Abstract
Apigenin (Api) and tt-farnesol (Far) are two naturally occurring agents that affect the development of cariogenic biofilms. Fluoride (F) interferes physicochemically with caries development and also exhibits antibacterial activity. We examined whether the association of Api and Far enhance the anti-caries properties of F by acting cooperatively on the expression of virulence of Streptococcus mutans. The biological effects of each of the agents were greatly enhanced when used in combination with F. In general, biofilms treated with Api and/or Far in combination with F displayed less biomass, insoluble glucans and iodophilic polysaccharides than did those treated with the test agents alone (P<0.05). The combination of the test agents with F was highly effective in preventing caries development in rats, especially Api+Far+F; and were comparable with those observed with chlorhexidine + F (positive control). Results from these studies show that apigenin and tt-farnesol may enhance the cariostatic effectiveness of fluoride.
Keywords: Apigenin, tt-Farnesol, Fluoride, S. mutans, biofilms
INTRODUCTION
Streptococcus mutans is the main pathogen responsible for the development of dental caries in humans (Tanzer, 1985; Loesche, 1986). The organism produces glucosyltransferases (GTFs), which catalyze synthesis of glucans from dietary carbohydrates, especially sucrose. Glucans are of central importance in adhesive interactions of S. mutans with tooth surface and other oral bacteria, and contribute to the formation of the matrix of dental biofilms (Yamashita et al., 1993). Furthermore, S. mutans survives and carries out glycolysis at low pH values attained within the matrix of the biofilms which results in demineralization of the adjacent dental enamel (Belli and Marquis, 1991; Bowen, 2002).
Recently, we have identified two potential anti-caries agents that are found in propolis, a natural beehive product (Koo et al., 2002; 2003a). Apigenin (4’, 5, 7-trihydroxyflavone) is a potent inhibitor of water insoluble glucans synthesis (Koo et al., 2002; 2003a); tt-farnesol (3,7,11-trimethyl-2,6,10-dodecatrien-1-ol), displays activities against streptococcal membranes by increasing their proton permeability and inhibits acid production by S. mutans within biofilms (Koo et al., unpublished data). Topical application of these compounds reduced incidence of dental caries with minimal effects on the viability of oral flora populations in vivo (Koo et al., 2003a).
Fluoride, in various vehicles, is the most effective anti-caries agent known (Clarkson, 2000; NIH, 2001). Nevertheless, dental caries remains a significant problem in many countries, including United States, and continues at a high level in susceptible subpopulations, especially, among economically underprivileged children (NIH, 2001). Fluoride exerts its major effect by reducing demineralization and enhancing remineralization of early carious lesions (Dawes and ten Cate, 1990). However, there is a plethora of evidence which shows that fluoride can affect the biological activities of cariogenic streptococci (Hamilton, 1990; Marquis et al., 2003). For example, fluoride inhibits acid production and the production of GTFs (Bowen and Hewitt, 1974; Marquis et al., 2003).
Enhancement of the protective effects of fluoride by including substances in preparations, which affect the virulence of cariogenic bacteria and/or enhance the antibacterial effects of fluoride, offer an attractive route to reduce prevalence of dental caries. It is generally accepted that effectiveness of fluoride could be enhanced when combined with additional cariostatic agents (NIH, 2001). However, most of the compounds tested thus far are broad-spectrum antimicrobials which suppress the resident flora (Caufield et al., 2001). In this study, we followed an alternative approach using apigenin and tt-farnesol to enhance the biological effects of fluoride against S. mutans by simultaneously acting on the development and virulence of cariogenic biofilms.
MATERIALS AND METHODS
Test Agents
Apigenin and tt-farnesol were obtained from Extrasynthese Co. (Genay-Sedex, France). Sodium fluoride and chlorhexidine were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). For this study, we tested 1 mM apigenin and 5 mM tt-farnesol, alone or in combination with sodium fluoride (250 ppm F); these concentrations were chosen based on data from our previous published and unpublished response to dose and animal studies (Koo et al., 2002; 2003a;b). The test agents were dissolved in 25% ethanol containing 1.25% dimethyl sulfoxide (DMSO) just prior to carrying out the experiments. Appropriate solvent controls were always included.
Biofilm Preparation and Treatments
Biofilms of S. mutans UA159 were formed on saliva-coated hydroxyapatite (sHA) discs (surface area of 2.7 ± 0.2 cm2, Clarkson Chromatography Products Inc., PA, USA) in batch cultures for 5 days as detailed elsewhere (Koo et al. 2003b; Chatfield et al., 2005). The sHA discs were generated by incubating the discs with clarified human whole saliva for 1h at 37°C. During the first 24 hours, the organisms were grown undisturbed to allow initial biofilm formation; the biofilms (24-h old) were then treated twice daily (one-minute exposure, at 10 a.m. and 4 p.m.) until the 5th day of the experimental period (126 h-old biofilms) with one of the following: i) 5 mM tt-farnesol (Far), ii) 1 mM Apigenin (Api), iii) 250 ppm fluoride (F), iv) Far + F, v) Api + F, vi) Api + Far + F, vii) vehicle control (25% ethanol containing 1.25% DMSO, as negative control), viii) CHX + F (as positive control). Each biofilm was exposed to the respective treatment a total of eight times. Biofilms assays were performed in quadruplicate in at least three different experiments.
Biofilm Analyses
At the end of the experimental period, the biofilms were gently washed in physiological saline (0.89% NaCl, w/v) to remove loosely adherent material. One set of biofilms was used for in situ measurements of pH by placing the tip of Beetrode pH electrode (World Precision Instruments, New Haven, CT, USA) into the matrix of the biofilms; a series of pH readings were recorded from 10 different sites (Li and Burne, 2001). Additional sets of biofilms were analyzed for: (i) biomass (dry-weight), (ii) number of viable cells, (iii) total protein (ninhydrin assay; Moore and Stein, 1954) and (iv) polysaccharide composition (soluble and insoluble glucans, and intracellular iodophilic polysaccharides) using colorimetric (Dubois et al., 1956; Di Persio et al., 1974) and scintillation counting (Koo et al., 2003b) methods.
Animal Studies
The animal experiment was performed using methods described previously (Koo et al., 2003a). At weaning, pups aged 21 days were infected by S. mutans UA159, and randomly placed into eight groups of 12 animals, and their teeth treated topically using a camel’s hair brush twice daily as follows: (1) 5 mM Far, (2) 1 mM Api, (3) 250 ppm F, (4) Far + F, (5) Api + F, (6) Api + Far + F, (7) vehicle control (25% ethanol containing 1.25% DMSO, as negative control), (8) CHX + F (as positive control). Each group of 12 animals was provided with National Institutes of Health diet 2000 (which contains 56% sucrose) and 5% sucrose water ad libitum. The experiment proceeded for 5 weeks, at the end of which the animals were killed by CO2 asphyxiation. The microbiological assessment and caries evaluation were carried out using previously described methods (Koo et al., 2003a). This study was reviewed and approved by the University of Rochester Committee on Animal Resources.
Statistical Analyses
For the in vitro studies, the data were analyzed using ANOVA, and the F-test was used to test any difference between the groups. When significant differences were detected, pairwise comparison was made between all the groups using Tukey’s method to adjust for multiple comparisons. For the animal studies, smooth-surface and sulcal caries scores were expressed as proportions of their maximum possible values (124 and 56). The data were subjected to ANOVA in the Tukey-Kramer Honest Standard Deviation (HSD) test for all pairs. Statistical software JMP version 3.1 (SAS Institute, Cary, NC, USA) was used to perform the analyses. The level of significance was set at 5% for both studies.
RESULTS
All the test agents, with the exception of fluoride alone, diminished the further accumulation of S. mutans biofilms compared with the vehicle control (P<0.05) (Table 1). The combinations of Api + F, and Api + Far + F were the most effective treatments and resulted in 40.7 % and 50.6 % less biomass (dry-weight) than the vehicle control treatment; concentration of protein was affected similarly. None of the test agents displayed bactericidal activity.
Table 1.
Streptococcus mutans UA159 biofilms composition and acidogenicity after treatments
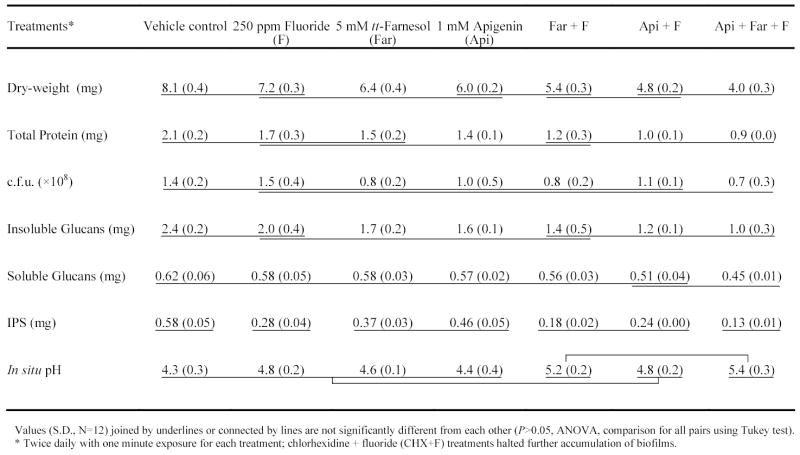
The total amount of polysaccharides in the biofilms was affected by treatments with the test agents. The amount of insoluble glucans in the biofilms treated with Api and Far, alone or in combinations, was significantly less than in those treated with vehicle control (P<0.05, Table 1); the combinations Api + F, and Api + Far + F were more effective than each of the test agents alone (P<0.05). The amount of water-soluble glucans in the biofilms was unaffected by the test agents, except those treated with the combination Api + Far + F. In contrast, the amount of iodophilic polysaccharides was drastically reduced by F, alone or in combinations (Api + F, Far + F and Api + Far + F) (51.7 to 77.6% reduction).
The acidogenicity of the treated-biofilms was reduced by Far + F and Api + Far + F only. The pH values measured in the biofilms’ matrix that were treated with the combinations were 0.9 –1.1 units higher than those treated with vehicle control 16 hours after last treatment (P<0.05, Table 1).
Our positive control CHX + F displayed bactericidal activity against the early-formed S. mutans biofilms, and halted the further accumulation of the biofilms (data not shown).
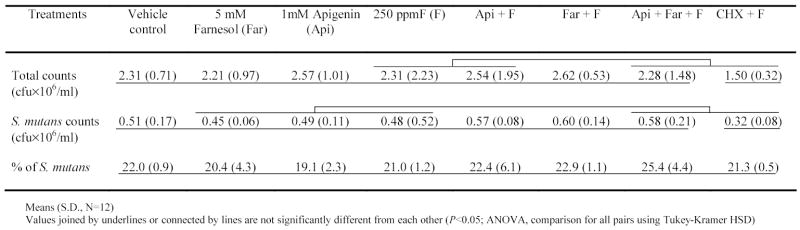
In the animal experiment, weight gains were not significantly different among the controls and test agents groups (P>0.05). The percentage of S. mutans UA159 recovered from the jaws of the rats was calculated from total cultivable flora and S. mutans population. The percentage of S. mutans in the animals’ plaque was similar among all groups, and ranged from 19.1% to 25.4% (Table 2). However, the group treated with CHX + F displayed significantly lower counts of both total and S. mutans populations compared to control group (P<0.05).
Table 2.
Effects of treatments on microbial population in the animals’ plaque.
The incidence of smooth-surface caries was reduced in all treatment groups compared with the control (30 to 65% reduction, P<0.05; Table 3), with exception of the group treated with 5 mM tt-farnesol. The smooth-surface caries severity scores were also significantly lower in all groups treated with the test agents (Table 3). However, only the groups treated with Api + Far + F, and CHX + F had less severe lesions than the test agents (Api or Far) or fluoride alone (at Ds level only, P<0.05). Furthermore, the animals treated with Api + Far + F was the only group free of Dx scores.
Table 3.
Effects of treatments on development (smooth surface and severity) of dental caries in rats.

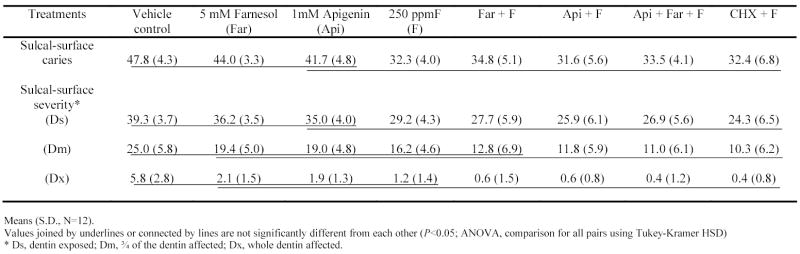
The incidence of sulcal-surface caries was significantly lower in the groups treated with F, either alone or in combinations than control (P<0.05; Table 4). The severity of sulcal lesions followed a similar pattern to that of sulcal-surface caries scores; animals treated with fluoride (alone or in combinations) exhibited lower scores at Ds level compared to control group (P<0.05). However, only the combinations Api + F, Api + Far + F and CHX + F were effective in reducing the sulcal-surface severity at Dm and Dx levels compared to control (P<0.05).
Table 4.
Effects of treatments on development (sulcal-surface caries and severity) of dental caries in rats.
DISCUSSION
The data show that the combination of Api and Far with F reduced the virulence of S. mutans significantly, which resulted in enhanced cariostatic properties without significant effect on the viability of the oral flora population (both total and S. mutans) in plaque from the animals. Among all the different combinations of the test agents, Api + Far + F displayed the maximum therapeutic effect in vivo, and its potency was comparable with our positive control CHX + F. It appears that Api and Far acting in concert with F on the virulence of S. mutans involved in the formation of cariogenic biofilm communities caused significant changes in the insoluble glucans and intracellular polysaccharides (IPS) content of the biofilms.
The putative pathways by which Api, Far and F affect the cariogenicity of S. mutans may involve several routes; we propose at least three: 1) inhibition of glucan synthesis; 2) disruption of acid production and acid tolerance, and 3) affecting IPS accumulation. Apigenin is a potent inhibitor of GTFs B and C either in solution or adsorbed on to a sHA surface, and also affects the expression of gtfB and gtfC genes (Koo et al., 2003a; unpublished data). These enzymes are responsible for the synthesis of insoluble glucans, which are critical in the expression of virulence in the pathogenesis of dental caries (Yamashita et al., 1993). In contrast, F and Far affect the synthesis of exopolysaccharides without direct effects on GTF activity (Bowen and Hewitt, 1974, Marquis et al., 2003). Enzyme secretion by bacterial cells is generally coupled to Δp, the proton-motive force, across the cell membrane. Because F and Far act to diminish Δp by increasing proton permeability and discharge of ΔpH across the cell membrane (Marquis et al., 2003), it is probable that they will affect the secretion of GTFs and thereby diminish the synthesis of glucans. Thus, Api and Far acting cooperatively with fluoride could reduce the amount of glucan in the biofilm. Our data support this hypothesis because it is evident that the amount of extracellular insoluble glucans in S. mutans biofilms was reduced in presence of Api + Far + F.
Bacteria such as the mutans streptococci can carry out glycolysis at low pH values even though glycolytic enzymes are not acid tolerant because they maintain ΔpH across the cell membrane with the interior more alkaline than the exterior. During glycolysis, protons are moved out of the cell through the proton-translocating, membrane F-ATPase. Fluoride short circuits this flow through the diffusion of HF into cell, which acidifies the cytoplasm and inhibits intracellular enzymes (Marquis et al., 2003). In contrast, tt-farnesol changes the permeability and fluidity of the cell membrane by its lipophilic properties, which favours localization in the membrane (Ramage et al., 2002; Koo et al., 2002; Inoue et al., 2004). Apigenin is without effect on proton-permeability of the membrane of S. mutans. However, Api exhibited moderate inhibitory effects on the activity of F-ATPase (25% inhibition), which could affect the acid-tolerance of S. mutans. Cytoplasmic acidification caused by these agents could disrupt the glycolytic acid production and the formation of intracellular iodophilic polysaccharides (IPS), a glycogen-like storage polymer (Hamilton, 1990). The IPS provide S. mutans with an endogenous source of carbohydrate which can be metabolized when exogenous fermentable substrate have been depleted in the oral cavity (Hamilton, 1976); as a result, IPS can promote the formation of dental caries by prolonging the exposure of tooth surfaces to organic acids and a concomitant lower fasting pH in the matrix of the plaque (Tanzer et al., 1976). The importance of IPS to S. mutans virulence supports previous reports in the literature which describes an association of these storage polysaccharides and dental caries in animals and in humans (Loesche et al., 1967; Tanzer et al., 1976; Spatafora et al., 1995). It is noteworthy that the biofilms with least amount of IPS has the highest pH values, especially those treated with Api + Far + F (Table 1). It is apparent that by disrupting the accumulation of IPS, the combination of agents is reducing the acidogenicity of the biofilms, and thereby, affecting the development of dental caries in rats. Whether these agents can affect the synthesis of extracellular or intracellular polysaccharides, or acid production by other cariogenic organisms await further evaluation.
The combination of these novel agents with fluoride may represent a potentially useful and an alternative approach to the current chemotherapeutic strategies to prevent this ubiquitous disease by reducing the expression of virulence of S. mutans without necessarily suppressing the resident oral flora. Although details of the toxicology of Api and Far were not investigated here, we did not observe any adverse reactions in our animal study. We are currently investigating the molecular mechanism(s) of action of these agents in combination.
Acknowledgments
This research was supported by USPHS Research Grant 1R03 DE015441-01 from the National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD 20892.
References
- Belli WA, Marquis RE. Adaptation of Streptococcus mutans and Enterococcus hirae to acid stress in continuous culture. Appl Environ Microbiol. 1991;57:1134–1138. doi: 10.1128/aem.57.4.1134-1138.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen WH, Hewitt MJ. Effect of fluoride on extracellular polysaccharide production by Streptococcus mutans. J Dent Res. 1974;53:627–629. [Google Scholar]
- Bowen WH. Do we need to be concerned about dental caries in the coming millennium? Crit Rev Oral Biol Med. 2002;13:126–131. doi: 10.1177/154411130201300203. [DOI] [PubMed] [Google Scholar]
- Caufield PW, Dasanayake AP, Li Y. The antimicrobial approach to caries management. J Dent Educ. 2001;65:1091–1095. [PubMed] [Google Scholar]
- Chatfield CH, Koo H, Quivey RG. The putative lytR ortholog in Streptococcus mutans plays a role in cell division and is growth-rate regulated. Microbiology. 2005;151:625–631. doi: 10.1099/mic.0.27604-0. [DOI] [PubMed] [Google Scholar]
- Clarkson JJ. International collaborative research on fluoride. J Dent Res. 2000;79:893–904. doi: 10.1177/00220345000790040301. [DOI] [PubMed] [Google Scholar]
- Dawes C, ten Cate JM. International symposium on fluorides: Mechanisms of action and recommendation for use. J Dent Res. 1990;69(spec issue):505–836. [Google Scholar]
- DiPersio JR, Mattingly SJ, Higgins ML, Shockman GD. Measurement of intracellular iodophilic polysaccharide in two cariogenic strains of Streptococcus mutans by cytochemical and chemical methods. Infect Immun. 1974;10:597–604. doi: 10.1128/iai.10.3.597-604.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- Hamilton IR (1976). Intracellular polysaccharide synthesis by cariogenic microorganisms. In: Proceedings in microbiology. Aspects of dental caries. Stiles HM, Loesche WJ, O’Brien TL, editors. Special supplement to Microbiology Abstracts, vol. 3. Information Retrieval, Inc. London, pp. 683–701.
- Hamilton IR. Biochemical effects of fluoride on oral bacteria. J Dent Res. 1990;69(spec issue):660–667. doi: 10.1177/00220345900690S128. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Shiraishi A, Hada T, Hirose K, Hamashima H, Shimada J. The antibacterial effects of terpene alcohols on Staphylococcus aureus and their mode of action. FEMS Microbiol Lett. 2004;237:325–331. doi: 10.1016/j.femsle.2004.06.049. [DOI] [PubMed] [Google Scholar]
- Koo H, Rosalen PL, Cury JA, Park YK, Bowen WH. Effects of compounds found in propolis on S. mutans growth and on glucosyltransferase activity. Antimicrob Agents Chemother. 2002;46:1302–1309. doi: 10.1128/AAC.46.5.1302-1309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo H, Pearson SK, Scott-Anne K, Abranches J, Cury JA, Rosalen PL, Park YK, Marquis, Bowen WH. Effects of apigenin and tt-farnesol on glucosyltransferase activity, biofilm viability and caries development in rats. Oral Microbiol Immunol. 2003a;17:337–343. doi: 10.1034/j.1399-302x.2002.170602.x. [DOI] [PubMed] [Google Scholar]
- Koo H, Hayacibara MF, Schobel BD, Cury JA, Rosalen PL, Park YK, Vacca Smith AM, Bowen WH. Inhibition of Streptococcus mutans biofilm accumulation and polysaccharide production by apigenin and tt-farnesol. J Antimicrob Chemother. 2003b;52:782–789. doi: 10.1093/jac/dkg449. [DOI] [PubMed] [Google Scholar]
- Li Y, Burne RA. Regulation of the gtfBC and ftf genes of Streptococcus mutans in biofilms in response to pH and carbohydrate. Microbiology. 2001;147:2841–2848. doi: 10.1099/00221287-147-10-2841. [DOI] [PubMed] [Google Scholar]
- Loesche WJ, Henry CA. Intracellular microbial polysaccharide production and dental caries in a Guatemalan Indian village. Archs Oral Biol. 1967;12:189–194. doi: 10.1016/0003-9969(67)90037-4. [DOI] [PubMed] [Google Scholar]
- Loesche WJ. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis RE, Clock SA, Mota-Meira M. Fluoride and organic weak acids as modulators of microbial physiology. FEMS Microbiol Rev. 2003;760:1–18. doi: 10.1111/j.1574-6976.2003.tb00627.x. [DOI] [PubMed] [Google Scholar]
- Moore S, Stein WH. A modified ninhydrin reagent for the photometric determination of amino acids and related compounds. J Biol Chem. 1954;211:907–913. [PubMed] [Google Scholar]
- National Institutes of Health. Diagnosis and Management of Dental Caries Throughout Life. NIH Consensus Statement. 2001;18(1):1–30. [PubMed] [Google Scholar]
- Ramage G, Saville SP, Wickes BL, Lopez-Ribot JL. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl Environ Microbiol. 2002;68:5459–5463. doi: 10.1128/AEM.68.11.5459-5463.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute Inc (ed) JMP.® User’s Guide: Version 2 of JMP, Cary, SAS Institute, NJ, 1989. Spatafora G, Rohrer K, Barnard D, Michalek S (1995). A Streptococcus mutans mutant that synthesizes elevated levels of intracellular polysaccharide is hypercariogenic in vivo. Infect Immun 63:2556–63. [DOI] [PMC free article] [PubMed]
- Tanzer JM, Freedman ML, Woodiel FN, Eifert RL, Rinehimer LA (1976). Association of Streptococcus mutans virulence with synthesis of intracellular polysaccharide. In: Proceedings in microbiology. Aspects of dental caries. Stiles HM, Loesche WJ, O’Brien TL, editors. Special supplement to Microbiology Abstracts, vol. 3. Information Retrieval, Inc. London, pp. 596–616.
- Tanzer JM, Freedman ML, Fitzgerald RJ (1985). Virulence of mutants defective in glucosyltransferase, dextran-mediated aggregation, or dextranase activity. In: Molecular Basis of Oral Microbial Adhesion. Mergenhagen SE, Rosan B, editors. American Society of Microbiology, Washington, DC, pp. 204–211.
- Yamashita Y, Bowen WH, Burne RA, Kuramitsu HK. Role of the Streptococcus mutans gtf genes in caries induction in the specific-pathogen-free rat model. Infect Immun. 1993;61:3811–3817. doi: 10.1128/iai.61.9.3811-3817.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]


