Abstract
RWJ-54428 (MC-02,479) is a new cephalosporin with activity against resistant gram-positive organisms, including methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci, and penicillin-resistant Streptococcus pneumoniae. The in vivo efficacy of RWJ-54428 was evaluated against gram-positive bacteria in four mouse models of infection. RWJ-54428 was effective in vivo against methicillin-susceptible and -resistant S. aureus in a mouse model of sepsis, with 50% effective doses being similar to those of vancomycin. In a single-dose neutropenic mouse thigh model of infection, RWJ-54428 at 30 mg/kg of body weight showed activity similar to that of vancomycin at 30 mg/kg against a strain of methicillin-resistant S. aureus. RWJ-54428 also showed a prolonged in vivo postantibiotic effect in this model. In a mouse model of pneumonia due to a penicillin-susceptible strain of Streptococcus pneumoniae, RWJ-54428 displayed efficacy and potency superior to those of penicillin G and cefotaxime. In a mouse model of pyelonephritis due to Enterococcus faecalis, RWJ-54428 had bactericidal effects similar to those of vancomycin and ampicillin, but at two- to threefold lower total daily doses. These studies show that RWJ-54428 is active in experimental mouse models of infection against gram-positive organisms, including strains resistant to earlier cephalosporins and penicillin G.
Infections due to multiresistant gram-positive organisms are increasing in frequency (13). The high incidence of methicillin resistance in hospitals (as well as its appearance in the outpatient setting) coupled with the emergence of vancomycin-intermediate Staphylococcus aureus (VISA), has further complicated the prevention and treatment of serious infections due to staphylococci (13). Furthermore, resistance to penicillin among strains of Streptococcus pneumoniae has been spreading worldwide (6), with a recent survey in the United States reporting that over 20% of S. pneumoniae isolates are resistant to penicillin (15). These reports emphasize the need for new antibacterials with activity against resistant gram-positive organisms.
A research program with the objective of discovering novel cephalosporins with broad activity against resistant gram-positive bacteria has been described previously and resulted in the discovery of RWJ-54428 (also known as MC-02,479; Fig. 1), which is in phase I clinical trials (5). In vitro studies show that RWJ-54428 has potent activity against staphylococci (including mec-positive, methicillin-resistant strains), penicillin-resistant pneumococci, and enterococci (1, 7, 11). RWJ-54428 has been shown to have bactericidal activity and a postantibiotic effect (PAE) in vitro (J. Blais, F. Malouin, K. Mathias, C. Chan, S. Chamberland, and V. J. Lee, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-179, 1997). This report describes the initial pharmacokinetics and in vivo antibacterial activity of RWJ-54428 in mouse models of infection due to S. aureus (including methicillin-resistant strains), S. pneumoniae, and Enterococcus faecalis.
FIG. 1.
Chemical structure of RWJ-54428 (MC-02,479).
(The work described in this paper was conducted as part of a research collaboration with the R. W. Johnson Pharmaceutical Research Institute. This work was presented in part at the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Ontario, Canada, 28 September to 1 October 1997.)
MATERIALS AND METHODS
Antimicrobial agents.
RWJ-54428 (MC-02,479) was synthesized at Essential Therapeutics, Inc., Mountain View, Calif. (5). All other antibiotics (ampicillin, cefazolin, cefotaxime, imipenem-cilastatin, penicillin G, quinupristin-dalfopristin, and vancomycin) were obtained from commercial sources.
Susceptibility testing.
MICs were determined by a broth microdilution assay according to NCCLS reference methods (9). Assays were performed with a final volume of 100 μl. The inocula were adjusted to yield a final cell density of ca. 5 × 105 CFU/ml. Antibiotics were diluted directly into 96-well microtiter plates by serial twofold dilution. Microtiter plates were read with a plate reader (Molecular Devices, Sunnyvale, Calif.) at 650 nm as well as by visual observation with a reading mirror.
Mouse model of sepsis.
All procedures were approved by the Essential Therapeutics Institutional Animal Care and Use Committee, as outlined by the Animal Welfare Act (9 C.F.R. Ch. 1, 1 January 2001 edition).
S. aureus Smith (ATCC 13709) and methicillin-resistant S. aureus (MRSA) 076 (courtesy of H. Chambers) were grown overnight at 37°C in brain heart infusion broth (BHIB). After 16 h of growth, they were subcultured into fresh BHIB and incubated for 4 to 5 h at 37°C. The cells were washed twice with phosphate-buffered saline (PBS) and adjusted to ca. 108 CFU/ml by correlation of the absorbance at 600 nm with predetermined plate counts. The inoculum was mixed with an equal volume of sterile 14% hog gastric mucin (12) and kept in an ice bath until it was used (<1 h). Male Swiss mice (age, 4 to 6 weeks; n = 10/group, with five groups for each compound; Charles River, Hollister, Calif.) were infected with an intraperitoneal dose of 0.5 ml of the bacterial suspension (ca. 1.4 × 107 CFU/mouse; 100 times the 100% lethal dose). Antibiotics were administered subcutaneously at 0 and 2 h postchallenge. The total dose required for survival of 50% of mice at 72 h (ED50) was determined by the probit method (10).
Pneumonia model.
S. pneumoniae ATCC 6301 was grown for 22 h in Todd-Hewitt broth at 35°C. The inoculum was adjusted to ca. 6 × 106 CFU/ml by correlation of the absorbance at 600 nm with predetermined plate counts. Male Swiss mice (age, 4 to 6 weeks; n = 10/group) were infected by intranasal instillation of 0.05 ml of inoculum (2). Two doses of each antibiotic were given subcutaneously at 24 and 30 h postinoculation. The animals were killed 18 h following the last dose by using carbon dioxide. The lungs were removed aseptically and homogenized with a Pro200 homogenizer (Pro Scientific, Monroe, Conn.) in 1 ml of ice-cold PBS. Serial 10-fold dilutions of the homogenized material were plated on sheep blood agar, and the colonies were counted.
Pyelonephritis model.
E. faecalis ATCC 23241 was grown aerobically overnight in BHIB at 35°C. Cells were harvested by centrifugation and adjusted to ca. 108 CFU/ml by correlation of the absorbance at 600 nm with predetermined plate counts. Male BALB/c mice (age, 4 to 6 weeks; n = 10/group; Charles River) were infected by injection of 0.1 ml of inoculum in the lateral tail vein. Antibiotic treatment was begun 24 h postchallenge. Antibiotics were administered subcutaneously three times a day (4 h apart) for 3 days (nine doses). The animals were killed 16 to 20 h following the last treatment by using CO2. Both kidneys were removed aseptically, combined, and homogenized in 1 ml of ice-cold PBS. Serial 10-fold dilutions of the homogenized material were plated on brain heart infusion agar, and the colonies were counted.
Neutropenic mouse thigh model.
Male Swiss mice (age, 4 to 6 weeks; n = 4/group) were made neutropenic by intraperitoneal injection of 150 mg of cyclophosphamide (Cytoxan; Mead Johnson, Princeton, N.J.) per kg of body weight on days 1 and 4. On day 5, the mice were infected by intramuscular injection of 0.1 ml of inoculum in each thigh (four thighs per group per time point). The inoculum consisted of MRSA strain COL (kindly provided by H. Chambers) or VISA strain HIP-5836 (courtesy of F. Tenover). The bacteria were grown overnight at 35°C in BHIB. On the following morning they were subcultured into fresh BHIB and incubated for 4 h at 35°C. The inoculum was adjusted to a final concentration of ∼5.0 × 106 CFU/ml by correlation of the absorbance at 600 nm with predetermined plate counts. A single dose of antibiotics was given subcutaneously 2 h postinfection. At various time points the mice were euthanized with CO2, and both thighs were removed aseptically and homogenized in 4 ml of ice-cold PBS. Serial 10-fold dilutions of the homogenized material were plated on Mueller-Hinton agar, and the colonies were counted. The maximum reduction in bacterial counts was determined by inspection of the log number of CFU per thigh versus time curves. The effective regrowth time measured the cycle of bacterial killing and regrowth following a single dose. It was calculated as the time required for bacteria to regrow to the inoculum observed prior to dosing (time zero) (4). The in vivo PAE was calculated by the equation PAE = T − C − M, where M represents the time that the non-protein-bound serum RWJ-54428 concentrations exceed the MIC, T is the time required for the counts in the thigh (log number of CFU) to increase by 1 log above the count closest to but not less than that at time M, and C is the time from time zero required for the untreated controls to increase by 1 log (3).
To determine the pharmacokinetic properties of the test agents in infected animals, mice were administered a single subcutaneous dose of RWJ-54428 at 10 or 50 mg/kg of body weight or vancomycin at 30 mg/kg on the fifth day after initiation of cyclophosphamide treatment (as described above). Groups of three mice each were killed at 0.08, 0.16, 0.25, 0.5, 0.75, 1.0, and 2.0 h after dosing. Blood samples (one sample from each animal) were collected by cardiac puncture. Concentrations in serum were fit by using WinNonlin software (Pharsight, Mountain View, Calif.). RWJ-54428 analytical standards (0.5 to 100 mg/liter) were prepared in fresh pooled mouse serum collected from untreated animals. The serum samples or standards were mixed with double the volume of 4% trichloroacetic acid, vortexed, and then centrifuged at 14,000 rpm for 10 min by using a refrigerated Eppendorf 5415c centrifuge set at 4 to 10°C. Aliquots of the supernatant (25 μl) were injected directly onto a high-pressure liquid chromatograph by using a temperature-controlled autoinjector set at 10°C. A standard curve of the peak area versus standard concentration was constructed, and the data were fit by weighted linear regression (MK model, version 5.0; Biosoft, Ferguson, Mo.). The concentration of RWJ-54428 in the serum samples was calculated from this standard curve. Serum vancomycin concentrations were measured by a bioassay. Bioassay plates for vancomycin concentration determination were prepared by inoculating a Bacillus subtilis ATCC 6633 spore suspension into antibiotic medium 8 (Difco). The suspension was poured onto a large bioassay dish and left to solidify for 30 min, and 6-mm-diameter sample wells were made with a hollow punch. Vancomycin analytical standards were prepared in 95% serum-5% water. Twenty microliters of a standard or a serum sample was added to a 6-mm-diameter well. The plates were then incubated overnight at 35°C, and zone diameters were measured with an electronic caliper. A standard curve of the zone diameter versus the standard concentration was constructed, and the data were fit by least-squares linear regression. The concentration of vancomycin in the serum samples was calculated from this standard curve.
RESULTS
Susceptibility studies.
The MICs of RWJ-54428 and other antibiotics for the in vivo test strains are shown in Table 1. For MRSA, RWJ-54428 was 8 to 32 times more active in vitro than the other beta-lactams tested. For S. pneumoniae, RWJ-54428 was as active as cefotaxime, and for E. faecalis, RWJ-54428 was at least eightfold more potent than the other agents tested.
TABLE 1.
In vitro antibacterial activities of RWJ-54428 and other drugs against strains used in animal models
| Antibiotic | MIC (μg/ml)
|
|||||
|---|---|---|---|---|---|---|
| MSSA Smith | MRSA COL | MRSA 076 | VISA HIP-5836 | S. pneumoniae ATCC 6301 | E. faecalis ATCC 23241 | |
| RWJ-54428 | 0.125 | 1 | 1 | 0.5 | 0.015 | 0.125 |
| Ampicillin | NDa | 16 | >32 | ND | ND | 2 |
| Cefazolin | 0.25 | >32 | >32 | ND | ND | ND |
| Cefotaxime | 2 | >32 | >32 | 8 | 0.015 | ND |
| Imipenem | 0.03 | 32 | 64 | 2 | ND | 1 |
| Penicillin G | 0.125 | 8 | 64 | ND | 0.06 | ND |
| Q-Db | ND | 0.06 | 0.25 | 0.25 | ND | 4 |
| Vancomycin | 1 | 1 | 0.5 | 8 | 0.25 | 1 |
ND, not determined.
Q-D, quinupristin-dalfopristin.
Mouse model of sepsis.
The effectiveness of RWJ-54428 and control compounds in sepsis studies is shown in Table 2. For the methicillin-susceptible S. aureus (MSSA) strain (Smith), the ED50 of RWJ-54428 was 1.3 mg/kg (95% confidence interval, 0.7 to 1.6), which was superior to that of cefotaxime, similar to that of vancomycin, but less than that of imipenem. In studies with MRSA 076, the ED50 of RWJ-54428 was similar to those of vancomycin and quinupristin-dalfopristin but superior to that of cefazolin.
TABLE 2.
In vivo efficacies of RWJ-54428 and comparator drugs in a mouse sepsis model
| Antibiotic | MIC (μg/ml) | ED50 (mg/kg) | 95% confidence interval for ED50 |
|---|---|---|---|
| S. aureus Smith | |||
| RWJ-54428 | 0.125 | 1.3 | 0.7-1.6 |
| Vancomycin | 1 | 2.6 | 1.1-3.9 |
| Imipenem | 0.03 | 0.1 | 0.02-0.3 |
| Cefotaxime | 2 | 4.7 | 1.6-10.2 |
| MRSA 076 | |||
| RWJ-54428 | 1 | 6.1 | 3.9-8.3 |
| Vancomycin | 0.5 | 6.5 | 4.5-12.0 |
| Quinupristin-dalfopristin | 0.25 | 9.9 | 3.4-16.5 |
| Cefazolin | >32 | >80 | NAa |
NA, not available.
Pneumonia model.
The activities of RWJ-54428, cefotaxime, and penicillin G in a mouse model of pneumonia caused by S. pneumoniae ATCC 6301 are shown in Table 3. RWJ-54428 treatment resulted in a significant decrease in the number of viable cells in the lungs at a total dose as low as 5 mg/kg. The efficacy of RWJ-54428 at 5 mg/kg/day was significantly greater than those of cefotaxime and penicillin G at 10 mg/kg/day, and RWJ-54428 was only slightly less effective than penicillin G and cefotaxime at 20 mg/kg/day.
TABLE 3.
In vivo antibacterial activities of RWJ-54428, cefotaxime, and penicillin G in a mouse model of pneumonia caused by S. pneumoniae ATCC 6301a
| Compound | MIC (μg/ml) | Total doseb (mg/kg) | Mean ± SD log CFU in lungsc |
|---|---|---|---|
| No treatment | 0 | 7.1 ± 0.5 | |
| RWJ-54428 | 0.015 | 5 | 4.8 ± 0.5** |
| 10 | <2.0d** | ||
| 20 | <2.0d** | ||
| Cefotaxime | 0.015 | 5 | 6.3 ± 0.2* |
| 10 | 5.1 ± 0.7** | ||
| 20 | 2.7 ± 1.1** | ||
| Penicillin G | 0.06 | 5 | 7.0 ± 0.2 |
| 10 | 6.1 ± 0.4* | ||
| 20 | 3.2 ± 0.9** |
The challenge dose was 3.0 × 105 CFU/mouse.
Doses were administered at 24 and 30 h postinoculation.
The numbers of viable CFU in the lungs were counted 18 h after the last dose. The difference between the control group and each treated group was determined by using a Peritz F test (∗, P < 0.05; ∗∗, P < 0.01).
The limit of detection was 2.0 log CFU/lung.
Pyelonephritis model.
The efficacies of RWJ-54428, ampicillin, and vancomycin in a mouse pyelonephritis study caused by a strain of E. faecalis are shown in Table 4. A significant decrease in the number of viable bacteria in the kidneys was observed with RWJ-54428 at total daily doses of ≥30 mg/kg, while significant decreases in bacterial counts were seen with ampicillin and vancomycin only with total daily doses of ≥300 and ≥90 mg/kg, respectively. The efficacy of RWJ-54428 at 90 mg/kg/day was similar to that observed with ampicillin at 300 mg/kg/day, and RWJ-54428 was slightly more effective than vancomycin at 180 mg/kg/day.
TABLE 4.
In vivo antibacterial activities of RWJ-54428, ampicillin, and vancomycin in a mouse model of pyelonephritis caused by E. faecalis ATCC 23241a
| Compound | MIC (μg/ml) | Total daily doseb (mg/kg) | Mean ± SD log CFU in kidneysc |
|---|---|---|---|
| No treatment | 0 | 6.8 ± 0.8 | |
| RWJ-54428 | 0.125 | 3 | 6.8 ± 0.4 |
| 30 | 5.9 ± 0.3* | ||
| 90 | 4.8 ± 0.3** | ||
| Ampicillin | 2 | 30 | 6.4 ± 0.8 |
| 150 | 6.3 ± 0.4 | ||
| 300 | 4.6 ± 1.2** | ||
| Vancomycin | 1 | 30 | 6.1 ± 0.3 |
| 90 | 5.6 ± 0.4* | ||
| 180 | 5.3 ± 0.4** |
The challenge dose was 1.2 × 108 CFU/mouse.
Doses were administered three times daily for 3 days, starting 24 h postinoculation.
The numbers of viable CFU in kidneys harvested 16 to 20 h after the last dose. The difference between the control and each treated group was determined by using a Peritz F test (∗, P < 0.05; ∗∗, P < 0.01).
Mouse pharmacokinetics and results in neutropenic mouse thigh model.
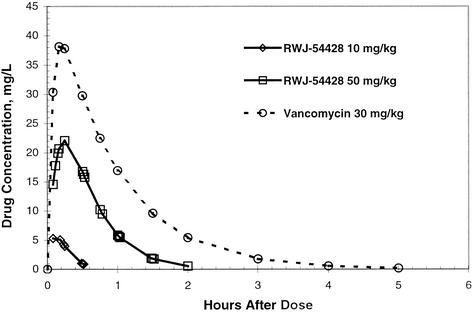
The serum RWJ-54428 and vancomycin concentrations following a single subcutaneous dose in neutropenic mice are shown in Fig. 2. The decline in concentrations was best described by a one-compartment model with first-order absorption. The values for the pharmacokinetic parameters are shown in Table 5. RWJ-54428 was absorbed rapidly after subcutaneous administration, with the times to the maximum concentrations of drug in serum ranging from 0.12 to 0.31 h. The clearances of RWJ-54428 from serum at each dose were within a twofold range.
FIG. 2.
Serum RWJ-54428 and vancomycin concentrations in mice after a single subcutaneous dose.
TABLE 5.
Pharmacokinetic parameters of RWJ-54428 and vancomycin in serum following a single subcutaneous injection in male neutropenic micea
| Compound, dose (mg/kg) | Mean wt (kg) | V/F (liters/kg) | Cmax (mg/liter) | Tmax (h) | AUC (μg · h/liter) | CL/F (liters/h/kg) | t1/2 (h) |
|---|---|---|---|---|---|---|---|
| RWJ-54428 | |||||||
| 10 | 0.022 | 0.6 | 5.6 | 0.12 | 1.84 | 4.9 | 0.08 |
| 50 | 0.022 | 1.3 | 22.1 | 0.25 | 16.8 | 3.0 | 0.29 |
| Vancomycin, 30 | 0.023 | 0.63 | 38.0 | 0.12 | 42.4 | 0.71 | 0.61 |
V/F, volume of distribution; Cmax, maximum concentration of drug in serum; Tmax, time to maximum concentration of drug in serum; AUC, area under the concentration-time curve; CL/F, clearance; t1/2, half-life.
The recovery of MRSA COL from infected thighs following a single dose of RWJ-54428, imipenem, and vancomycin are shown in Table 6. Single 30-mg/kg doses of RWJ-54428 or vancomycin produced a prolonged effect against MRSA COL. Imipenem was not bactericidal at a single dose of 30 mg/kg. The durations of the antibacterial effects in infected thighs were prolonged for both agents. In vivo PAEs also increased with dose and were 2.3 and 6.5 h for RWJ-54428 at doses of 10 and 30 mg/kg, respectively. Calculated effective regrowth times also increased with dose and ranged between 4.4 and 8 h for RWJ-54428 and 8 to 16 h for vancomycin.
TABLE 6.
In vivo activities of RWJ-54428, imipenem, and vancomycin in a neutropenic mouse thigh model caused by MRSA COL
| Compound | MIC (μg/ml) | Dose (mg/kg) | Max Δ log CFU/thigha | ERTb (h) | In vivo PAE (h) |
|---|---|---|---|---|---|
| No treatment | 0 | 2.7 | NAc | NA | |
| RWJ-54428 | 1 | 1 | −0.3 | 4.4 | NDd |
| 10 | −0.5 | 5 | 2.3 | ||
| 30 | −0.6 | 8 | 6.5 | ||
| Imipenem | 32 | 30 | 0.6 | 0 | 0 |
| Vancomycin | 1 | 20 | −0.5 | 8 | 18 |
| 30 | −0.7 | 16 | >20 |
Max Δ log CFU/thigh, maximum reduction in bacterial counts compared to those for the controls at the start of therapy.
ERT, effective regrowth time.
NA, not available.
ND, not determined.
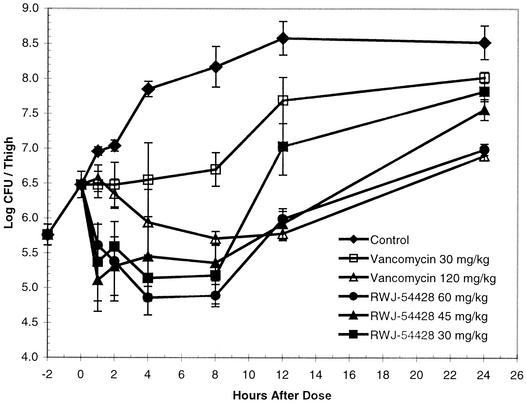
The effects of RWJ-54428 and vancomycin against VISA HIP-5836 are shown in Fig. 3. A single 30-mg/kg dose of RWJ-54428 produced a maximum bactericidal effect of 1.3 log CFU/thigh. In contrast, a single 120-mg/kg dose of vancomycin produced a reduction of only 0.8 log CFU/thigh.
FIG. 3.
Efficacies of single subcutaneous doses of either RWJ-54428 or vancomycin against vancomycin-intermediate resistant S. aureus HIP-5836 in a neutropenic mouse thigh infection model.
DISCUSSION
RWJ-54428 is a new cephalosporin with activity against gram-positive organisms. It has shown excellent activity in vitro against a broad spectrum of gram-positive organisms including MRSA, methicillin-resistant S. epidermidis, methicillin-resistant coagulase-negative staphylococci, VISA, penicillin-susceptible and -resistant pneumococci, group A and group B streptococci, penicillin-susceptible and -resistant viridans group streptococci, and vancomycin-susceptible and -resistant enterococci (1).
Cephalosporins are typically considered to have no clinically important activities against enterococci or mecA-positive (methicillin-resistant) staphylococci. In contrast to earlier cephalosporins studied for the treatment of infections caused by MRSA, RWJ-54428 has a high affinity to PBP 2a, the mecA gene product associated with resistance to methicillin and other beta-lactams (F. Malouin et al., unpublished data). This improved affinity translates into activity in vitro and in vivo against problematic gram-positive pathogenic bacteria. In animal models of infection due to MSSA or MRSA, RWJ-54428 showed efficacy and potency similar to those of vancomycin and was over fourfold more potent than vancomycin against the VISA strain in the neutropenic thigh model. RWJ-54428 showed efficacy superior to those of both penicillin G and cefotaxime against a strain of S. pneumoniae in a mouse pneumonia model. RWJ-54428 reduced bacterial counts to below the limit of detection (2.0 log CFU/lung) at a total dose of 10 mg/kg. Neither penicillin G nor cefotaxime reduced bacterial counts to this level at total doses of up to 20 mg/kg. RWJ-54428 was effectively used to treat an infection caused by a strain of E. faecalis in a mouse model of pyelonephritis. The activity of RWJ-54428 in this model was comparable to those of vancomycin and ampicillin in overall bactericidal effects, but the activity was achieved at two- to threefold lower doses.
RWJ-54428 showed extended in vivo PAEs (6 to 10.5 h). While the in vivo PAEs of RWJ-54428 for MRSA COL are not as long as those of vancomycin, they are longer than those reported for FK037 (3.4 h) and are longer than those reported for penicillin G, nafcillin, and cefazolin (1.4, 3.0, and 4.5 h, respectively) against MSSA ATCC 25923 (8, 14). Further studies to define the relevance of a prolonged in vivo PAE and the target pharmacokinetic-pharmacodynamic parameter for efficacy (e.g., the percentage of the dosing interval at which concentrations exceed the MIC) are required.
Acknowledgments
The assistance of Thamil Annamalai, Johanne Blais, Sarah Bond, Suzanne Chamberland, Sharon Chen, Doug Clark, Erik Corcoran, Keith Huie, Craig Park, and Vrushali Tembe is gratefully acknowledged.
REFERENCES
- 1.Chamberland, S., J. Blais, M. Hoang, C. Dinh, D. Cotter, E. Bond, C. Gannon, C. Park, F. Malouin, and M. N. Dudley. 2001. In vitro activity of RWJ-54428 (MC-02,479) against multi-resistant gram-positive bacteria. Antimicrob. Agents Chemother. 45:1422-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins, M. S., A. Edwards, R. E. Roby, N. S. Mehton, and D. Ladehoff. 1989. Pseudomonas immune globulin therapy improves survival in experimental Pseudomonas aeruginosa bacteremic pneumonia. Antibiot. Chemother. 42:184-192. [DOI] [PubMed] [Google Scholar]
- 3.Craig, W. A., and B. Vogelman. 1987. The postantibiotic effect. Ann. Intern. Med. 106:900-902. [DOI] [PubMed] [Google Scholar]
- 4.Hanberger, H., E. Svensson, M. Nilsson, L. E. Nilsson, E. G. Hornsten, and R. Maller. 1993. Effects of imipenem on Escherichia coli studied using bioluminescence, viable counting and microscopy. J. Antimicrob. Chemother. 31:245-260. [DOI] [PubMed] [Google Scholar]
- 5.Hecker, S. J., T. W. Glinka, A. Cho, Z. J. Zhang, M. E. Price, S. Chamberland, D. Griffith, and V. J. Lee. 2000. Discovery of RWJ-54428 (MC-02,479), a new cephalosporin active against resistant gram-positive bacteria. J. Antibiot. 53:1272-1281. [DOI] [PubMed] [Google Scholar]
- 6.Heffelfinger, J. D., S. F. Dowell, J. H. Jorgensen, K. P. Klugman, L. R. Mabry, D. M. Musher, J. F. Plouffe, A. Rakowsky, A. Schuchat, and C. G. Whitney. 2000. Management of community-acquired pneumonia in the era of pneumococcal resistance: a report from the Drug-Resistant Streptococcus pneumoniae Therapeutic Working Group. Arch. Intern. Med. 160:1399-1408. [DOI] [PubMed] [Google Scholar]
- 7.Johnson, A. P., M. Warner, M. Carter, and D. M. Livermore. 2002. In vitro activity of cephalosporin RWJ-54428 (MC-02,479) against multidrug-resistant gram-positive cocci. Antimicrob. Agents Chemother. 46:321-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mine, Y., Y. Watanabe, H. Sakamoto, K. Hatano, Y. Wakai, T. Kamimura, S. Tawara, S. Matsumoto, F. Matsumoto, and S. Kuwahara. 1993. In vivo antibacterial activity of FK037, a novel parenteral broad-spectrum cephalosporin. J. Antibiot. 46:88-98. [DOI] [PubMed] [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution of antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed. Approved standard M7-A4, vol. 17, no. 2. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 10.Pasiello, A. P., J. M. Essigman, and G. N. Wogan. 1977. Rapid and accurate determination of median lethal dose (LD50) and its error with a small computer. J. Toxicol. Environ. 3:797-809. [DOI] [PubMed] [Google Scholar]
- 11.Swenson, J. M., and F. C. Tenover. 2002. In vitro activity of a new cephalosporin, RWJ-54428, against streptococci, enterococci and staphylococci, including glycopeptide-intermediate Stapylococcus aureus. J. Antimicrob. Chemother. 49:845-850. [DOI] [PubMed] [Google Scholar]
- 12.Teng, N. H., H. S. Kaplan, J. M. Herbert, C. Moore, H. Douglas, A. Wunderlich, and A. I. Braude. 1975. Protection against gram-negative bacteremia and endotoxemia with human monoclonal IgM antibodies. Proc. Natl. Acad. Sci. USA 82:1790-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tenover, F. C. 1999. Implications of vancomycin resistant Staphylococcus aureus. J. Hosp. Infect. 43(Suppl.):S3-S7. [DOI] [PubMed]
- 14.Vogelman, B., S. Gudmundsson, J. Turnidge, J. Leggett, and W. A. Craig. 1988. In vivo postantibiotic effect in a thigh infection in neutropenic mice. J. Infect. Dis. 157:287-298. [DOI] [PubMed] [Google Scholar]
- 15.Whitney, C. G., M. M. Farley, J. Hadler, L. H. Harrison, C. Lexau, A. Reingold, L. Lefkowitz, P. R. Cieslak, M. Cetron, E. R. Zell, J. H. Jorgensen, and A. Schuchat. 2000. Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States. N. Engl. J. Med. 343:1917-1924. [DOI] [PubMed] [Google Scholar]