Abstract
The unicellular acidophilic red alga Galdieria sulphuraria is a facultative heterotroph with a complex uptake system for sugars and polyols, consisting of at least 14 transporters. Upon transfer to heterotrophic conditions, these transporters were induced simultaneously. Once induced, transporters for common hexoses and pentoses are apparently not down-regulated under heterotrophic conditions. Uptake of deoxysugars (FUC/Rha), however, was repressed by substrates metabolized via gluco-, galacto-, glycero-, or hexokinase, whereas substrates phosphorylated by xylulokinase had no effect. This indicates that several sugar kinases play a key role in sugar sensing. In contrast, polyol transporters were repressed only by glucose or its analogs but not by other sugars. This repression does not involve the activity of kinases. Most likely this type of glucose sensing is independent of metabolism and takes place prior to or during uptake. In its natural environment, these two different sensing mechanisms would enable the alga to utilize a mixture of different substrates in a most economic way by repressing dispensible transporters.
It is important for plants to regulate their metabolism according to the supply of nutrients. Availability of sugars generally up-regulates heterotrophic metabolism, the synthesis of storage compounds, and sugar transport, whereas photosynthesis is down-regulated. Depletion of sugars has the opposite effects. Sugar sensing is the basis of these “feast and famine” responses (Koch, 1996), and it is, therefore, one of the crucial regulatory processes. The final step in sugar sensing is the regulation of gene expression by transcription factors (Nehlin and Ronne, 1990). Models for sugar sensing are largely derived from the yeast (Saccharomyces cerevisiae) system, in which a number of transcription factors and their regulation are already known (Gancedo, 1998). Although these final steps in the signal transduction cascade are widely accepted for higher plants (Jang and Sheen, 1997) and animals (Matschinsky et al., 1998) also, the introductory steps in the signal cascade are still discussed with much controversy (Halford et al., 1999). The most frequently discussed pathways include (a) hexokinase as a bifunctional enzyme with catalytic and regulatory activity (Entian and Fröhlich, 1984; Trumbly, 1992; Ronne, 1995; Jang and Sheen, 1997), (b) direct sensing via cytosolic extensions of transporter homologs (Özcan et al., 1996a, 1996b; Lalonde et al., 1999), (c) sensing via metabolic flux rates and cellular energy charge (Sierkstra et al., 1993), and (d) the generation of a cAMP signal by both a G-protein-coupled receptor system for detection of extracellular Glc and the activity of an intracellular hexokinase (Rolland et al., 2000). In yeast, cAMP has long been identified as a second messenger in sugar sensing (Beullens et al., 1988; Thevelein and de Winde, 1999; Rolland et al., 2000). The involvement of a G protein-coupled receptor system and hexokinase in the generation of a cAMP signal (Rolland et al., 2000) demonstrates a cross-talk between different signal transduction cascades. It has often been emphasized that cooperation of several pathways is very likely, because no single parameter accounts for all the observed effects in the field of sugar sensing (Koch, 1996; Smeekens and Rook, 1997; Gancedo, 1998). Sugar transporters with a sensing function, like the yeast transporter homolog Snf3, obviously provide the cell with information about incoming sugars (Özcan et al., 1996a, 1996b; Lalonde et al., 1999). However, additional information on metabolic flux seems crucial for sugar sensing. The hexokinase-dependent sensing pathway is directly linked to the flux of sugars. Again, this is based on observations made for yeast (Trumbly, 1992; Ronne, 1995) but seems to be partially conserved in all eukaryotes (Koch, 1996; Jang and Sheen, 1997; Smeekens and Rook, 1997). Hexokinase, reportedly, has a catalytic as well as a regulatory function, the latter being based on a protein kinase activity (Entian and Fröhlich, 1984) that controls the interaction of a second protein kinase (Snf1) and its regulatory subunit (Snf4; Jiang and Carlson, 1996). The protein kinase activity of the Snf1/Snf4 complex then acts on transcription factors, such as Mig1, which in response regulate gene activity (Gancedo, 1998). Most current models favor hexokinase as the primary sugar sensor. However, hexokinase is not the only sugar-phosphorylating enzyme. In yeast, hexokinase 2 seems to be the major sensing kinase, whereas hexokinase 1 and glucokinase 1 are involved in different sensing cascades (De Winde et al., 1996) and are both not as well characterized as hexokinase 2. In plants, investigation of the role of sugar kinases other than hexokinase in sensing has just begun (Pego and Smeekens, 2000). This may be because substrates of the hexokinase (Glc and Fru) also represent the most important sugars in plants. However, to shed more light on the sensing properties of kinases, a comparison between different kinases—sensing and nonsensing—is crucial. For these studies, the unicellular, acido-, and thermophilic red alga G. sulphuraria is an ideal organism. It occurs worldwide mostly in hot, acidic sulfur springs and solfatara soils with temperatures up to 56°C and pH values between 0.05 and 3 (Doemel and Brock, 1970). G. sulphuraria grows autotrophically as well as heterotrophically on at least 27 different sugars and polyols (Gross and Schnarrenberger, 1995), as well as on amino acids and organic acids (Rigano et al., 1976). To use all of these different compounds, the alga had to develop not only the enzymatic machinery for their metabolism but also a versatile uptake system (Oesterhelt et al., 1999).
With a complex mixture of compounds, the alga should be able to select for certain substrates to be efficient. This requires a sugar-sensing mechanism, which, for example, should enable the alga to distinguish between most and least desirable substrates. Taking advantage of the versatility of carbon metabolism in G. sulphuraria, it is possible to investigate sugar sensing and signaling pathways in wild-type cells. Considering the extreme conditions at the habitat of G. sulphuraria, it may well be possible that mechanisms of sugars sensing evolved differently from higher plants.
For G. sulphuraria it has been shown that enzymes, necessary for metabolizing the wide range of substrates, are constitutively expressed, including four different hexo- and pentulokinases (Gross and Oesterhelt, 1999). On the other hand, when transferred from autotrophic to heterotrophic conditions, cells exhibit lag phases of between 1 and 45 d, depending on the substrate supplied (Gross and Schnarrenberger, 1995). This lag phase under heterotrophic conditions can be attributed to the induction of an uptake system that consists of several different sugar and polyol transporters (Oesterhelt et al., 1999). More than 14 transporters are responsible for the uptake of substrates in G. sulphuraria, and the optimal regulation of these transporters is the basis for heterotrophic growth of the alga. It has been shown previously (Oesterhelt et al., 1999) that the uptake system of G. sulphuraria is induced under heterotrophic conditions in the presence of a metabolizable substrate and not by darkness alone. The induction pattern of transporters depends on the substrate supplied for heterotrophic growth and shall be further discussed in this paper.
RESULTS
Induction of Sugar and Polyol Uptake
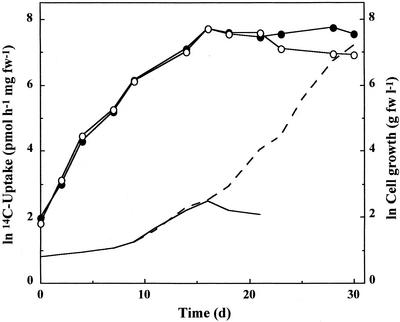
When autotrophic cells of G. sulphuraria MBI (Marine Biotechnology Institute, Tokyo) were transferred to heterotrophic conditions on Glc, a lag phase of about 6 d preceded exponential cell growth. Uptake capacity of cells for [14C]Glc, however, started to increase with the onset of heterotrophic conditions and continued until saturation of uptake was reached after about 18 d and then remained constant for several days even in the absence of substrate (Fig. 1). It has been shown previously (Oesterhelt et al., 1999) that maximum transport capacities of cells will exceed uptake requirements for exponential cell growth and that these surplus capacities are obviously used for the accumulation of storage compounds.
Figure 1.
Uptake capacity of cells for [14C]Glc upon transfer from autotrophic to heterotrophic conditions on Glc. Uptake was monitored in a batch culture with 25 mm Glc as substrate (solid line, cell growth; ○, Glc uptake) and in a continuous culture with additional Glc supplied at the end of exponential cell growth (broken line, cell growth; ●, Glc uptake). Experiments were repeated at least three times. fw, Fresh weight.
The uptake system of G. sulphuraria for sugars and polyols is not induced by darkness; rather, the presence of a metabolizable substrate is necessary for induction (Oesterhelt et al., 1999). However, general heterotrophic metabolism is not the key to induction because heterotrophic growth on amino acids did not induce uptake of sugars or polyols (data not shown), whereas growth on sugars and polyols caused induction of transporters.
Repression of Fuc Uptake
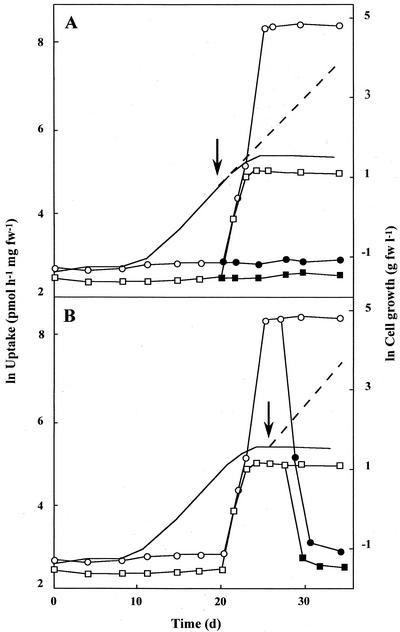
Depending on the substrate supplied, different subsets of the 14 sugar and polyol transporters were expressed in G. sulphuraria. In cells grown on Glc, uptake of [14C]Glc (maximum transport capacity: 4,000 pmol h−1 mg−1 fresh weight), [14C]Fru (1,600 pmol h−1 mg−1 fresh weight), [14C]mannitol (980 pmol h−1 mg−1 fresh weight), [14C]sorbitol (1,030 pmol h−1 mg−1 fresh weight) and [3H]Ara (1,180 pmol h−1 mg−1 fresh weight) was induced with the onset of heterotrophic conditions. In contrast, [14C]Fuc and [3H]Rha were not taken up by Glc-grown cells. However, once substrate depletion was reached, uptake of these deoxysugars was rapidly induced within 3 d (maximum transport capacities: 5,000 and 150 pmol h−1 mg−1 fresh weight, respectively; Fig. 2A). Following the addition of Glc, the uptake of Fuc and Rha was again repressed within 2 to 3 d (Fig. 2B).
Figure 2.
Repression of deoxysugar uptake by Glc. Cells were transferred from autotrophic to heterotrophic conditions with 25 mm Glc as substrate (solid line, cell growth; ○, □, uptake). Additional Glc (arrow) was supplied before (A) and after (B) substrate depletion (broken line, cell growth; ●, ▪, uptake). Uptake capacities of cells for [14C]Fuc (○, ●) and [3H]Rha (□, ▪) were monitored throughout culturing conditions. Experiments were repeated at least three times. fw, Fresh weight.
For dulcitol-grown cells, uptake of [14C]Glc, [14C]Fru, [14C]mannitol, [14C]Fuc, and [3H]Rha was induced with the onset of heterotrophic conditions (Fig. 3A). At the end of induction, uptake capacities remained constant for several days and were comparable with those reached in Glc-grown cells. When dulcitol-grown cells with maximal uptake capacities for [3H]Rha were transferred to Glc, uptake of Rha was completely repressed within 3 d (Fig. 3B). These data show that the expression of Fuc and Rha uptake is regulated in different ways by Glc and dulcitol.
Figure 3.
Induction of transporters on dulcitol. Cells were transferred from autotrophic to heterotrophic conditions with 25 mm dulcitol as substrate (solid line, cell growth). A, Uptake capacities of cells for [14C]Glc (●), [14C]Fru (▴), [14C]mannitol (▵), [14C]Fuc (○), and [3H]Rha (□) were monitored. B, Uptake of [3H]Rha was monitored during exponential cell growth on dulcitol (solid line, cell growth; □, Rha uptake) and after transfer of cells to Glc (arrow) (broken line, cell growth; ▪, Rha-uptake). Experiments were repeated at least three times. fw, Fresh weight.
We tested a number of other substrates and nonmetabolizable sugars for their effect on the repression of Fuc uptake (Table I). Common hexoses, such as Man, Fru, Gal, and sorbose, repressed Fuc uptake, as has been shown for Glc. Deoxysugars and pentoses had no repressive effect. Polyols can be divided into two groups: Mannitol and sorbitol repressed Fuc uptake, whereas arabitol and xylitol did not. Methylated sugars (3-O-methyl-Glc [MOG]; methyl-Man) were taken up by cells but did not serve as substrates for heterotrophic growth, regardless of preculturing conditions (data not shown). It is surprising that the effect of these methylated sugars on the uptake of Fuc was not uniform. 1-O-Methyl-Glc (methyl-Glc) and methyl-Man repressed uptake of [14C]Fuc, whereas MOG had no effect (Table I).
Table I.
Effect of substrates and Glc analogs on the activity of the Fuc transporter of G. sulphuraria
| Substrate | Fuc Uptake
|
|||
|---|---|---|---|---|
| Repression | No repression | |||
| % | % | |||
| Hexoses | l-Sorbose | 0 | Tagatose | 100 |
| Gal | 1 | |||
| Glc | 3 | |||
| Man | 7 | |||
| Fru | 28 | |||
| Deoxysugars | l-Rha | 100 | ||
| Quinovose | 95 | |||
| Pentoses | l-Ara | 100 | ||
| Xylose | 70 | |||
| Disaccharides | Trehalose | 0 | ||
| Polyols | Sorbitol | 13 | l-Ara | 124 |
| Mannitol | 27 | Xylitol | 70 | |
| Dulcitol | 70 | |||
| C3 compounds | Glycerol | 2 | Pyruvate | 86 |
| Methylated sugars | Methyl-Glc | 8 | MOG | 80 |
| Methyl-Man | 25 | |||
Cells were grown on Glc (exponential phase) and transferred to a different substrate. After 3 d, uptake of [14C]Fuc was tested. Uptake of cells transferred to autotrophic medium was taken as 100%. Results are given for one representative set of data. Experiments were repeated at least twice.
Metabolism of Methylated Glc Analogs
The different response of cells to Glc analogs prompted us to analyze the metabolism of methylated sugars. After incubation of Glc-grown cells with methyl(∝-d-[U-14C]gluco)pyranoside ([14C]methyl-Glc), 3-O-methyl-d-[U-14C]Glc ([14C]MOG), and [14C]Glc overnight, cells were extracted and cell compounds were separated into a soluble and an insoluble fraction. Soluble compounds were further separated into sugars, sugar phosphates, and sugar nucleotides by HPLC. Soluble and insoluble fractions were analyzed for their 14C content, and the loss of radioactivity was calculated. In Table II, the metabolization patterns of methyl-Glc and MOG are compared with that of Glc. A significant pool of labeled sugar phosphates or nucleotides was not apparent for any of the three substrates. Only feeding of [14C]Glc led to a strong accumulation of insoluble products (47% of total uptake). A significant loss of radioactivity, observed when feeding [14C]Glc (45%) and [14C]methyl-Glc (67%), was due to CO2 evolution (Table II). The evolution of CO2 from methyl-Glc was not due to a breakdown of this compound in the acid medium used because the presence of living cells was necessary for CO2 release. However, methyl-Glc could have been partially degraded and only certain products metabolized by the cells. Therefore, we incubated methyl-Glc overnight in medium of pH 2 and 7 and analyzed the medium by HPLC. No additional peaks or quantitative differences were observed in the two treatments, indicating the acid stability of methyl-Glc (data not shown).
Table II.
Distribution of 14C after feeding Glc and Glc analogs to cells
| 14C-Labeled Substrate | Radioactivity
|
||
|---|---|---|---|
| Soluble | Insoluble | Loss | |
| % | |||
| Glc | 7 ± 1 | 47 ± 7 | 45 ± 8 |
| Methyl-Glc | 16 ± 8 | 17 ± 8 | 67 ± 3 |
| MOG | 77 ± 8 | 9 ± 7 | 14 ± 5 |
Glc-grown cells were fed [14C]Glc, [14C]methyl-Glc, and [14C]MOG for 20 h. Cells were extracted, and cell extracts were separated into a soluble and an insoluble fraction. The total uptake of 14C was taken as 100%. sd of four independent experiments is given.
A key step for metabolism of methyl-Glc would be phosphorylation. Therefore, we tested the phosphorylation of methyl-Glc and MOG by crude cell extracts of G. sulphuraria and, for comparison, commercially available hexokinase (yeast). No phosphorylated products were detected with either substrate after 2 h of incubation with yeast hexokinase. In contrast, using crude algal cell extract, methyl-Glc was phosphorylated (data not shown). The kinase responsible for the phosphorylation of methyl-Glc has not been identified yet. Most likely, glucokinase generates methyl-Glc-6-P, which is then converted to methyl-Fru-6-P and cleaved by aldolase to yield glycer-aldehyde-3-P and 1-O-methyl-hydroxyacetone. The triose-P from this reaction is further metabolized to eventually give CO2. MOG was not phosphorylated by the algal extract.
Regulation of Other Transporters
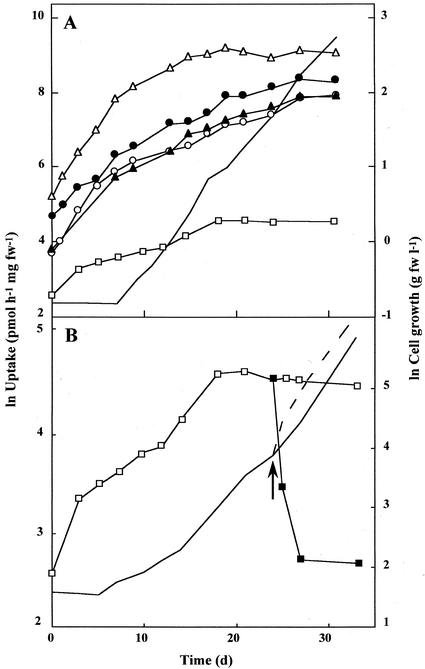
To compare the regulation of deoxysugar uptake with that of other substrates, we monitored the uptake capacities for [14C]Glc, [14C]Fru, [14C]mannitol, [14C]sorbitol, [3H]Ara, and [3H]Rha in cells that had been precultivated on Glc and then transferred to a different substrate (Table III). Uptake of [14C]Glc, [14C]Fru, and [3H]Ara was not repressed by any substrate supplied. Uptake of [3H]Rha was strongly repressed by Glc, Fru, Man, methyl-Man, methyl-Glc, mannitol, sorbitol, and trehalose, whereas arabitol and MOG did not cause repression. In contrast, uptake of [14C]mannitol and [14C]sorbitol was repressed by MOG, Glc, and methyl-Glc, whereas methyl-Man, Man, and trehalose did not repress polyol uptake.
Table III.
Effect of substrates and Glc analogs on the activity of sugar and polyol transporters
| Substrate | Uptake of 14C- and 3H-Labeled Substrates
|
||||||
|---|---|---|---|---|---|---|---|
| Glca | Frua | Mannitola | Sorbitola | Arab | Rhab | Fuca | |
| None | 100c | 100d | 100e | 100f | 100g | 100h | 100i |
| Glc | 293 | 144 | 42 | 46 | 264 | 6 | 3 |
| Fru | 365 | 762 | 197 | 384 | 366 | 15 | 28 |
| Man | 400 | 517 | 115 | 150 | 395 | 13 | 8 |
| Methyl-Man | 256 | 219 | 96 | 100 | 327 | 15 | 25 |
| Methyl-Glc | 216 | 245 | 59 | 56 | 276 | 9 | 8 |
| MOG | 231 | 75 | 33 | 29 | 208 | 85 | 80 |
| Arabitol | 303 | 589 | 230 | 591 | 204 | 121 | 124 |
| Mannitol | 380 | 480 | 208 | 399 | 239 | 12 | 27 |
| Sorbitol | 210 | 479 | 133 | 220 | 263 | 9 | 13 |
| Trehalose | nd | nd | 77 | 99 | nd | 15 | 0 |
Cells were grown on Glc and transferred from the exponential phase to a different substrate. After 3 d, uptake of 14C- and 3H-labeled substrates was tested. Uptake of cells transferred to autotrophic medium was taken as 100%. Results are given for one representative set of data. Experiments were repeated four times. nd, Not determined.
14C-labeled.
3H-labeled.
1.4 nmol h−1 mg−1 fresh wt.
1.11 nmol h−1 mg−1 fresh wt.
2.36 nmol h−1 mg−1 fresh wt.
2.25 nmol h−1 mg−1 fresh wt.
0.45 nmol h−1 mg−1.
0.34 nmol h−1 mg−1 fresh wt.
27.3 nmol h−1 mg−1 fresh wt.
In summary, regulation of Rha and Fuc uptake are identical. Uptake of Glc, Fru, and Ara is not repressed by any of the substrates tested, whereas mannitol and sorbitol uptake is repressed by Glc, methyl-Glc, and MOG. Three different regulative patterns for sugar uptake can, therefore, be distinguished in G. sulphuraria.
DISCUSSION
Induction of Sugar and Polyol Uptake
G. sulphuraria is a facultative heterotrophic red alga utilizing a unique spectrum of substrates, including hexoses, pentoses, deoxysugars, polyols, disaccharides, and amino acids. The transporters necessary for the uptake of sugars and polyols are not regulated in a “one-substrate-one-transporter” manner but are usually induced en bloc under heterotrophic conditions. Only metabolizable sugars and polyols cause this induction. However, heterotrophic metabolism alone is not sufficient for induction because growth on amino acids does not induce uptake of sugars or polyols.
Repression of Deoxysugar Transporters
To be most efficient, cells should be able to distinguish between different substrates and regulate their uptake system accordingly, e.g. a repression of transporters for less preferred substrates. Accordingly, we observed a substrate repression of Fuc and Rha transporters when cells were grown on Glc. The same repression was caused by a number of other hexoses (Gal, Fru, Man, sorbose), polyols (sorbitol, mannitol, glycerol), and trehalose, as well as the sugar analogs methyl-Glc and methyl-Man. In contrast, xylitol, arabitol, tagatose, Rha, Fuc, quinovose (6-deoxy-Glc), and MOG did not lead to substrate repression.
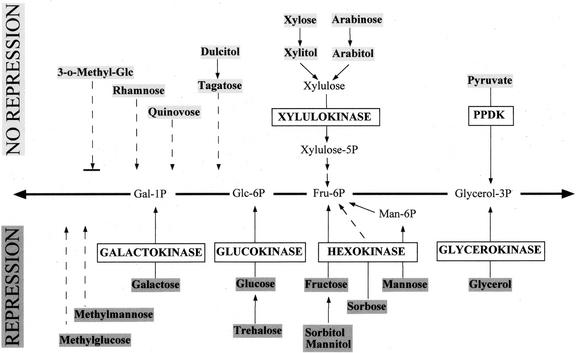
To elucidate the mechanism for this sugar sensing, we compared the metabolic pathways for these two groups of substrates (Fig. 4). Obviously, the position at which a substrate enters the central cell metabolism is not relevant, because several substrates (e.g. Gal, mannitol, glycerol) enter at different points, and yet all evoke substrate repression. On the other hand, mannitol and arabitol both enter the central cell metabolism eventually via Fru-6-P but evoke different cellular responses.
Figure 4.
Role of different kinases in the expression of deoxysugar transporters in G. sulphuraria. The entry of the various substrates into the glycolytic and gluconeogenetic metabolism of the alga is shown. Uncertain metabolic steps are indicated by dotted lines. Substrates shaded in dark gray lead to a repression of deoxysugar transporters; substrates shaded in light gray did not. It appears that activity of galactokinase, glucokinase, hexokinase, and glycerokinase leads to repression, whereas xylulokinase and PPDK have no such effect.
A unifying trait of all substrates causing repression of Fuc uptake is a direct or indirect phosphorylation by hexokinase or glycerokinase. So far, we have identified three hexokinases in G. sulphuraria: a glucokinase, a galactokinase (W. Gross, unpublished results), and a hexokinase for Fru and Man (Heilmann et al., 1997). Substrates that are not metabolized via a hexo- or glycerokinase did not repress the transporters for Fuc and Rha, e.g. xylitol and arabitol, which are metabolized via a xylulokinase, dulcitol, and its direct product tagatose, as well as deoxysugars (Rha, Fuc, quinovose) and pyruvate, which is phosphorylated by pyruvate, orthophosphate dikinase (PPDK).
Methylated sugars have long been a tool to study sugar sensing, and their effect on transporter repression has, therefore, been studied in G. sulphuraria. We could clearly distinguish different cellular responses to the different forms of methylated Glc. Methyl-Glc caused substrate repression of deoxysugar transport, whereas MOG did not. Neither sugar analog serves as a substrate for heterotrophic growth, and yet methyl-Glc is phosphorylated and partially metabolized in G. sulphuraria as indicated by the production of 14CO2 after feeding [14C]methyl-Glc. This metabolism has to include phosphorylation, again pointing to the key role of kinases in sugar sensing. For methyl-Man, we have to postulate the same phosphorylation and metabolism because it also leads to substrate repression of deoxysugar transport. It remains to be elucidated which kinase phosphorylates methyl-Glc, but it has to belong to the “sensing kinases” as opposed to the xylulokinase or the PPDK.
Repression of Polyol Transporters
Similar to the repression of deoxysugar uptake, polyol transporters were also repressed by Glc. However, the mechanism of sensing is different. Neither phosphorylation nor general metabolic activity seems to be the key to this type of sugar sensing. It appears that for this type of repression the Glc moiety itself is recognized, independently of metabolism. A sensing Glc transporter (Lalonde et al., 1999), reporting the influx or presence of Glc (and its methylated analogs), could explain this regulation of polyol uptake. Influx of Glc molecules results in repression of polyol transporters. A distinction among Glc, methyl-Glc, and MOG is, therefore, not possible. Intracellular Glc, however, is not sensed, because trehalose does not repress polyol uptake. Trehalose is taken up as a disaccharide in G. sulphuraria and is intracellularly cleaved by a trehalase (W. Gross and J. Knirr, unpublished results). This demonstrates the main difference between the repression of deoxysugar and polyol uptake. Trehalose leads to substrate repression of deoxysugar transport because the phosphorylation of intracellular Glc is sensed. In the case of polyol uptake regulation, intracellular processes are obviously not involved.
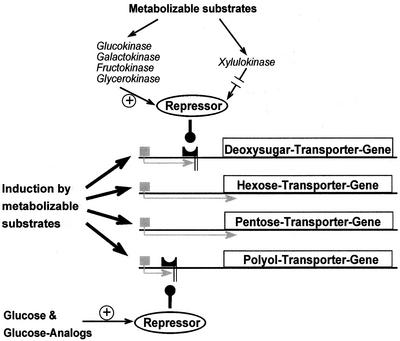
In summary, three different mechanisms of the regulation of transporters for sugar and polyol uptake emerge (Fig. 5): (a) Transporters for common hexoses and pentoses are not repressed by any substrate, (b) polyol uptake is repressed specifically by Glc, and (c) deoxysugar uptake is repressed via the activity of hexokinases and glycerokinase but not xylulokinase.
Figure 5.
A model for the regulation of sugar/polyol transporters in G. sulphuraria. Metabolizable substrates induce the whole spectrum of transporters. Polyol transporters are repressed by Glc and its analogs that are apparently sensed prior or during uptake. Deoxysugar transporters are repressed by substrates metabolized via hexokinases or the glycerokinase. Activity of xylulokinase does not cause repression. We postulate a transcriptional regulation of transporters by repressors.
Ecological Implications
At its natural habitat, G. sulphuraria grows mainly in cryptoendolithic cell mats (Gross and Oesterhelt, 1999) and uses heterotrophic metabolism to survive light-limited conditions. The substrate for heterotrophic growth apparently derives from decaying cells within this mat. To make economic use of this mixture of substrates, the alga represses transporters for less preferred substrates when Glc is present. After depletion of Glc, the repression of polyol uptake ceases. Finally, when other common hexoses, such as Fru, Man, and Gal, are consumed, the deoxysugar transporters are no longer repressed. This three-step cascade ensures a direction of metabolism toward the most efficient energy source at any given point.
MATERIALS AND METHODS
Plant Material
Stock cultures of strain Galdieria sulphuraria MBI were obtained from the MBI (Tokyo; Oesterhelt et al., 1999). G. sulphuraria strain 074 W was isolated from Java, Indonesia (Gross and Schnarrenberger, 1995), and G. sulphuraria strain AZ was isolated from the Azores (Sao Miguel).
The alga was grown autotrophically in an inorganic culture medium (Gross and Schnarrenberger, 1995). Cultures were supplied with air enriched with 2% CO2 through an inlet at the bottom of the culture vessel and illuminated with white incandescent light of 80 μmol m−2 s−1. Heterotrophic cultures were grown at 25°C in 2 l Erlenmeyer flasks and shaken at 120 rpm on a rotary table in the dark. The inorganic culture medium was supplemented with 25 mm substrate. Cell density was determined spectrophotometrically at 800 nm (Gross and Schnarrenberger, 1995). Cells were harvested by centrifugation at 3,000g for 5 min, washed with inorganic culture medium, and immediately used for uptake studies.
Uptake of 14C-Labeled Substrates
Unless indicated otherwise, cells from the exponential growth phase were used to analyze the uptake of d-[U-14C]Glc (111 MBq mmol−1), [14C]MOG (4 GBq mmol−1), [14C]methyl-Glc (11 GBq mmol−1; Amersham International, Buckinghamshire, UK), l-[1-14C]Fuc (2 GBq mmol−1), l-[3H (g)]Rha (185 GBq mmol−1; American Radiolabeled Chemicals, St. Louis), d-[1-14C]mannitol (2 GBq mmol−1), l-[1-3H]Ara (111 GBq mmol−1; Moravek Biochemicals, Brea, CA) and d-[U-14C]Fru (11 GBq mmol−1; DuPont de Nemours, Wilmington, DE). The assay mixture (0.5 mL) contained 24 mg (fresh weight) of cells and 1 mm sugar, polyol, or Glc analog in an inorganic salt medium (3.7 kBq of [14C]Glc, [14C]MOG, [14C]Fuc, [14C]mannitol, [14C]Fru, 22 kBq [14C]methyl-Glc, or 5.55 kBq [3H]Rha, and [3H]Ara). Cells were incubated for 30 min at 25°C unless indicated otherwise. After incubation, the cell suspension was placed onto 0.5 mL of silicone oil (Density = 1.05; Fluka, Neu-Ulm, Germany) in an Eppendorf tube. Cells were centrifuged for 10 s at 10,000g through the silicone oil layer, washed twice with distilled water, and were extracted for 20 min at 100°C in 0.5 mL of 50% (v/v) ethanol. After centrifugation, 3 mL of scintillation fluid were added to the supernatant (0.5 mL), and radioactivity was measured in a scintillation counter. As a control, cells were centrifuged immediately after the addition of labeled sugar.
Quantification of 14CO2
Cells (200 mg fresh weight) were incubated in inorganic salt medium, supplied with 0.25 mm labeled substrate (3.7 kBq of [14C]Glc, [14C]MOG; 22 kBq of [14C]methyl-Glc) and constantly bubbled with air. CO2 was trapped in 25% (v/v) ethanolamine (in ethanol) and analyzed for radioactivity in a scintillation counter. After 24 h, cells were harvested, washed, and extracted for 20 min at 100°C in 0.5 mL of 50% (v/v) ethanol. Soluble and insoluble cellular fractions were analyzed for their 14C content.
Separation of Soluble Cellular Components
During feeding experiments with 14C-labeled substrates, cells were shaken on a rotary table (120 rpm) for 20 h and centrifuged through a layer of silicone oil (uptake assay and cell treatment as described above). The pellet was resuspended in 50% (v/v) ethanol and frozen at −70°C for 30 min. After centrifugation, the insoluble fraction was washed once with 80% (v/v) ethanol and once with H2O. The pellet was resuspended in 0.5 mL of H2O and analyzed for radioactivity in a scintillation counter. Soluble fractions were pooled, dried under vacuum, dissolved in 50 μL of H2O, and used for analysis of metabolites.
Separation of Sugars, Sugar Phosphates, and Sugar Nucleotides
An aliquot of 20 μL was loaded onto a Partisil SAX HPLC column (10 μm; 250 × 4.6 mm; Alltech, Deerfield, IL). The column was run at room temperature at 1 mL min−1. Following injection, sugars were washed from the column for 8 min with 10 mm NaH2PO4·H3PO4, pH 2.56. Sugar phosphates and sugar nucleotides were eluted with a linear gradient from 10 to 100 mm NaH2PO4·H3PO4, pH 2.56 (30 min). Fractions (1 mL) were collected and analyzed for radioactivity in a scintillation counter.
Separation of Sugars
An aliquot of 20 μL was loaded onto a PL Hi-Plex Ca2+ HPLC column (300 × 7.7 mm; Polymer Lab, Shropshire, UK) with double-distilled water as a mobile phase at 85°C. The effluent of the column was monitored at 30°C by a refractive index detector connected to an integrator. Compounds were identified by comparison with the retention time of standard sugars and polyols. Fractions (1 mL) were collected and analyzed for radioactivity in a scintillation counter.
In Vitro Sugar Phosphorylation
Heterotrophically grown cells (0.2 g fresh weight) from the exponential growth phase on Glc were homogenized in 1.5 mL of 50 mm Tris-HCl, pH 7.5, containing 5 mm MgCl2 and protease inhibitors (Mini Complete, Boehringer Mannheim/Roche, Basel), using a Mini Bead Beater (Biospec Products, Bartlesville, OK) and 1 mL of glass beads (0.25-mm diameter). Homogenization was done in 15 cycles of 20 s with 20-s pauses for efficient cooling in an ice bath. The homogenate was centrifuged at 4°C for 15 min at 37,000g, and 0.8 mL of the supernatant was used for in vitro phosphorylation. After addition of [14C]methyl-Glc (1 mm; 22 kBq) and ATP (2 mm), the assay was incubated for 2 h at room temperature. Proteins were precipitated by adding 0.5 volumes of 96% (v/v) ethanol. After centrifugation (15 min at 37,000g), the supernatant was vacuum dried and dissolved in 100 μL of H2O, and an aliquot of 20 μL was analyzed with a Partisil SAX-HPLC column.
For comparison, in vitro phosphorylation by yeast hexokinase was tested for Glc, methyl-Glc, and MOG. For each assay, 1 mm 14C-labeled substrate (3.7 kBq of [14C]Glc, [14C]MOG; 22 kBq of [14C]methyl-Glc) was supplied with ATP (2 mm) and commercially available yeast hexokinase (1 unit, Boehringer Mannheim/Roche). After 6 h of incubation, samples were treated and analyzed by HPLC as described above.
ACKNOWLEDGMENTS
We thank Dr. H. Ikemoto (MBI, Heita, Japan) for a stock culture and Mrs. Sandra Berger for excellent technical assistance.
Footnotes
This work was supported in part by the Deutsche Forschungsgemeinschaft (grant no. SFB 429).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010553.
LITERATURE CITED
- Beullens M, Mbonyi K, Geerts L, Gladines D, Detremerie K, Jans AWH, Thevelein JM. Studies on the mechanism of the glucose-induced cAMP signal in glycolysis and glucose repression mutants of the yeast Saccharomyces cerevisiae. Eur J Biochem. 1988;172:227–231. doi: 10.1111/j.1432-1033.1988.tb13877.x. [DOI] [PubMed] [Google Scholar]
- De Winde JH, Crauwels M, Hohmann S, Thevelein JM, Winderickx J. Differential requirements of the yeast kinases for sugar sensing in establishing the catabolite-repressed state. Eur J Biochem. 1996;241:633–643. doi: 10.1111/j.1432-1033.1996.00633.x. [DOI] [PubMed] [Google Scholar]
- Doemel WN, Brock TD. The upper temperature limit of Cyanidium caldarium. Arch Microbiol. 1970;72:326–332. doi: 10.1007/BF00409031. [DOI] [PubMed] [Google Scholar]
- Entian KD, Fröhlich KU. Saccharomyces cerevisiae mutants provide evidence of hexokinase PII as a bifunctional enzyme with catalytic and regulatory domains for triggering carbon catabolite repression. J Bacteriol. 1984;158:29–35. doi: 10.1128/jb.158.1.29-35.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gancedo JM. Yeast carbon catabolite repression. Microbiol Mol Biol Rev. 1998;62:334–361. doi: 10.1128/mmbr.62.2.334-361.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross W, Oesterhelt C. Ecophysiological studies on the red alga Galdieria sulphuraria isolated from south-west Iceland. Plant Biol. 1999;1:694–700. [Google Scholar]
- Gross W, Schnarrenberger C. Heterotrophic growth of two strains of the acido-thermophilic red alga Galdieria sulphuraria. Plant Cell Physiol. 1995;36:633–638. [Google Scholar]
- Halford NG, Purcell PC, Hardie DG. Is hexokinase really a sugar sensor in plants? Trends Plant Sci. 1999;4:117–120. doi: 10.1016/s1360-1385(99)01377-1. [DOI] [PubMed] [Google Scholar]
- Heilmann I, Schnarrenberger C, Gross W. Mannose metabolizing enzymes from the red alga Galdieria sulphuraria. Phytochemistry. 1997;45:903–906. [Google Scholar]
- Jang JC, Sheen J. Sugar sensing in higher plants. Trends Plant Sci. 1997;2:208–214. [Google Scholar]
- Jiang R, Carlson M. Glucose regulates protein interactions within the yeast SNF1 protein kinase complex. Genes Dev. 1996;10:3105–3115. doi: 10.1101/gad.10.24.3105. [DOI] [PubMed] [Google Scholar]
- Koch KE. Carbohydrate-modulated gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:509–540. doi: 10.1146/annurev.arplant.47.1.509. [DOI] [PubMed] [Google Scholar]
- Lalonde S, Boles E, Hellmann H, Barker L, Patrick JW, Frommer WB, Ward JM. The dual function of sugar carriers: transport and sugar sensing. Plant Cell. 1999;11:707–726. doi: 10.1105/tpc.11.4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matschinsky FM, Glaser B, Magnuson MA. Pancreatic beta-cell glucokinase: closing the gap between theoretical concepts and experimental realities. Diabetes. 1998;47:307–315. doi: 10.2337/diabetes.47.3.307. [DOI] [PubMed] [Google Scholar]
- Nehlin JO, Ronne H. Yeast MIG1 repressor is related to the mammalian early growth response and Wilms' tumour finger proteins. EMBO J. 1990;9:2891–2898. doi: 10.1002/j.1460-2075.1990.tb07479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt C, Schnarrenberger C, Gross W. Characterization of a sugar/polyol-uptake system in the red alga Galdieria sulphuraria. Eur J Phycol. 1999;34:271–277. [Google Scholar]
- Özcan S, Dover J, Rosenwald AG, Wolfl S, Johnston M. Two glucose transporters in Saccharomyces cerevisiae are glucose sensors that generate a signal for induction of gene expression. Proc Natl Acad Sci USA. 1996a;93:12428–12432. doi: 10.1073/pnas.93.22.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özcan S, Dover J, Rosenwald AG, Wolfl S, Johnston M. Two glucose transporters in Saccharomyces cerevisiae are glucose sensors that generate a signal for induction of gene expression. Proc Natl Acad Sci USA. 1996b;93:12428–12432. doi: 10.1073/pnas.93.22.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pego JV, Smeekens SCM. Plant fructokinases: a sweet family get-together. Trends Plant Sci. 2000;5:531–536. doi: 10.1016/s1360-1385(00)01783-0. [DOI] [PubMed] [Google Scholar]
- Rigano C, Fuggi A, Di Martino Rigano V, Aliotta G. Studies on utilization of 2-ketoglutarate, glutamate and other amino acids by the unicellular alga Cyanidium caldarium. Arch Microbiol. 1976;107:133–138. doi: 10.1007/BF00446832. [DOI] [PubMed] [Google Scholar]
- Rolland F, de Winde JH, Lemaire K, Boles E, Thevelein JM, Winderickx J. Glucose-induced cAMP signalling in yeast requires both a G-protein coupled receptor system for extracellular glucose detection and a separable hexose kinase-dependent sensing process. Mol Microbiol. 2000;38:348–358. doi: 10.1046/j.1365-2958.2000.02125.x. [DOI] [PubMed] [Google Scholar]
- Ronne H. Glucose repression in fungi. Trends Genet. 1995;11:12–17. doi: 10.1016/s0168-9525(00)88980-5. [DOI] [PubMed] [Google Scholar]
- Sierkstra LN, Silljé HHW, Verbakel JMA, Verrips CT. The glucose-6-phosphate-isomerase reaction is essential for normal glucose repression in Saccharomyces cerevisiae. Eur J Biochem. 1993;214:121–127. doi: 10.1111/j.1432-1033.1993.tb17903.x. [DOI] [PubMed] [Google Scholar]
- Smeekens S, Rook F. Sugar sensing and sugar-mediated signal transduction in plants. Plant Physiol. 1997;115:7–13. doi: 10.1104/pp.115.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevelein JM, de Winde JH. Novel sensing mechanisms and targets for the cAMP-protein kinase A pathway in the yeast Saccharomyces cerevisiae. Mol Microbiol. 1999;33:904–918. doi: 10.1046/j.1365-2958.1999.01538.x. [DOI] [PubMed] [Google Scholar]
- Trumbly RJ. Glucose repression in the yeast Saccharomyces cerevisiae. Mol Microbiol. 1992;6:15–21. doi: 10.1111/j.1365-2958.1992.tb00832.x. [DOI] [PubMed] [Google Scholar]