Abstract
Ribavirin in combination with alpha 2 interferon is the consensus treatment for chronic hepatitis C. However, recent preliminary pharmacological studies have suggested that the bioavailability of ribavirin displays great interindividual variability. In order to monitor serum ribavirin levels during combination treatment, we developed and validated a quantitative assay using an approach adaptable for routine hospital laboratories. The method involved solid-phase extraction on phenyl boronic acid cartridges followed by high-performance liquid chromatography with a C18-bonded silica column and a mobile phase containing 10 mM ammonium phosphate buffer (with the pH adjusted to 2.5) and UV detection (207 nm). The sensitivity, recovery, linearity of the calibration curve, intra- and interassay accuracies, precision, and stability at 4°C were consistent with its use in the laboratory routine. In addition, other nucleoside analogues sometimes used with ribavirin in patients coinfected with hepatitis C virus (HCV) and human immunodeficiency virus did not interfere with the quantification of ribavirin levels. The ribavirin concentration was quantified in 24 serum samples from patients with chronic hepatitis C treated with a combination of ribavirin and alpha 2 interferon. The mean serum ribavirin concentration was 2.67 ± 1.06 μg/ml (n = 24) at week 12 of treatment (W12) and 3.24 ± 1.35 μg/ml (n = 24) at week 24 of treatment (W24). In addition, ribavirin concentrations displayed high interindividual variabilities: the coefficients of variation of the serum ribavirin concentrations adjusted to the administered dose were 44 and 48% at W12 and W24, respectively. Moreover, the ribavirin concentration was higher in patients with a sustained virological response (n = 11) than in patients with treatment failure (n = 13), with significant intergroup differences at W12 (P = 0.030) and W24 (P = 0.049). The present study describes a simple analytical method for the quantification of ribavirin in human serum that could be a useful tool for the monitoring of ribavirin concentrations in HCV-infected patients in order to improve the efficacy and safety of therapy with ribavirin plus interferon.
In patients chronically infected with hepatitis C virus (HCV), combination therapy with ribavirin plus alpha 2 interferon has greatly improved the rates of sustained biochemical and virological response compared to those achieved with interferon monotherapy (3, 6, 12, 14). Unfortunately, more than 40% of treated patients either remain nonresponsive or have relapses with this treatment. Although some epidemiological factors support the predictive value of the treatment in terms of the long-term outcome, no clear explanation for the lack of efficacy in some patient subgroups has been provided. Resistance to interferon is the hypothesis that has been the most investigated, but other mechanisms including those related to ribavirin should also be closely examined.
The pharmacokinetics of ribavirin were initially investigated in studies with healthy volunteers and patients after the administration of either a single dose or multiple doses (4). Although intermediate bioavailability and a high level of interindividual variability were described, no further evaluation was performed with patients with chronic HCV infection receiving combination therapy. However, the extensive use of this drug, which induces numerous side effects, requires complementary pharmacological investigations in order to adjust the dose of the drug according to individual patient characteristics. For instance, no data on the bioavailability of ribavirin from large-scale clinical trials of the efficacy of combination treatment have been published. One reason could be that no standard assay for plasma ribavirin concentration determination was available for routine laboratories, although descriptions of some analytical methods have been published, including radioimmunoassay (1), high-performance liquid chromatography (HPLC) with UV (5, 7, 10, 13, 19, 20) or mass spectrometric (15) detection, and gas chromatography-mass spectrometry (16). However, these methods differ according to their sensitivities, specificities, and difficulty (time and cost).
The main purpose of the present study was to adapt and validate a routine assay for the quantification of ribavirin in serum for the management of therapy in patients with chronic HCV infection. In this regard, an HPLC method with commercially available reagents was chosen since its use is feasible in most hospital laboratories. In order to illustrate this technique, we retrospectively tested a small number of treated patients and compared their serum ribavirin concentrations to their response to treatment.
MATERIALS AND METHODS
Chemicals and instruments.
All chemicals were of HPLC or reagent grade and were obtained from Prolabo (Paris, France). Ribavirin (1-beta-d-ribofuranosyl-1H-1,2,4 triazole-3-carboxamide) (purity, 99%) and 3-methylcytidine methosulfate (purity, 98%), which was used as an internal standard (IS), were purchased from Sigma (Saint-Quentin Fallavier, France). Zidovudine, lamivudine, and abacavir were from Glaxo Wellcome (London, United Kingdom); acyclovir was from Glaxo Wellcome (Marly le Roi, France); and zalcitabine and ganciclovir were from Roche (Neuilly-sur-Seine, France). Didanosine and stavudine were from Bristol-Myers Squibb (Princeton, N.J.).
Phenyl boronic acid cartridges (Bond Elute phenyl boronic acid [100 mg/10 ml]), which were used for solid-phase extraction (SPE), were obtained from Varian (Harbor City, Calif.) Sample Preparation Product. The cartridges were positioned on a Vac Elut vacuum manifold from the Varian Sample Preparation Product. Stock solutions of ribavirin (10 mg/ml) and 3-methylcytidine methosulfate (10 mg/ml) were prepared in distilled water and were stored in 1.0-ml aliquots at −20°C until use. Working solutions of ribavirin (100 and 10 μg/ml) and 3-methylcytidine methosulfate (100 μg/ml) were prepared weekly by dilution of stock solutions in methanol. Stock solutions of anti-human immunodeficiency virus (anti-HIV) nucleoside analogues (0.1 mg/ml) were prepared in methanol, stored at −20°C until use, and then diluted in distilled water to 30 μg/ml for abacavir and to 10 μg/ml for all the other nucleoside analogues.
HPLC apparatus and conditions.
The HPLC system used consisted of the following: an M-510 HPLC pump (Waters Associates, Milford, Mass.) set to deliver 0.7 ml/min, which led to a typical back pressure of 1,500 lb/in2; a Rheodyne 7125 injection system with an injection volume of 20 μl; a C18-bonded silica column with 5-μm beads (3.9 by 300 mm; Novapak); an L4000 UV detector (Merck, Darmstadt, Germany); and a Shimadzu model C-R3A integrator.
The wavelength was fixed at 207 nm, and the sensitivity was 0.005 absorbance units. The mobile-phase solvent consisted of 10 mM ammonium phosphate buffer (the pH was adjusted to 2.5 with orthophosphoric acid). After the run analysis, the HPLC column was stored in an acetonitrile-water (50:50; vol/vol) mixture.
Solid-phase extraction.
The extraction procedure that follows was performed by a methodology derived from a previous report (7). The assay was calibrated by using five nonzero calibrators containing 0.2, 0.5, 1, 3, and 5 μg of ribavirin per ml and the IS (2.5 μg/ml). The calibrators were obtained by diluting the working solutions with blank bovine serum (Biotrol, Biotrol Diagnosis, Chennevrières, France). Previous experiments demonstrated that double blanks were similar in human serum and bovine serum (data not shown).
The samples (1 ml) were diluted (1:4) in 250 mM ammonium acetate buffer (the pH was adjusted to 8.5 with ammoniac). The diluted samples were loaded onto phenyl boronic acid cartridges that had been pretreated with 1 ml of 3% formic acid in methanol, followed by 5 ml of 250 mM ammonium acetate buffer (pH 8.5). The samples were drained away and the cartridges were washed five times with 1-ml aliquots of 250 mM ammonium acetate buffer. Ribavirin and the IS were subsequently eluted with 1 ml of 3% formic acid into glass tubes. The effluents were dried under nitrogen and reconstituted with 200 μl of the mobile phase. Twenty microliters of the reconstituted samples was injected onto the HPLC column.
Validation of the method.
The validation of the method was performed in accordance with Food and Drug Administration guidelines (17, 18). The linearity was tested by assaying a set of five calibrators (0.2, 0.5, 1, 3, and 5 μg/ml) six times and by calculating the measured concentration of each calibrator from the calibration curve. A plot of the percent deviation from the calculated concentration against the expected concentration was drawn and inspected for trends.
Within-day precision was calculated by repeated analysis of spiked serum samples (0.2, 1, and 3 μg/ml) during 1 working day by the same operator. Between-day precision was calculated by analysis of serum samples spiked with the same concentration of ribavirin, with one analysis being performed a day.
The stability of ribavirin in serum was assessed by five replicate analyses of three samples spiked with ribavirin (0.2, 1, and 3 μg/ml). The samples were analyzed immediately or after storage at 4°C for 24, 48, 72, or 96 h, respectively.
To determine the stability of ribavirin after freezing-thawing, control plasma samples spiked with 2 μg of ribavirin per ml were compared with samples of the same batch that were frozen at −20°C and thawed at ambient temperature. This freeze-thaw cycle was repeated three times. In addition, the stability of ribavirin in dried residues after SPE was tested with four replicates spiked with 0.2, 1, and 3 μg of ribavirin per ml. Each sample was extracted, dried under nitrogen, and analyzed immediately or after storage at −20°C for 24, 48, or 72 h, respectively.
In order to investigate the influence of the matrix on the concentration of ribavirin, the specificity of the assay was investigated by analyzing blank plasma from six different human plasma samples spiked with 2 μg of ribavirin per ml.
Lastly, some nucleoside inhibitors most likely to be encountered in the plasma of HCV-positive patients as a result of concomitant therapy were screened by the HPLC assay. Drugs were tested as pure solutions diluted in methanol so that the concentrations were 30 μg/ml for abacavir and 10 μg/ml for the other nucleoside analogue tested; the drugs were then injected into the system, and their peak retention times were compared with the peak retention time of ribavirin. An interfering drug was defined as a molecule that exhibited a retention time within 0.3 min of that of ribavirin or the IS.
Demographic and clinical characteristics of the patients tested.
Twenty-four patients with chronic HCV infection previously treated with combination therapy were selected for the analysis, provided that blood samples recovered on week 12 of treatment (W12) and week 24 of treatment (W24) were available from a stored serum bank. The patients' characteristics are summarized in Table 1. All patients were infected with a genotype 1 isolate of HCV and were drug-naive patients receiving their first anti-HCV treatment, consisting of interferon plus ribavirin at the doses usually prescribed at that time, as follows: subcutaneous injection of standard alpha 2 interferon at 3 million IU three times per week and 1,000- or 1,200-mg ribavirin tablets per day according to their weight (less than or more than 75 kg, respectively). Because of side effects caused by the drugs, two patients were receiving a reduced ribavirin dose (600 mg/day) at the time of assay for ribavirin quantification. All the patients were negative for HBsAg and HIV infection. The clinical outcome analysis defined among the 24 patients two subgroups of patients with equilibrated responses: 11 patients had sustained virological responses and 13 patients had treatment failures (the patients were either nonresponders or relapsers). Responders were defined as patients with a negative test result for HCV RNA at the end of therapy (48 weeks, which is the period of treatment for the responders at present), and nonresponders were defined as patients with a positive test result for HCV RNA at the end of a course of 24 weeks of therapy.
TABLE 1.
Clinical characteristics of patients with chronic HCV infection and treated with ribavirin-interferon combination therapya
| Patients characteristic | All patients (n = 24) | Responders (n = 11) | Nonresponders (n = 13) |
|---|---|---|---|
| Mean (minimum-maximum) age (yr) | 44.3 (24-67) | 39 (24-67) | 49 (35-62) |
| Mean (minimum-maximum) wt (kg) | 73.8 kg (50-112) | 76 (50-112) | 72 (51-100) |
| Mean (minimum-maximum) Knodell score | 9.7 (4-15) | 9.5 (6-15) | 9.5 (4-15) |
| Mean (minimum-maximum) Métavir | |||
| Activity | 2.0 (1-3) | 2 (1-3) | 2 (1-3) |
| Fibrosis | 2.1 (0-4) | 2.1 (1-3) | 2 (0-4) |
| Mean (minimum-maximum) ALAT (ULN)b | 2.2 (0.94-4.7) | 2.3 (0.9-4.1) | 2.1 (0.9-1.7) |
| Mean (minimum-maximum) viral load IU/ml | 1,238,767 IU/ml (8,000-5,078,650) | 1,126,218 IU/ml (8,000-3,160,000) | 1,334,000 IU/ml (111,300-5,078,650) |
| Ribavirin dose (mg/day) | 1,000 (n = 9), 1,200 (n = 2) | 600 (n = 2, adjusted for adverse effects), 1,000 (n = 8), 1,200 (n = 3) |
All patients were infected with genotype 1 HCV isolates and were drug naive and receiving their first standard combination therapy.
ALAT, alanine aminotransferase; ULN, upper limit of normality.
Blood samples were collected from October 1998 to August 2000 in Vacutainer tubes without additives. After centrifugation the serum was transferred to another tube and stored at −80°C until analysis. Ribavirin quantification was carried out with serum samples drawn 2 to 4 h after the morning administration of ribavirin at W12 and W24 of a 48-week combination treatment.
No unusual investigations and the administration of no specific drug was requested from the patients for the purpose of this study, which conformed with local ethical considerations and the principles outlined in the Declaration of Helsinki.
Statistical analysis.
For each series of experiments, the variability of the ribavirin concentration was estimated by determination of the relative standard deviation (17, 18). The dose-normalized concentration of ribavirin was expressed as the concentration in serum (in micrograms per milliliter) adjusted to the daily ribavirin dose according to the patient's weight (milligrams per day per kilogram of body weight).
For the retrospective comparison of serum ribavirin concentrations according to the outcome for the patients, a nonparametric Mann-Whitney test was used in order to avoid the making of assumptions about the distributions of the measured variables. P values <0.05 were considered significant.
RESULTS
Ribavirin assay validation.
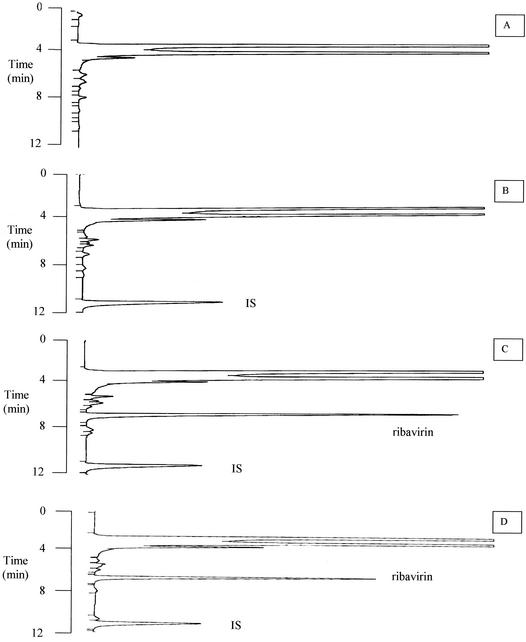
Typical chromatograms of blank serum, blank serum with IS, serum supplemented with a medium-range calibrator, and patient serum containing ribavirin are shown in Fig. 1. The retention times of ribavirin and the IS were 6.7 and 11.0 min, respectively.
FIG. 1.
Typical HPLC chromatograms of extracts of control human serum (A), control human serum spiked with 2.5 μg of IS per ml (B), control human serum spiked with 2.5 μg of IS per ml and 3.0 μg of ribavirin per ml (C), and serum from a patient receiving ribavirin (ribavirin concentration, 2.5 μg/ml) (D).
A plot of the ratio of the height of ribavirin/height of the IS versus the ribavirin concentration provides a linear relationship described by the equation y = 0.977 (± 0.161) x − 0.0173 (± 0.095), with r equal to 0.988 ± 0.007. This linear correlation was verified over a range of concentrations from 0.1 to 10.0 μg/ml.
Extraction recovery, expressed as a percentage, was defined as the ratio of the slope of the calibration curve for the extracted analytes to the slope of the calibration curve for the nonextracted analytes. The recovery was 87%.
Within-day and between-day precisions are given in Table 2. The lower limit of quantification and the limit of detection were set at 0.2 and 0.06 μg/ml, respectively, for extracted samples.
TABLE 2.
Within-day and between-day variabilities of ribavirin concentrations in serum samples measured by HPLC with UV detectiona
| Spiked concn (μg/ml) | Relative % standard deviationb
|
|
|---|---|---|
| Within-day precision | Between-day precision | |
| 0.2 | 4.8 (0.2 ± 0.0) | 17.8 (0.2 ± 0.0) |
| 1 | 11.2 (0.9 ± 0.1) | 9.4 (1.0 ± 0.1) |
| 3 | 6.9 (3.1 ± 0.1) | 8.9 (2.8 ± 0.2) |
Five serum samples were tested.
The values in parentheses are means ± standard errors of the mean accuracy (in micrograms per milliliter).
The concentrations of ribavirin in serum stored at 4°C were not reduced over 96 h; the coefficient of variation of the slopes of the linear regression lines was 14%.
In contrast, storage of dried residues at −20°C for 72 h induced a gradual decrease in the ribavirin concentration. The slopes of the linear regression lines were 0.944 for fresh samples and 0.787, 0.623, and 0.591 after storage at −20°C for 24, 48, and 72 h, respectively. After storage for 72 h at −20°C, the concentration of ribavirin was reduced by 37% compared with the concentration in fresh samples.
Three freeze-thaw cycles with spiked samples induced a trend toward a loss of ribavirin. The concentration of ribavirin was reduced by 33% compared with the concentration in fresh samples after three freeze-thaw cycles.
There was no influence of the matrix on the concentration of ribavirin (coefficient of variation, 6%). In addition, the nucleoside analogues didanosine, stavudine, zidovudine, lamivudine, abacavir, zalcitabine, acyclovir, and ganciclovir did not interfere with the analytical method.
Serum ribavirin concentrations during combination treatment.
The mean absolute concentration of ribavirin at W12 was 2.67 ± 0.21 μg/ml (n = 24). The ribavirin concentration at W24 (3.23 ± 0.28 μg/ml; n = 24) was similar to that at W12. The mean relative concentrations of ribavirin adjusted according to the dose of ribavirin received by the patient (in milligrams per kilogram per day) were 0.20 ± 0.02 μg/ml/dose (n = 24) at W12 and 0.24 ± 0.02 μg/ml/dose (n = 24) at W24. The coefficients of variation for these adjusted concentrations were 44 and 48% at W12 and W24, respectively. The ranges of ribavirin concentrations at W12 and W24 were 0.07 to 0.35 and 0.08 to 0.48 μg/ml/dose, respectively.
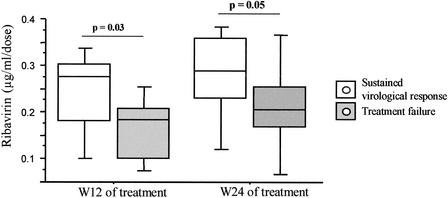
In order to evaluate the interest in quantification of ribavirin in serum in the management of patients treated for HCV infection, we retrospectively compared the concentrations of ribavirin according to the outcome status of the patients tested. In the responder group of patients (n = 11), the relative concentrations of ribavirin were 0.24 ± 0.03 and 0.28 ± 0.03 μg/ml/dose at W12 and W24, respectively, while in patients with treatment failures (n = 13) they were 0.16 ± 0.20 and 0.21 ± 0.32 μg/ml/dose at W12 and W24, respectively. These relative concentrations of ribavirin were significantly different between patients who were responders and those who were nonresponders (P < 0.030 at W12 and P < 0.049 at W24 [Mann-Whitney test]) (Fig. 2).
FIG. 2.
Concentration of ribavirin in sera from 11 responders and 13 nonresponders after 12 and 24 weeks of ribavirin and interferon combination therapy. Data are presented as box plots in which 50% of the values lie within the box. The horizontal lines drawn through the middle of the boxes represent the median values. The top and bottom of each box are the 10th and 90th percentiles of all values, respectively. P values were 0.03 and 0.05 for responder patients versus nonresponder patients after 3 and 6 months of treatment, respectively.
DISCUSSION
This study presents the validation of a simple bioanalytical method for the quantification of ribavirin in human serum. Its feasibility for the monitoring of drug therapy for HCV-infected patients was also investigated.
The method was adapted from a previous report (7) by changing the detection wavelength (207 nm) and modifying the SPE and purification steps. Indeed, a methanolic solution containing 3% formic acid was used to elute ribavirin in the SPE procedure in order to improve the evaporation step. Finally, the stability of ribavirin during sample storage and interference with other nucleoside analogues were assessed.
The method described here yielded linear results, with a lower limit of quantification of 0.2 μg/ml with samples from which ribavirin was extracted. In addition, the limit of detection (0.06 μg/ml) was in the same range as those previously described for HPLC methods with UV detection (19, 20). The sensitivity, recovery, linearity of the calibration curve, and intra-and interassay precisions of the present method agreed with the values specifically recommended for validation of bioanalytical methods (17, 18) and allowed the quantification of ribavirin over a wide range of clinically relevant concentrations (9).
No interference by other nucleotide analogues was found. This specificity is due to the SPE procedure, which is based on the principle that ribavirin and other molecules with vicinal hydroxyl groups specifically bind to phenyl boronic acid at high pH. Since didanosine, stavudine, zidovudine, lamivudine, abacavir, zalcitabine, acyclovir, and ganciclovir have no vicinal hydroxyl group in their structures, analytical interference was no longer expected. It was important to validate this point, since patients coinfected with HCV and HIV are more frequently treated with a combination of ribavirin and other nucleoside inhibitors. This combination treatment sometimes results in increased toxicity for mitochondria (10) that could be managed by adaptation of the dose to match the clinical characteristics of these patients.
Analysis of the stability of ribavirin under various storage conditions showed that the ribavirin concentration remains stable in serum stored for 4 days at 4 or −20°C, whereas it tends to decrease in dried residues stored at −20°C for 3 days as well as in samples undergoing three freeze-thaw cycles. These data suggest that storage of dried residues as well as freeze-thaw cycles should be avoided for the preservation of ribavirin in human serum, whereas storage of serum samples at 4 or −20°C ensures the good preservation of ribavirin for 4 days.
It has been suggested that only 27% of the pharmacokinetic variability in ribavirin clearance relies on the main cofactors influencing this clearance, including body weight, gender, age, and serum creatinine levels (8). Therefore, the bioavailability of ribavirin is probably controlled by many other, unknown parameters. In this context, a reliable and simple method for the quantification of ribavirin in serum could be highly helpful for the management of patients with HCV infection receiving combination treatment. Indeed, not only response rates but also the frequency of adverse events are probably dependent on the interindividual variability of serum ribavirin concentrations. The monitoring of serum ribavirin concentrations could improve the efficacy and the safety of the combination treatment. The minimum dose of ribavirin required for efficacy has not been clinically established. Initial clinical trials have adopted a standard dose defined on the basis of the results of pilot studies (2, 4). The recommended dose of ribavirin was chosen to be 1,000 or 1,200 mg according to the patient's weight (less than or more than 75 kg, respectively). A subsequent recommendation for adaptation of the dose according to body weight was made by the manufacturer (800, 1,000, and 1,200 mg for body weights of <65, 65 to 85, and >85 kg, respectively). To our knowledge, these recommendations are based not on direct measurements of ribavirin concentrations but on extrapolation of statistical relations among dose, body weight, and response status.
Thus, the analytical method for the quantification of ribavirin presented in this paper could be a useful tool that can be used to define the minimum efficient dose of ribavirin that takes into account all the factors affecting pharmacokinetic variability instead of body weight only. Subsequently, it will probably be used to define new rules for dose adjustment for the management of ribavirin-related adverse events.
The secondary objective of the study was to explore a possible relation between the bioavailability of ribavirin and the efficacy of combination therapy. Our study population was chosen in order to represent the most likely group of patients who could benefit from monitoring of ribavirin concentrations: patients infected with HCV genotype 1, patients undergoing first treatment for cure, and patients undergoing treatment with the standard combination of interferon and ribavirin. The response status of these patients was defined as treatment success (patients with sustained virological responses) or treatment failure (nonresponders and patients who were responders but who then had relapses). Our data suggest that high serum ribavirin levels could be one factor predictive of long-term treatment success. A recent clinical trial with pegylated interferon supports our observation by showing that the doses of the two medications (ribavirin and interferon) received by the patient are significant predictors of a sustained virological response (P = 0.015 for ribavirin and P = 0.002 for pegylated interferon) (11). Another study confirmed that the ribavirin concentration at treatment week 4 is positively correlated to the initial virological response (a negative result by an HCV-specific reverse transcription-PCR at week 24) (8). Our data suggest that this relation could be extended to the sustained virological response (a negative PCR result at week 24 of posttreatment follow-up). In this context, monitoring of the ribavirin concentration at the time of treatment initiation could help to adapt the ribavirin dose to the patient's pharmacological characteristics in order to improve efficacy in the case of low bioavailability or to prevent severe adverse events in the case of high bioavailability.
In conclusion, the present study describes a simple analytical method for the quantification of ribavirin in human serum that could be a useful tool for the monitoring of ribavirin concentrations in patients with chronic HCV infection in order to improve the efficacy and the safety of therapy with ribavirin plus interferon.
Acknowledgments
This study was supported by a grant from Schering-Plough Laboratories.
REFERENCES
- 1.Austin, R. K., P. E. Trefts, M. Hintz, J. D. Connor, and M. F. Kagnoff. 1983. Sensitive radioimmunoassay for the broad-spectrum antiviral agent ribavirin. Antimicrob. Agents Chemother. 24:696-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braconier, J. H., O. Paulsen, K. Engman, and A. Widell. 1995. Combined alpha-interferon and ribavirin treatment in chronic hepatitis C: a pilot study. Scand. J. Infect. Dis. 27:325-329. [DOI] [PubMed] [Google Scholar]
- 3.Davis, G. L. 1999. Combination therapy with interferon alfa and ribavirin as retreatment of interferon relapse in chronic hepatitis C. Semin. Liver Dis. 19:49-55. [PubMed] [Google Scholar]
- 4.Glue, P. 1999. The clinical pharmacology of ribavirin. Semin. Liver Dis. 19:17-24. [PubMed] [Google Scholar]
- 5.Granich, G. G., D. J. Krogstad, J. D. Connor, K. L. Desrochers, and C. Sherwood. 1989. High-performance liquid chromatography (HPLC) assay for ribavirin and comparison of the HPLC assay with radioimmunoassay. Antimicrob. Agents Chemother. 33:311-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heathcote, J. 2000. Antiviral therapy for patients with chronic hepatitis C. Semin. Liver Dis. 20:185-199. [DOI] [PubMed] [Google Scholar]
- 7.Homma, M., A. L. Jayewardene, J. Gambertoglio, and F. Aweeka. 1999. High-performance liquid chromatographic determination of ribavirin in whole blood to assess disposition in erythrocytes. Antimicrob. Agents Chemother. 43:2716-2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jen, J. F., P. Glue, S. Gupta, D. Zambas, and G. Hajian. 2000. Population pharmacokinetic and pharmacodynamic analysis of ribavirin in patients with chronic hepatitis C. Ther. Drug Monit. 22:555-565. [DOI] [PubMed] [Google Scholar]
- 9.Khakoo, S., P. Glue, L. Grellier, B. Wells, A. Bell, C. Dash, I. Murray-Lyon, D. Lypnyj, B. Flannery, K. Walters, and G. M. Dusheiko. 1998. Ribavirin and interferon alfa-2b in chronic hepatitis C: assessment of possible pharmacokinetic and pharmacodynamic interactions. Br. J. Clin. Pharmacol. 46:563-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lafeuillade, A., G. Hittinger, and S. Chadapaud. 2001. Increased mitochondrial toxicity with ribavirin in HIV/HCV coinfection. Lancet 357:280-281. [DOI] [PubMed] [Google Scholar]
- 11.Manns, M. P., J. G. McHutchison, S. C. Gordon, V. K. Rustgi, M. Shiffman, R. Reindollar, Z. D. Goodman, K. Koury, M. Ling, and J. K. Albrecht. 2001. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 358:958-965. [DOI] [PubMed] [Google Scholar]
- 12.McHutchison, J. G., and T. Poynard. 1999. Combination therapy with interferon plus ribavirin for the initial treatment of chronic hepatitis C. Semin. Liver Dis. 19:57-65. [PubMed] [Google Scholar]
- 13.Paroni, R., C. R. Sirtori, C. Borghi, and M. G. Kienle. 1987. High-performance liquid chromatographic determination of ribavirin in serum and urine and of its urinary metabolite 1,2,4-triazole-3-carboxamide. J. Chromatogr. 420:189-196. [DOI] [PubMed] [Google Scholar]
- 14.Poynard, T., P. Marcellin, S. S. Lee, C. Niederau, G. S. Minuk, G. Ideo, V. Bain, J. Heathcote, S. Zeuzem, C. Trepo, J. Albrecht, et al. 1998. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. Lancet 352:1426-1432. [DOI] [PubMed] [Google Scholar]
- 15.Preston, S. L., G. L. Drusano, P. Glue, J. Nash, S. K. Gupta, and P. McNamara. 1999. Pharmacokinetics and absolute bioavailability of ribavirin in healthy volunteers as determined by stable-isotope methodology. Antimicrob. Agents Chemother. 43:2451-2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roboz, J., and R. Suzuki. 1978. Determination of 1-beta-d-ribofuranosyl-1,2,4-triazole-3-carboxamide (virazole) in blood and urine by chemical ionization-mass fragmentography. J. Chromatogr. 160:169-179. [DOI] [PubMed] [Google Scholar]
- 17.Shah, V. P., K. K. Midha, S. Dighe, I. J. McGilveray, J. P. Skelly, A. Yacobi, T. Layloff, C. T. Viswanathan, C. E. Cook, R. D. McDowall, et al. 1991. Analytical methods validation: bioavailability, bioequivalence and pharmacokinetic studies. Conference report. Eur. J. Drug Metab. Pharmacokinet. 16:249-255. [DOI] [PubMed] [Google Scholar]
- 18.Shah, V. P., K. K. Midha, J. W. Findlay, H. M. Hill, J. D. Hulse, I. J. McGilveray, G. McKay, K. J. Miller, R. N. Patnaik, M. L. Powell, A. Tonelli, C. T. Viswanathan, and A. Yacobi. 2000. Bioanalytical method validation—a revisit with a decade of progress. Pharm. Res. 17:1551-1557. [DOI] [PubMed] [Google Scholar]
- 19.Smith, R. H., and B. E. Gilbert. 1987. Quantification of ribavirin in biological fluids and tissues by high-performance liquid chromatography. J. Chromatogr. 414:202-210. [DOI] [PubMed] [Google Scholar]
- 20.Svensson, J. O., A. Bruchfeld, R. Schvarcz, and L. Stahle. 2000. Determination of ribavirin in serum using highly selective solid-phase extraction and high-performance liquid chromatography. Ther. Drug Monit. 22:215-218. [DOI] [PubMed] [Google Scholar]