Abstract
OBJECTIVE
This study investigated (1) the effect of octreotide-LAR (Sandostatin-LAR®Depot; Novartis) on the enteroinsular axis in a biracial cohort of severely obese adults, (2) whether octreotide suppression of insulin secretion occurs by both a direct β-cell effect and through mediating a glucagon-like peptide 1 (GLP-1) response, and (3) whether differences in GLP-1 concentrations could explain racial differences in insulin concentrations.
DESIGN
Prospective, open-label trial using a pre–post test design. SETTING: Single university, clinical research center.
SUBJECTS
In all, 42 healthy, severely obese Caucasian and African-American (AA) adults (93% female, 64% Caucasian, age = 37.8 ± 1.2 y, weight = 123 ± 4.2 kg, BMI = 44.5 ± 1 kg/m2), recruited through physician referral and newspaper ads, participated in the study.
INTERVENTIONS
Indices of β-cell activity, insulin and GLP-1 response before and during a 75-gm oral glucose tolerance test were determined before and after 24 weeks of octreotide-LAR.
RESULTS
AA exhibited higher β-cell activity, and insulin and GLP-1 concentrations than Caucasians. Octreotide-LAR suppressed the insulin and GLP-1 levels in both groups.
Keywords: insulin, somatostatin, octreotide, glucagon-like peptide-1, race
Introduction
Hyperinsulinemia is the hallmark of obesity and several of the obesity-related diseases including type II diabetes mellitus (T2DM) and cardiovascular disease (CVD). Whether the hyperinsulinemia is primary or secondary to obesity is still in debate. African-Americans (AA) have a higher prevalence of obesity, T2DM and CVD, and higher resting and stimulated insulin concentrations than obese Caucasians, which cannot be explained by the severity of obesity or the degree of insulin sensitivity. The enteroinsular axis (EIA) is responsible for >50% of the postprandial insulin release, and glucagon-like peptide-1 (GLP-1) is the major determinant of early insulin secretion in response to a mixed meal.1 It has been suggested that the somatostatin analogue, octreotide, decreases glucose-stimulated insulin response in a dose-dependent way through a specific subset of somatostatin receptors on pancreatic β cells.2–5 This study investigated (1) the effect of octreotide-LAR (Sandostatin-LAR®Depot; Novartis) on the EIA in a biracial cohort of severely obese adults, (2) whether octreotide suppression of insulin secretion occurs by both a direct β-cell effect and through mediating GLP-1 response, and (3) whether differences in GLP-1 concentrations could explain racial differences in insulin concentrations.
Materials and methods
In all, 42 healthy, severely obese Caucasian and AA adults (93% female, 64% Caucasian, age = 37.8 ± 1.2 y, weight = 123 ± 4.2 kg, BMI = 44.5 ± 1 kg/m2) participating in an open-label trial of octreotide-LAR (Sandostatin-LAR®Depot; Novartis) were evaluated. After undergoing a complete history and physical, anthropometric measurements and a 3-day food record was obtained. Macro- and micro-nutrient analyses of energy intake were determined by the software program Ohio Distinctive Software (Columbus, OH, USA). A 75 gm 3-h oral glucose tolerance test (OGTT) was performed following an overnight fast to evaluate insulin, glucose and GLP-1 responses. Blood samples were obtained at 0, 15, 30, 60, 90, 120, 150 and 180 min.
Serum glucose (mM/l) was measured by the glucose oxidase method. Insulin (μU/ml) was measured by standard double-antibody radioimmunoassay (RIA) and total GLP-1 (pM) was measured by double antibody RIA (Linco Research; St Louis, MO, USA). For 24 weeks, subjects received octreotide-LAR 40 mg i.m. every 28 days and ursodeoxycholic acid (Actigall®, Novartis). The OGTT was repeated at 0 and 24 weeks with the computation of corrected insulin response at 30 min (CIR30),6 a measure of β-cell activity, whole-body insulin sensitivity index (WBISI),7 and area under the curve (AUC) for insulin8 and GLP-1.
Results
Baseline age, weight, waist-to-hip ratio, BMI, glucose tolerance, insulin resistance and diet were similar between AA and Caucasians (see Table 1); 32% of the Caucasians and 31% of the AA subjects had impaired glucose tolerance. AA exhibited higher insulin secretion than Caucasians (CIR30: 2.26 ± 0.4 vs 1.0 ± 0.1, P < 0.01; insulin AUC: 23974 ± 4828 vs 14654 ± 1512; P = 0.02); and higher GLP-1 secretion (fasting: 6.7 ± 2.5 vs 4.5 ± 1.1, P = 0.01; GLP-1AUC: 1174.7 ± 412 vs 822.4 ± 191, P = 0.02). After 24 weeks of octreotide-LAR (see Table 1 and Figure 1) body weight and BMI were significantly reduced in Caucasians only. Glucose AUC and energy intake were significantly affected in both racial groups. However, neither group exhibited changes in the percentage of energy from macronutrients. Changes in WBISI, CIR30 and insulin AUC were significant in both groups (P < 0.01, Figure 1); however, AA continued to have higher CIR30 than Caucasians (0.68 ± 0.14 vs 0.30 ± 0.40, P < 0.01). For the entire cohort, octreotide-LAR reduced the GLP-1 response during the OGTT (ΔGLP-1AUC = −298 ± 129, P < 0.05). Both fasting GLP-1 and GLP-1AUC were significantly decreased in Caucasians (Δ fasting GLP-1 = −1.04 ± 0.4 and Δ GLP-1AUC = −216 ± 68, both P < 0.05) but not in AA (Δ fasting GLP-1 = −2.1 ± 1.9 and Δ GLP-1AUC = −433 ± 325, both P = NS). For the entire cohort, baseline CIR30 was significantly associated with insulin AUC (r = 0.77, P = < 0.0001) and GLP-1 (r = 0.31 P < 0.0001). After 24 weeks of octreotide-LAR, ΔCIR30 correlated with Δ insulin AUC (r = 0.31, P < 0.0001), ΔGLP-1 (r = 0.37, P < 0.01) and ΔGLP-1AUC (r = 0.39, P < 0.01).
Table 1.
Characteristics of subjects at baseline and after 24 weeks of octreotide LAR therapy
|
AAs (n = 16)
|
Caucasians (n = 26)
|
|||
|---|---|---|---|---|
| Baseline | Week 24 | Baseline | Week 24 | |
| Weight (kg) | 123 ± 5 | 121 ± 5 | 123 ± 6 | 118 ± 6* |
| Body mass index (kg/m2) | 46.4 ± 1.7 | 45.7 ± 1.8 | 43.6 ± 1.4 | 42.0 ± 1.4** |
| Waist-to-hip ratio | 0.86 ± 0.02 | 0.84 ± 0.01 | 0.82 ± 0.01 | 0.80 ± 0.01 |
| Glucose AUC | 1202 ± 45.6 | 1433 ± 45.6* | 1248 ± 36 | 1536 ± 36* |
| WBISI | 2.54 ± 0.43 | 3.10 ± 0.42* | 3.16 ± 0.29 | 4.2 ± 0.50* |
| Total kilojoules | 10486 ± 846 | 7334 ± 720** | 9059 ± 557 | 6442 ± 402* |
| % Carbohydrates | 44.6 ± 1.7 | 41.6 ± 2.9 | 46.2 ± 1.5 | 41.8 ± 2.7 |
| % Fat | 39.3 ± 1.6 | 40.3 ± 1.4 | 37.9 ± 1.3 | 39.8 ± 2.0 |
| % Protein | 15.9 ± 0.8 | 17.3 ± 1.1 | 16.4 ± 0.7 | 18.3 ± 1.2 |
All values expressed as mean ± s.e.m. ANOVA, P = nonsignificant between groups at like times.
P < 0.01
P < 0.05 within racial groups from baseline to week 24.
Figure 1.
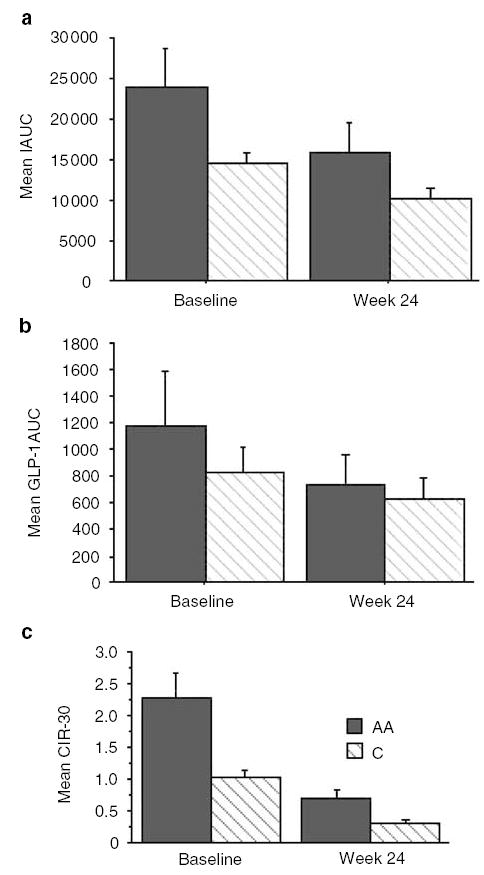
Changes in (a) insulin AUC (IAUC), (b) EIA (GLP-1AUC), and (c) β-cell activity (CIR30) in 16 AA (gray bar) and 26 Caucasian (striped bar) obese adults before and after 24 weeks of octrotide-LAR. Error bars denote standard error of the mean. ANOVA with repeated measures document significant differences between racial group in IAUC (P = 0.02), GLP1-AUC (P = 0.02) and CIR30 (P < 0.01) at baseline, and differences in CIR330 (P < 0.01) at week 24. For both racial groups, significant changes in IAUC (P < 0.01), GLP1-AUC (P < 0.01) and CIR30 (P < 0.01) occurred following treatment with octreotide-LAR.
Discussion
The results of our study indicate that at comparable levels of body weight, nutritional intake and insulin sensitivity, obese AA had higher fasting and stimulated GLP-1 and insulin concentrations compared to Caucasians. In agreement with other reports,2 our findings support a direct effect of octreotide on β-cell activity. Our findings also suggest that octreotide reduces glucose-stimulated GLP-1 response, which further contributes to the insulin suppression.
Octreotide equally suppressed β-cell activity and GLP-1 response in both racial groups. However, after 24 weeks of octreotide-LAR, β-cell activity, insulin and GLP-1 concentrations remained higher in AA than Caucasians.
Our results do not explain whether the above variations are associated with EIA hyperactivity, GLP-1 hypersecretion, differences in metabolic clearance or their combinations. Increased GLP-1 levels may account for the enhanced first and second phase of insulin secretion in AA and the exacerbated insulin response in obese subjects. GLP-1 promotes adipogenesis through its effects on insulin sensitivity, stimulation of fatty acid synthesis in adipose tissue9 and attenuation of the lipolytic action of glucagons.10,11 Obese animals and humans have increased GLP-1 concentrations in response to fat and glucose intake1,12,13 and an exacerbated insulin secretion in response to lower concentration of GLP-1.14 The higher prevalence of CVD and T2DM in AA may be explained by the hyperinsulinemic state fostered by higher GLP-1 concentrations as well as through direct GLP-1 mediation. GLP-1 receptors are present in the heart, and the infusion of GLP-1 causes an increase in the blood pressure and heart rate, which persists even after Adrenergic and cholinergic blockade Infusion of exendins 9–39 (a GLP-1 antagonist) prevents the rise in the blood pressure and heart rate, indicating a potential direct Cardiovascular effects of GLP-1.15–19 GLP-1 receptor (GLP-1R) desensitization may play a role in the genesis of T2DM and consequently the secretory activity of the β-cells. The accumulation of GLP-1 after DPPIV degradation in the circulation may also induce T2DM. GLP-1 (9–36) amide could antagonize the antidiabetes effects of GLP-1 (7–36).20 The validity of these pathogenetic mechanisms in humans in vivo remains to be determined.
Although energy intake during the study was significantly affected in both groups as suggested by the analysis of food records; changes in body weight and BMI did not reflect the magnitude of such restriction. When asked to record dietary intake, individuals may modify their eating habits or represent their diet in a more positive way, including under-reporting intake. No between-group differences in energy and macronutrients intake were observed at any time point. Based on the findings that both groups reported similar reductions in energy and macronutrient intake, we assume that any inaccuracy in self-reporting dietary intake was reflected in both groups.
In conclusion, obese AAs have higher fasting and stimulated GLP-1 and insulin concentrations compared to Caucasians. Octreotide may affect insulin secretion by both a direct β-cell effect as well as mediating GLP-1 response. Racial differences in GLP-1 concentrations or activity may be one mechanism contributing to the higher prevalence of hyperinsulinemia-associated disorders, including obesity, CVD and type II diabetes in AAs.
Acknowledgments
Funding for this study was provided in part by Novartis Pharmaceuticals Corporation and the University of Tennessee Health Science Center General Clinical Resource Center (UTHSC GCRC) [USPH RR000211]. We would also like to thank the nurses in the UTHSC GCRC and the HELP Center for their assistance with the study. This work was supported in part by University of Tennessee GCRC 5M01RR 00211, and by a research grant from Novartis Pharmaceuticals Corporation.
References
- 1.Kieffer TJ, Habener JF. The glucagon-like peptides. Endocr Rev. 1999;20:876–913. doi: 10.1210/edrv.20.6.0385. [DOI] [PubMed] [Google Scholar]
- 2.Bertoli A, Magnaterra R, Borboni P, Marini MA, Barini A, Fusco A, Bollea MR. Dose-dependent effect of octreotide on insulin secretion after OGTT in obesity. Horm Res. 1998;49:17–21. doi: 10.1159/000023120. [DOI] [PubMed] [Google Scholar]
- 3.Giustina A, Girelli A, Buffoli MG, Cimino A, Legati F, Valentini U, Giustina G. Low-dose octreotide is able to cause a maximal inhibition of the glycemic responses to a mixed meal in obese type 2 diabetic patients treated with insulin. Diabetes Res Clin Pract. 1991;14:47–54. doi: 10.1016/0168-8227(91)90052-f. [DOI] [PubMed] [Google Scholar]
- 4.Lunetta M, Di Mauro M, Le Moli R, Burrafato S. Long-term octreotide treatment reduced hyperinsulinemia, excess body weight and skin lesions in severe obesity with Acanthosis nigricans. J Endocrinol Invest. 1996;19:699–703. doi: 10.1007/BF03349042. [DOI] [PubMed] [Google Scholar]
- 5.Rohrer SP, Birzin ET, Mosley RT, Berk SC, Hutchins SM, Shen DM, Xiong Y, Hayes EC, Parmar RM, Foor F, Mitra SW, Degrado SJ, Shu M, Klopp JM, Cai SJ, Blake A, Chan WW, Pasternak A, Yang L, Patchett AA, Smith RG, Chapman KT, Schaeffer JM. Rapid identification of subtype-selective agonists of the somatostatin receptor through combinatorial chemistry. Science. 1998;282:737–740. doi: 10.1126/science.282.5389.737. [DOI] [PubMed] [Google Scholar]
- 6.Sluiter WJ, Erkelens DW, Terpstra P, Reitsma WD, Doorenbos H. Glucose tolerance and insulin release, a mathematical approach. II. Approximation of the peripheral insulin resistance after oral glucose loading. Diabetes. 1976;25:245–249. doi: 10.2337/diab.25.4.245. [DOI] [PubMed] [Google Scholar]
- 7.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 8.Toft I, Bonaa KH, Lindal S, Jenssen T. Insulin kinetics, insulin action, and muscle morphology in lean or slightly overweight persons with impaired glucose tolerance. Metabolism. 1998;47:848–854. doi: 10.1016/s0026-0495(98)90125-1. [DOI] [PubMed] [Google Scholar]
- 9.Oben J, Morgan L, Fletcher J, Marks V. Effect of the enteropancreatic hormones, gastric inhibitory polypeptide and glucagon-like polypeptide-1(7–36) amide, on fatty acid synthesis in explants of rat adipose tissue. J Endocrinol. 1991;130:267–272. doi: 10.1677/joe.0.1300267. [DOI] [PubMed] [Google Scholar]
- 10.Delgado E, Luque MA, Alcantara A, Trapote MA, Clemente F, Galera C, Valverde I, Villanueva-Penacarrillo ML. Glucagon-like peptide-1 binding to rat skeletal muscle. Peptides. 1995;16:225–229. doi: 10.1016/0196-9781(94)00175-8. [DOI] [PubMed] [Google Scholar]
- 11.Dupre J, Greenidge N, McDonald TJ, Ross SA, Rubinstein D. Inhibition of actions of glucagon in adipocytes by gastric inhibitory polypeptide. Metabolism. 1976;25:1197–1199. doi: 10.1016/s0026-0495(76)80002-9. [DOI] [PubMed] [Google Scholar]
- 12.Ebert R, Frerichs H, Creutzfeldt W. Impaired feedback control of fat induced gastric inhibitory polypeptide (GIP) secretion by insulin in obesity and glucose intolerance. Eur J Clin Invest. 1979;9:129–135. doi: 10.1111/j.1365-2362.1979.tb01678.x. [DOI] [PubMed] [Google Scholar]
- 13.Yip RG, Wolfe MM. GIP biology and fat metabolism. Life Sci. 2000;66:91–103. doi: 10.1016/s0024-3205(99)00314-8. [DOI] [PubMed] [Google Scholar]
- 14.Verdich C, Toubro S, Buemann B, Lysgard Madsen J, Juul Holst J, Astrup A. The role of postprandial releases of insulin and incretin hormones in meal-induced satiety–effect of obesity and weight reduction. Int J Obes Relat Metab Disord. 2001;25:1206–1214. doi: 10.1038/sj.ijo.0801655. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto H, Lee CE, Marcus JN, Williams TD, Overton JM, Lopez ME, Hollenberg AN, Baggio L, Saper CB, Drucker DJ, Elmquist JK. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest. 2002;110:43–52. doi: 10.1172/JCI15595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barragan JM, Rodriguez RE, Blazquez E. Changes in arterial blood pressure and heart rate induced by glucagon-like peptide-1-(7–36) amide in rats. Am J Physiol. 1994;266:E459–66. doi: 10.1152/ajpendo.1994.266.3.E459. [DOI] [PubMed] [Google Scholar]
- 17.Edwards CM, Edwards AV, Bloom SR. Cardiovascular and pancreatic endocrine responses to glucagon-like peptide-1(7–36) amide in the conscious calf. Exp Physiol. 1997;82:709–716. doi: 10.1113/expphysiol.1997.sp004059. [DOI] [PubMed] [Google Scholar]
- 18.Wei Y, Mojsov S. Tissue-specific expression of the human receptor for glucagon-like peptide-I: brain, heart and pancreatic forms have the same deduced amino acid sequences. FEBS Lett. 1995;358:219–224. doi: 10.1016/0014-5793(94)01430-9. [DOI] [PubMed] [Google Scholar]
- 19.Edwards CM, Todd JF, Ghatei MA, Bloom SR. Subcutaneous glucagon-like peptide-1 (7–36) amide is insulinotropic and can cause hypoglycaemia in fasted healthy subjects. Clin Sci (Lond) 1998;95:719–724. doi: 10.1042/cs0950719. [DOI] [PubMed] [Google Scholar]
- 20.Wettergren A, Wojdemann M, Holst JJ. The inhibitory effect of glucagon-like peptide-1 (7–36) amide on antral motility is antagonized by its N-terminally truncated primary metabolite GLP-1 (9–36)amide. Peptides. 1998;19:877–882. doi: 10.1016/s0196-9781(98)00020-5. [DOI] [PubMed] [Google Scholar]


