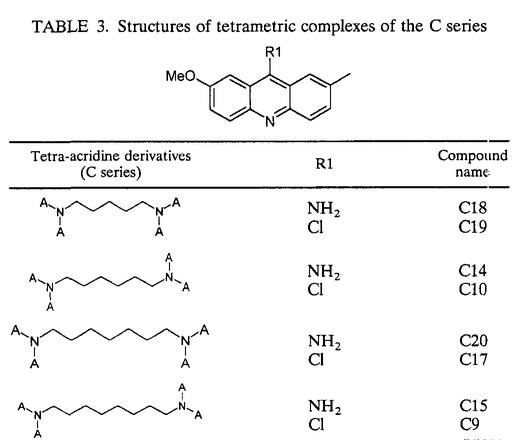
Series of 7-substituted 9-chloro and 9-amino-2-methoxyacridines (A series) together with bis- and tetra-acridine derivatives (B and C series) linked by various carbon chains were synthesized in the Laboratoire de Valorisation de la Chimie Fine, Université d'Aix-Marseille III (Site de Saint Jérome, Marseilles, France). The chemical structures of these compounds are described in Tables 1, 2, and 3. The synthesis of acridine derivatives and analysis of purity, including all nuclear magnetic resonance spectral data, were previously described by Sourdon et al. (26). Amphotericin B (Sigma, St. Louis, Mo.), pentamidine (Sigma), allopurinol (Sigma), ketoconazole (Calbiochem, San Diego, Calif.), and carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP) (Sigma) were used as standard drugs for positive controls. All compounds were dissolved in sterile dimethyl sulfoxide (DMSO) (analytical grade; Sigma) and stored frozen at −70°C until used. Antileishmanial activity was assessed with the referenced strain of L. infantum (MHOM/FR/78/LEM75).
TABLE 1.
Structures of 7-substituted 9-amino and 9-chloro-2-methoxyacridines of the A series
Mathematical models for prediction of physicochemical and biological properties.
Predicted values calculated for lipophilicity (log P, representing the n-octanol-water partition coefficient) and solubility in water (log S) were estimated by mathematical methods with ALOGPS version 2.0 software as described by Tetko et al. (29). Prediction of the acid-base ionization constant pKa of acridine derivatives was performed with Pallas 2.1 software (Compudrug International Inc., San Francisco, Calif.).
Predicted values of antileishmanial activity were investigated by using the chemistry software server PASS for prediction of biological activity spectra (http://www.ibmh.msk.su/PASS/), according to the mathematical model and the database developed by Poroikov et al. (24) and Lagunin et al. (18).
Antileishmanial activity against promastigotes.
L. infantum promastigotes in late log phase were incubated in RPMI medium supplemented with 12% fetal calf serum at an average of 105 cells/ml, and a range of acridine concentrations were aseptically incorporated into duplicate cultures (final DMSO concentration, less than 5%). Following a 48-h incubation at 25°C, promastigote growth was estimated by counting parasites with an hemacytometer. The 50% inhibitory concentration (IC50) was defined as the concentration of drug necessary to inhibit 50% of parasite growth.
Antileishmanial activity against intracellular amastigotes.
Intracellular amastigote cultures were performed in human monocyte-derived macrophages as previously described by Ogunkolade et al. (23). Maturation of monocytes into adherent macrophages was performed by treating exponentially growing monocytes (105 cells/ml) with 1 μM phorbol myristate acetate (Sigma). After a 48-h incubation at 37°C (5% CO2) in chamber slides (Fisher, Paris, France), cells were rinsed with fresh medium and suspended in RPMI medium containing stationary-phase promastigotes (cell/promastigotes ratio of 1:10). After a 24-h incubation at 37°C (5% CO2), promastigotes were removed by four successive washes with fresh medium. Adapted dilutions of chemical compounds were added in duplicate chambers, and cultures were incubated for 96 h at 37°C (5% CO2). Negative controls treated with solvent (DMSO) and positive controls containing a range of amphotericin B (Sigma) concentrations were added to each set of experiments. At the end of the incubation period, cells were harvested with analytical grade methanol (Sigma) and stained with 10% Giemsa stain (Eurobio, Paris, France). The percentage of infected macrophages in each assay was determined microscopically at a magnification of ×1,000. The IC50 was defined as the concentration of drug necessary to produce a 50% decrease of infected macrophages.
Toxicity against human monocytes.
The in vitro toxicities of acridine derivatives were assessed on human monocytes maintained in RPMI medium (Eurobio) supplemented with 10% fetal calf serum (Eurobio) at 37°C in 5% CO2 and replicated every 7 days. A range of concentrations was incorporated in late-log-phase monocytes (105 cells/ml), and cultures were incubated at 37°C with 5% CO2. After a 72-h incubation, cell growth was measured by counting monocytes in an hemacytometer. The IC50 was defined as the concentration of drug required to induce a 50% decrease of cell growth. An in vitro selective index (SI), corresponding to the ratio between antiparasitic and cytotoxic activities, was calculated according to the following formula: SI = IC50 observed for human monocytes/IC50 observed for intracellular amastigotes.
Effects of 9-amino derivatives on promastigote cell cycle and protein synthesis.
Duplicate cultures of L. infantum promastigotes (105 cells/ml) in late log phase were incubated at 25°C in RPMI medium supplemented with 12% fetal calf serum, and various concentrations of acridine derivatives were incorporated into the cultures. After a 28-h incubation, the cell cycle and total protein content were assessed as described by Crissman et al. (6). Cells were fixed with 70% methanol, centrifuged at 1,500 rpm in a Jouan C3i centrifuge for 5 min, and suspended in 1 ml of RNase solution (50 μg/ml in phosphate-buffered saline [PBS] buffer) at 37°C for 30 min. DNA was stained with 10 μl of propidium iodide (1 mg/ml), total protein was stained with 10 μl of fluorescein isothiocyanate (1 mg/ml), and cells were analyzed by flow cytometry. Fluorescences measured in parasites incubated with 7.5 μM pentamidine and 0.35 mM allopurinol were taken as positive controls.
Effects of 9-amino derivatives on membrane potential.
Membrane potential was analyzed according to the method described by Shapiro (25) and adapted by Azas et al. (4), using a carbocyanine dye, 3,3′-dipentyloxacarbocyanine iodide. Duplicate cultures of promastigotes in the exponential growth phase containing a range of acridine concentrations were incubated for 3 h at 25°C. At the end of the incubation period, 3,3′-dipentyloxacarbocyanine iodide was incorporated into each assay at a final concentration of 0.5 μM, and promastigotes were analyzed by flow cytometry. The fluorescence measured in parasites incubated with 7.5 μM amphotericin B was taken as a positive control.
Effects of 9-amino derivatives on intracellular lipid droplets.
Neutral lipid and polar lipid droplets were assessed as described by Greenspan et al. (15). L. infantum promastigotes (105 cells/ml) in late log phase were incubated at 25°C in RPMI medium supplemented with 12% fetal calf serum, and various concentrations of acridine compounds were added in duplicate. After a 48-h incubation, cells were centrifuged at 1,500 rpm for 5 min and suspended in PBS buffer. Nile Red (Sigma) at a final concentration of 1 μg/ml was incorporated into each assay, and cells were incubated at room temperature for 7 min and washed three times with PBS buffer. Lipid droplets were analyzed by flow cytometry. The fluorescence measured in parasites incubated with 5 μM ketoconazole was taken as a positive control.
Effects of 9-amino derivatives on mitochondrial potential.
Variations in the mitochondrial potential induced by acridine derivatives were measured by tetramethylrosamine (Molecular Probes) incorporation. Duplicate promastigote cultures (5 × 105 cells/ml) in exponential log phase were treated with various concentrations of acridine derivatives and incubated at 25°C for 30 min. At the end of the incubation period, parasites were harvested by 10 min of centrifugation (1,500 rpm) and suspended in fresh medium containing tetramethylrosamine (Sigma) at a final concentration of 200 nM. Following 30 min of incubation at 25°C, cells were centrifuged, rinsed with fresh medium, and analyzed by flow cytometry. The fluorescence measured in parasites incubated with 7.5 μM FCCP was taken as a positive control.
Flow cytometric analysis.
Promastigotes were analyzed on a Facscan analytical flow cytometer (Becton-Dickinson, Paris, France) equipped with a 15-mV, 488-nm, air-cooled argon ion laser. Ten thousand cells were acquired for each analysis on the basis of their green or red fluorescence intensity. The percentages of parasites in the different phases of the cell cycle were analyzed by using Modfit software (Becton-Dickinson).
Statistical analysis.
The Spearman rank correlation test was used for studying coupled variables. Analysis was performed with Statgraphics plus software (Statistical Graphics Corporation, Englewood Cliffs, N.J.).
RESULTS
Complete data on the physicochemical properties and antiproliferative activities of the 7-subsituted 9-chloro- and 9-amino-2-methoxyacridines (A series) as well as the corresponding dimeric and tetrameric derivatives (B and C series) are summarized in Tables 4, 5, and 6, respectively. The lipophilicity of each chemical compound was estimated by prediction of the n-octanol-water partition coefficient, log P, which is defined as the ratio of the concentration in an immiscible solvent such as n-octanol to the concentration in the aqueous phase. All of the partition coefficients reported in Tables 4, 5, and 6 were greater than 1, indicating that acridines (A series), bisacridines (B series), and tetra-acridines (C series) exhibited high lipophilicity. Nevertheless, the lipophilicity varied according to molecule length and depended on the nature of the 9-substituted group: monoacridines displayed lower lipophilicity than bis- and tetra-acridines, and compounds bearing a 9-amino group showed lower lipophilicity than derivatives bearing a 9-chloro group. The data also demonstrated that all chemical compounds exhibited weak solubility in water, and as expected, solubility decreased with increasing lipophilicity. Predicted values of biological activities were obtained by comparing the chemical structure of each compound with structures or substructures of more than 30,000 well-known biologically active drugs. These values represented the probability Pa of each compound being active and illustrated its degree of similarity with well-known antileishmanial molecules. On this basis, a Pa of >0.7 signified that the corresponding compound was very likely to show activity in experiments, a Pa of >0.5 and <0.7 indicated that the compound was likely to show activity in experiments, and a Pa of <0.5 implied that the compound was unlikely to show activity in experiments. The predicted probabilities presented in Table 4 varied between 0.45 and 0.8, showing that almost all acridines were likely to have significant antileishmanial activity. Two compounds, A2 and A3, displayed higher Pa (0.748 and 0.764, respectively), indicating that their chemical structures were closely similar to those of active compounds. The predicted values calculated for bisacridines (Table 5) were higher than 0.7, while those for tetra-acridines were lower than 0.3. These results suggested that in contrast to the case for acridines and bisacridines, which were very likely to demonstrate antiparasitic activity, tetra-acridines were not very similar to known active drugs. Prediction of the acid-base ionization constant pKa was performed for acridine derivatives bearing a 9-amino group (Table 4); all of the predicted pKas except those of A3 and A43 varied between 6 and 7.
TABLE 4.
Toxicities and antileishmanial activities of 7-substituted 9-chloro and 9-aminoacridines of the A series
| Compound | R1 | R2 | Physicochemical propertiesa
|
Pab | Toxicity (IC50, μM) | Antileishmanial activity (IC50, μM)c
|
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Log P | Log S | pKa | PRO | AMA | SI | |||||
| A2 | NH2 | CH3 | 3.20 | −3.94 | 7.17 | 0.748 | 73.4 | 0.5 | 0.4 | 183.5 |
| A3 | NH2 | CH3OH | 2.05 | −3.90 | 14.14 | 0.764 | 56.6 | 5.4 | 4.1 | 13.8 |
| A4 | NH2 | CH3Br | 3.66 | −5.19 | 6.60 | 0.650 | 86.2 | 3.6 | 4.6 | 18.7 |
| A48 | NH2 | C4H8O3 | 2.88 | −4.63 | 6.83 | 0.667 | 54.4 | 2.4 | 1.3 | 41.8 |
| A66 | NH2 | C6H12O2 | 3.67 | −5.00 | 6.83 | 0.660 | 53.9 | 3.1 | 2.5 | 21.5 |
| A65 | NH2 | C7H14O2 | 3.99 | −5.22 | 6.83 | 0.631 | 5.4 | 2.2 | Toxic | |
| A57 | NH2 | C9H10O2 | 4.33 | 5.77 | 6.65 | 0.658 | 6.9 | 0.4 | 0.2 | 34.5 |
| A64 | NH2 | C9H9FO2 | 4.50 | −6.36 | 6.65 | 0.536 | 19.9 | 1.3 | 0.6 | 33.2 |
| A55 | NH2 | C9H9ClO2 | 4.91 | −6.48 | 6.65 | 0.702 | 152.2 | 76.1 | 45.6 | 3.3 |
| A62 | NH2 | C10H12O3 | 4.23 | −6.04 | 6.67 | 0.669 | 3.2 | 1.9 | Toxic | |
| A8 | Cl | CH3 | 4.35 | −5.04 | 0.686 | 171.6 | 1.1 | 2.3 | 74.6 | |
| A43 | Cl | CH3OH | 3.27 | −4.81 | 13.99 | 0.713 | 91.3 | 182.6 | 9.1 | 10.1 |
| A6 | Cl | CH3Br | 4.99 | −6.18 | 0.596 | 95.1 | 50.5 | 14.8 | 6.4 | |
| A50 | Cl | C4H8O3 | 4.19 | −5.61 | 0.616 | 226.1 | 150.7 | 75.3 | 3.0 | |
| A53 | Cl | C6H12O2 | 4.90 | −5.95 | 0.613 | 109.2 | 50.9 | 42.5 | 2.5 | |
| A61 | Cl | C7H14O2 | 5.07 | −6.19 | 0.587 | 209.6 | 27.9 | 36.4 | 5.7 | |
| A52 | Cl | C9H10O2 | 5.31 | −6.96 | 0.605 | 132.3 | 136.5 | Toxic | ||
| A44 | Cl | C9H9FO2 | 5.00 | −7.28 | 0.482 | 189.4 | 197.1 | 44.2 | 4.3 | |
| A46 | Cl | C9H9ClO2 | 5.42 | −5.38 | 0.644 | 155.3 | 156.3 | 155.4 | ||
| A54 | Cl | C10H12O3 | 5.31 | −6.97 | 0.615 | 122.5 | 125.4 | 122.6 | ||
Log P lipophilicity; log S, solubility in water.
Pa, predicted value for antileishmanial activity.
PRO, activity towards Leishmania promastigotes; AMA, activity towards Leishmania amastigotes; SI, ratio between antileishmanial activity on amastigotes and toxicity.
TABLE 5.
Toxicities and antileishmanial activities of dimeric complexes of the B seriesa
| Compound | R1 | Log P | Log S | Pa | Toxicity (IC50, μM) | Antileishmanial activity (IC50, μM)
|
||
|---|---|---|---|---|---|---|---|---|
| Promastigotes | Amastigotes | SI | ||||||
| B28 | NH2 | 4.82 | −7.95 | 0.757 | 8.9 | 5.2 | Toxic | |
| B16 | NH2 | 5.61 | −7.88 | 0.753 | 127.4 | 1.3 | 0.8 | 159.2 |
| B29 | NH2 | 6.55 | −7.78 | 0.753 | 8.1 | 8.8 | Toxic | |
| B12 | Cl | 6.58 | −9.26 | 0.709 | 125.1 | 24.1 | 10.3 | 12.1 |
| B11 | Cl | 7.22 | −9.17 | 0.706 | 159.3 | 60.2 | 56.2 | 2.8 |
| B13 | Cl | 7.89 | −9.04 | 0.706 | 114.4 | 115.3 | 118.2 | |
Log P, lipophilicity; log S, solubility in water; Pa, predicted values for antileishmanial activity; SI, ratio between antileishmanial activity on amastigotes and toxicity.
The toxicities of chemical compounds were assessed with the human monocyte cell line THP1. The results demonstrated that many acridine derivatives of the A, B, and C series displayed weak toxicity towards human cells, with IC50s greater than 50 μM. However, they suggested that the biological properties depended greatly on the nature of the 9-substituted group: compounds bearing a 9-amino group appeared to be more active on human cells than compounds bearing a 9-chloro group. Antileishmanial activity was explored for both extracellular promastigote and intracellular amastigote forms. The results displayed in Tables 4, 5, and 6 indicate that acridines (A series), bisacridines (B series), and tetra-acridines (C series) could significantly inhibit the growth of Leishmania parasites. 9-Amino derivatives displayed higher antileishmanial activity than 9-chloro derivatives, suggesting that the 9-amino group was responsible for strong interactions with macromolecules or intracellular structures. Two compounds of the A series, A2 and A57, showed strong antileishmanial activity, with an IC50s lower than 1 μM on both promastigote and amastigote forms; however, only compound A2 exhibited a significant SI, of 183.5. The results for bisacridines revealed that compound B16 exhibited a potent antileishmanial activity with a significant SI of 159.2, while data for tetra-acridines established that compound C20 presented a strong antileishmanial activity with a selective index of 139.4. Toxicity and antileishmanial activity did not depend on the physicochemical properties of the compounds, since no correlation could be found by the Spearman rank correlation test (P > 0.05) between lipophilicity (log P) or acid-base ionization constant (pKa) and the IC50 obtained with human cells, Leishmania promastigotes, or Leishmania amastigotes. On the other hand, no significant correlation could be found between predicted probabilities (Pa) and biological activities revealed in experiments.
The effects of 7-substituted 9-amino-2-methoxyacridines and corresponding bis- and tetra-acridines on Leishmania biochemical pathways are reported in Table 7. Experiments were performed with comparisons to well-known inhibitors of Leishmania biochemical pathways, such as the inhibitor of DNA synthesis pentamidine, the inhibitor of protein synthesis allopurinol, the pore-forming antibiotic amphotericin B, the inhibitor of ergosterol synthesis ketoconazole, and the inhibitor of mitochondrial activity FCCP.
TABLE 7.
Effects of 7-substituted 9-aminomethoxyacridines and related bis- and tetra-acridines on Leishmania biochemical pathways
| Compound | Effect at the IC50 (% compared to control culture) on:
|
|||||
|---|---|---|---|---|---|---|
| S + G2M phase | Protein content | Membrane potential | Polar lipids | Neutral lipids | Mitochondrial potential | |
| A2 | 76.9 | 78.2 | 100.3 | 152.3 | 114.2 | 99.9 |
| A3 | 80.2 | 77.4 | 99.2 | 98.4 | 99.5 | 100.1 |
| A4 | 84.5 | 82.6 | 100.4 | 102.5 | 100.4 | 98.9 |
| A48 | 96.2 | 82.9 | 100.1 | 128.6 | 105.8 | 95.8 |
| A66 | 78.6 | 83.8 | 99.3 | 62.1 | 85.3 | 100.2 |
| A65 | 86.5 | 80.3 | 100.1 | 99.8 | 100.2 | 100.4 |
| A57 | 79.7 | 86.5 | 98.2 | 96.5 | 100.4 | 99.6 |
| A64 | 77.5 | 94.1 | 99.1 | 80.2 | 92.1 | 93.5 |
| A55 | 95.2 | 100.1 | 100.1 | 97.6 | 95.4 | 98.4 |
| A62 | 81.4 | 77.3 | 99.7 | 99.1 | 96.3 | 100.4 |
| B28 | 85.2 | 76.7 | 99.7 | 155.2 | 113.4 | 101.1 |
| B16 | 75.3 | 72.8 | 96.4 | 115.3 | 106.4 | 97.6 |
| B29 | 81.8 | 84.7 | 98.6 | 194.2 | 124.6 | 98.4 |
| C19 | 83.4 | 73.3 | 100.1 | 98.8 | 100.2 | 102.3 |
| C20 | 77.1 | 92.2 | 99.9 | 100.1 | 99.1 | 96.3 |
| C14 | 87.1 | 63.5 | 99.3 | 105.5 | 98.6 | 98.6 |
| C15 | 84.3 | 76.1 | 98.6 | 99.5 | 100.3 | 101.2 |
| Pentamidine | 58.6 | |||||
| Allopurinol | 51.1 | |||||
| Amphotericin B | 55.2 | |||||
| Ketoconazole | 71.3 | 92.4 | ||||
| FCCP | 10.2 | |||||
The effects of acridine derivatives on DNA synthesis were estimated by cell cycle assessments after 28 h of treatment (Table 7). All acridine derivatives induced a dose-dependent decrease of the S plus G2M phases of the cell cycle, indicating a significant DNA synthesis inhibition. On the other hand, none of the compounds appeared to be as efficient as pentamidine at the IC50. The most important decreases in DNA synthesis were observed for compounds A2, B16, and C20 (76.9, 75.3, and 77.1%, respectively, compared to the control culture), while compounds A48 and A55 displayed a very slight effect. No correlation could be obtained between the percentages of inhibition of the S plus G2M phases and the corresponding antileishmanial activities by the Spearman correlation test (P > 0.5).
Assessment of intracellular protein contents (Table 7) was performed in comparison to the purine analogue allopurinol. The results demonstrated that acridine derivatives also disturbed protein metabolism, since almost all compounds of the A, B, and C series induced a dose-dependent decrease in protein amounts. The most active compound, C14, produced a 37% protein inhibition compared to the control culture, illustrating a strong alteration of protein metabolism. However, no correlation could be obtained between protein decrease and antileishmanial activity (P > 0.5) by the Spearman correlation test.
Nile red was used for studying lipid metabolism (Table 7). This vital dye emits a predominantly red fluorescence in polar hydrophobic domains (phospholipids) and a yellow fluorescence in neutral hydrophobic domains. The effects of acridine derivatives on phospholipid amounts were quantified by the dye-related red fluorescence. After a 24-h treatment, only a few derivatives of the A series were shown to modify lipid metabolism: compounds A2 and A48 induced a significant increase of phospholipid contents (152.3 and 128.6%, respectively, compared to the control culture), while A66 and A64 significantly reduced this parameter (62.1 and 80.2%, respectively, compared to the control culture). Surprisingly, all bisacridines (B series) favored the accumulation of phospholipids within parasites, while tetra-acridines (C series) appeared to be inefficient. The effects of acridine derivatives on neutral lipids were measured by the dye-related yellow-green fluorescence. The results demonstrated that acridines (A2 and A48) and bisacridines (B28, B16, and B29) responsible for phospholipid accumulation also induced a slight increase of neutral lipid droplets, while compound A66, which was shown to inhibit phospholipid synthesis, did not modify the neutral lipid amount. No correlation between lipid droplet amounts and IC50s obtained for promastigotes could be observed by the Spearman rank correlation test, suggesting that lipid metabolism did not represent the main biochemical target of acridine compounds.
Plasma membrane and mitochondrial membrane potentials were investigated for identifying pore-forming compounds and inhibitors of energetic metabolism (Table 7). The results showed that acridine derivatives did not affect either membrane integrity or mitochondrial activity, since the majority of the promastigote population maintained a normal mitochondrial or plasma membrane potential even at concentrations higher than antiproliferative values.
DISCUSSION
The results observed in the present study clearly confirmed that several compounds of the 2-methoxyacridine series together with their corresponding dimeric and tetrameric derivatives could exert in vitro activities against protozoa of the genus Leishmania. Nevertheless, they also indicated that biological properties varied considerably according to the chemical nature of each compound.
Among derivatives of the monoacridine series, both 9-substituted (R1) and 7-substituted (R2) groups were shown to play an important role in the ability of the compounds to inhibit parasite and cell growth. Compounds bearing a 9-amino group were more active than the corresponding 9-chloro-substituted derivatives, suggesting that although no significant correlation could be found between the ionization constant pKa and biological abilities, ionization events, which are responsible for accumulation of acridine compounds in the food vacuoles of parasites (13), could greatly influence antileishmanial activity. Compounds bearing a 7-methyl group exhibited the most selective antileishmanial activity, revealing that conformational properties related to the length and the position of the 7-substituted group could also modulate the reactivities of the compounds with parasite structures or macromolecules; according to this hypothesis, simple planar molecules better interfered with cellular targets than crowded structures.
7-Methyl-9-amino-2-methoxyacridine (A2) and 7-methyl-9-chloro-2-methoxyacridine (A8), the most efficient antileishmanial compounds of the monoacridine series, were used as starting materials for the synthesis of dimeric and tetrameric derivatives with multiple binding sites. Various alkanediamines (butanediamine, hexanediamine, heptanediamine, and octanediamine) were used as linkers for acridine moieties. Among dimeric derivatives, compound B16, corresponding to 7-methyl-9-amino-2-methoxyacridines joined by an hexanediamine side chain, displayed the most selective antileishmanial activity (SI = 159.2), while compound C20, corresponding to 7-methyl-9-amino-2-acridines joined by an heptanediamine linker, was the most efficient member of the tetrameric series (SI = 139.4). The significant influence of the 9-substituted group (R1) on biological properties was confirmed with dimeric and tetrameric derivatives, since 9-aminoacridine moieties remained more active than 9-chloro moieties. Moreover, the distance between the acridine rings represented another important parameter. In dimeric derivatives, the optimal distance was found by lengthening the linker chain from four carbons to six, while a seven-carbon linker was necessary for a maximal effect in tetrameric derivatives. These data confirmed that strong interactions with cellular entities could be obtained with molecules containing two or more acridine rings (5); however, no evolution from monofunctional to bifunctional interaction could be found, since monoacridines remained more efficient antileishmanial compounds than their corresponding dimeric and tetrameric complexes.
It is well established that the cytostatic activities of acridines towards tumor cells depend on the capacity of the molecules to prevent nucleic acid synthesis by intercalating with the base pairs of the DNA helix (8). On this basis, it has been proposed that the higher-order structure of DNA could be better controlled by a complexation using ligands with multiple binding sites and that bisintercalation with polymeric molecules could greatly increase antitumor properties (5, 19). This possibility was extensively verified with human cells: bis or tetra-acridines with multiple binding sites, which were considered as alternative methods for avoiding the efflux mechanism in multi-drug-resistant cells, were shown to be more effective antagonists of DNA metabolism (2, 3,9). However, the consequences of intercalation and bisintercalation for cell or parasite growth were less evident, suggesting that other cellular targets could interact with acridine rings (14). In order to identify these possible targets, we explored the effects of 7-substituted 9-aminoacridines and their corresponding dimeric and tetrameric derivatives on various Leishmania-specific entities or biochemical pathways.
Inhibition of DNA synthesis represents the most probable mechanism of acridine toxicity towards Leishmania (20, 30, 31): as expected, all acridine derivatives induced a dose-dependent drop in DNA synthesis, indicating strong interactions with DNA metabolism. Although no correlation could be found between DNA synthesis inhibition and antiproliferative activity, compounds A2, B16, and C20, the most selective antileishmanial derivatives, showed the strongest DNA-inhibitory properties. These results indicated that DNA metabolism would be the most important target for antileishmanial activity, but they suggested that additional cytoplasmic targets might interfere with this mechanism. Interactions of acridines with protein metabolism and enzymatic activities have also been considered an important aspect of acridine toxicity (10, 32); acridines have been shown to bind with proteins, to interfere with transcription, and to inhibit various enzymes vital to living organisms, such as protein kinase C. The acridine-induced reduction of protein amounts observed in the present study confirmed this ability, although it could not fully explain toxicity towards Leishmania. Inhibition of energetic metabolism was considered as another possible mechanism of toxicity towards Leishmania, since ultrastructural alterations in Leishmania mitochondrion could be observed following in vitro acridine treatment (20). This hypothesis was not confirmed by our results: none of the acridine derivatives tested could primarily affect the activity of mitochondria within promastigotes. Nevertheless, intercalation of acridine rings with kinetoplastic DNA in a compartment of the mitochondrion could modify mitochondrion morphology and hamper division of the parasite (13, 20). On the other hand, additional side effects, probably due to the strong lipophilic properties of the compounds, were shown to produce variations in intracellular lipid amounts.
In conclusion, the results observed in the present study confirmed that the promising antileishmanial activity of acridine compounds could be related to a multitarget mechanism of action. Their effects on DNA synthesis raised the possibility that the primary target involves DNA metabolism through intercalation with base pairs or inhibition of processes involving DNA-protein interaction, such as topoisomerase and telomerase activities (1, 27), while secondary effects could involve various other biochemical pathways, including protein and lipid metabolism. Additional experiments with in vitro or in vivo models should be performed in order to better define the antileishmanial activities of acridines and to identify their different mechanisms of action towards human cells and parasites.
TABLE 2.
Structures of dimeric complexes of the B series
TABLE 3.
Structures of tetrametric complexes of the C series
TABLE 6.
Toxicities and antileishmanial activities of tetrameric complexes of the C seriesa
| Compound | R1 | Log P | Log S | Pa | Toxicity (IC50, μM) | Antileishmanial activity (IC50, μM)
|
||
|---|---|---|---|---|---|---|---|---|
| Promastigotes | Amastigotes | SI | ||||||
| C19 | NH2 | 7.96 | −9.22 | <0.3 | 95.4 | 2.4 | 1.6 | 59.6 |
| C20 | NH2 | 8.42 | −9.16 | <0.3 | 69.7 | 0.9 | 0.5 | 139.4 |
| C14 | NH2 | 8.24 | −9.19 | <0.3 | 70.6 | 9.4 | 7.4 | 9.5 |
| C15 | NH2 | 8.45 | −9.12 | <0.3 | 45.9 | 0.9 | 0.6 | 76.5 |
| C17 | Cl | 9.95 | −10.63 | <0.3 | 65.1 | 68.4 | 51.4 | 1.2 |
| C18 | Cl | 9.75 | −10.69 | <0.3 | 66.6 | 65.1 | 30.2 | 2.2 |
| C9 | Cl | 10.05 | −10.59 | <0.3 | 64.2 | 66.4 | 44.1 | 1.4 |
| C10 | Cl | 9.85 | −10.65 | <0.3 | 65.8 | 68.1 | 28.8 | 2.3 |
Log P, lipophilicity; log S, solubility in water; Pa, predicted values for antileishmanial activity; SI, ratio between antileishmanial activity on amastigotes and toxicity.
REFERENCES
- 1.Adams, A., J. M. Guss, W. A. Denny, and L. P. Wakelin. 2002. Crystal structure of 9-amino-N-[2-(4-morpholinyl)ethyl]-4-acridinecarboxamide bound to d(CGTACG)2: implications for structure-activity relationships of acridinecarboxamide topoisomerase poisons. Nucleic Acids Res. 30:719-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alberti, P., J. Ren, M. P. Teulade-Fichou, L. Guittat, J. F. Riou, J. Chaires, C. Helene, J. P. Vigneron, J. M. Lehn, and J. L. Mergny. 2001. Interaction of an acridine dimer with DNA quadruplex structures. J. Biomol. Struct. Dyn. 19:505-513. [DOI] [PubMed] [Google Scholar]
- 3.Antonini, I., P. Polucci, A. Magnano, and M. Sante. 2001. Synthesis, antitumor cytotoxicity and DNA-binding of novel N-5,2-di(ω-aminoalkyl)-2,6-dihydropyrazolo[3,4,5-k]acridine-5-carbomamides. J. Med. Chem. 56:3329-3333. [DOI] [PubMed] [Google Scholar]
- 4.Azas, N., C. Di Giorgio, F. Delmas, M. Gasquet, and P. Timon-David. 1997. Leishmania infantum promastigotes: flow cytometry as a possible tool for assessing the effects of drugs on cellular functions. Exp. Parasitol. 87:1-7. [DOI] [PubMed] [Google Scholar]
- 5.Chen, T. K., R. Fico, and E. S. Canellakis. 1978. Diacridines, bifunctional intercalators. Chemistry and antitumor activity. J. Med. Chem. 21:868-874. [DOI] [PubMed] [Google Scholar]
- 6.Crissman, H. A., Z. Darzynkiewicz, J. A. Steinkamp, and R. A. Tobey. 1990. Simultaneous fluorescent labelling of DNA, RNA, and protein. Methods Cell Biol. 33:305-314. [DOI] [PubMed] [Google Scholar]
- 7.Croft, S. L., and Y. Yardley V. 2002. Chemotherapy of leishmaniasis. Curr. Pharmacol. 8:319-342. [DOI] [PubMed] [Google Scholar]
- 8.Demeunynck, M., F. Charmantray, and A. Martelli. 2001. Interest of acridine derivatives in the anticancer chemotherapy. Curr. Pharmacol. Des. 7:1703-1724. [DOI] [PubMed] [Google Scholar]
- 9.Denny, W. A., G. J. Atwell, B. C. Baguley, and L. P. Wakelin. 1985. Potential antitumor agents. Synthesis and antitumor activity of new classes of diacridines. The importance of the linker chain rigidity for DNA binding kinetics and biological activity. J. Med. Chem. 28:1568-1574. [DOI] [PubMed] [Google Scholar]
- 10.Finlay, G. J., and B. C. Baguley. 2000. Effects of protein binding on the in vitro activity of antitumour acridine derivatives and related anticancer drugs. Cancer Chemother. Pharmacol. 45:417-422. [DOI] [PubMed] [Google Scholar]
- 11.Fischer, C., A. Voss, and J. Engel. 2001. Development status of miltefosine as first oral drug in visceral and cutaneous leishmaniasis. Med. Microbiol. Immunol. (Berlin) 190:85-87. [DOI] [PubMed] [Google Scholar]
- 12.Gamage, S. A., D. P. Figgit, S. J. Wojcik, R. K. Ralph, A. Ransijn, J. Mauel, V. Yardley, D. Snowdon, S. L. Croft, and W. Denny. 1997. Structure-activity relationships for the antileishmanial and antitrypanosomal activities of 1′-substituted 9-anilino-acridines. J. Med. Chem. 40:2634-2642. [DOI] [PubMed] [Google Scholar]
- 13.Girault, S., S. Delarue, P. Grellier, A. Berecibar, L. Maes, L. Quirijnen, P. Lemiere, M. A. Debreu-Fontaine, and C. Sergheraert. 2001. Antimalarial in-vivo activity of bis(9-amino-6-chloro-2-methoxyacridines). J. Pharm. Pharmacol. 53:935-938. [DOI] [PubMed] [Google Scholar]
- 14.Girault, S., P. Grellier, A. Berecibar, L. Maes, E. Mouray, P. Lemiere, M. A. Debreu, E. Davioud-Charvet, and C. Sergheraert. 2000. Antimalarial, antitrypanosomal, and antileishmanial activities and cytotoxicity of bis(9-amino-6-chloro-2-methoxyacridines): influence of the linker. J. Med. Chem. 43:2646-2654. [DOI] [PubMed] [Google Scholar]
- 15.Greenspan, P., E. P. Mayer, and S. D. Fowler. 1985. Nile red: a selective fluorescent stain for intracellular lipid droplets. J. Cell Biol. 100:965-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grimaldi, R., G. R. Tesh, and C. McMahon-Pratt. 1989. A review of the geographic distribution and epidemiology of leishmaniasis in the New World. Am. J. Trop. Med. Hyg. 41:687.. [DOI] [PubMed] [Google Scholar]
- 17.Herwalt, B. L. 1999. Leishmaniasis. Lancet 354:1191-1199. [DOI] [PubMed] [Google Scholar]
- 18.Lagunin, A., A. Stepanchikova, D. Filimonov, and V. Poroikov. 2000. PASS: prediction of activity spectra for biologically active substances. Bioinformatics 16:747-748. [DOI] [PubMed] [Google Scholar]
- 19.Le Pecq, J. B., M. Le Bret, M. Barbet, and B. P. Roques. 1975. DNA polyintercalating drugs: DNA binding of diacridine derivatives. Proc. Natl. Acad. Sci. USA 72:2915-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mesa-Valle, C. M., J. Castilla-Calvente, M. Sanchez-Moreno, V. Moraleda-Lindez, J. Barbe, and A. Osuna. 1996. Activity and mode of action of acridine compounds against Leishmania donovani. Antimicrob. Agents Chemother. 40:684-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mrozek, A., J. Karolak-Wojciechowska, N. Bsiri, and J. Barbe. 2000. Quantitative analysis of structure-activity relationship in the acridine series. 1. Antiparasitic 9-thioaryl-acridine derivatives. Acta Pol. Pharm. 57:345-351. [PubMed] [Google Scholar]
- 22.Murray, H. W. 2000. Treatment of visceral leishmaniasis (kala-azar): a decade of progress and future approaches. Int. J. Infect. Dis. 4:158-177. [DOI] [PubMed] [Google Scholar]
- 23.Ogunkolade, B. W., I. Colomb-Valet, L. Monjour, A. Rhodes-Feuillette, J. P. Abita, and D. Frommel. 1990. Interactions between the human monocytic leukaemia THP1 cell line and Old and New World species of Leishmania. Acta Trop. 47:171-176. [DOI] [PubMed] [Google Scholar]
- 24.Poroikov, V. V., D. Filimonov, Y. Borodina, A. Lagunin, and A. Kos. 2000. Robustness of biological activity spectra predicting by computer program pass for non-congeneric sets of chemical compounds. J. Chem. Inform. Comput. Sci. 40:1349-1355. [DOI] [PubMed] [Google Scholar]
- 25.Shapiro, H. M. 2000. Microbial analysis at the single-cell level: tasks and techniques. J. Microbiol. Methods 42:3-16. [DOI] [PubMed] [Google Scholar]
- 26.Sourdon, V., S. Mazoyer, V. Pique, and J. P. Galy. 2001. Synthesis of new bis- and tetra-acridines. Molecules 6:673-682. [Google Scholar]
- 27.Spicer, J. A., S. A. Gamage, G. J. Finlay, and W. A. Denny. 2002. Synthesis and evaluation of unsymmetrical bis(arylcarboxamides) designed as topoisomerase-targeted anticancer drugs. Bioorg. Med. Chem. 10:19-29. [DOI] [PubMed] [Google Scholar]
- 28.Sundar, S. 2001. Drug resistance in Indian visceral leishmaniasis. Trop. Med. Int. Health 6:849-854. [DOI] [PubMed] [Google Scholar]
- 29.Tekto, I. V., Y. Tanchuk, and A. Villa. 2001. Prediction of n-octanol/water partition coefficients from PHYSPROP database using artificial neural networks and E-state indices. J. Chem. Infect. Comput. Sci. 41:1407-1421. [DOI] [PubMed] [Google Scholar]
- 30.Werbovetz, K. A., E. K. Lehnert, T. L. Macdonald, and R. D. Pearson. 1992. Cytotoxicity of acridine compounds for Leishmania promastigotes in vitro. Antimicrob. Agents Chemother. 36:495-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Werbovetz, K. A., P. G. Spoors, R. D. Pearson, and T. L. Macdonald. 1994. Cleavable complex formation in Leishmania chagasi treated with anilinoacridines. Mol. Biochem. Parasitol. 65:1-10. [DOI] [PubMed] [Google Scholar]
- 32.Wilmanska, D., M. Czyz, K. Studzian, M. K. Piestrzeniewicz, and M. Gniazdowski. 2001. Effects of anticancer drugs on transcription in vitro. Z. Naturforsch. 56:886-891. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization. 2002. Programme for the surveillance and control of leishmaniasis. World Health Organization, Geneva, Switzerland. [Online.] http://www.who.int/emc/diseases/leish/leishmaniasis.pdf.