Abstract
Mast cells play a pivotal role in immediate hypersensitivity and chronic allergic reactions that can contribute to asthma, atopic dermatitis, and other allergic diseases. Since mast cell numbers are increased at sites of inflammation in allergic diseases, pharmacologic intervention into the proliferation, migration, and survival (or apoptosis) of mast cells could be a promising strategy for the management of allergic diseases. Mast cells differentiate from multipotent hematopoietic progenitors in the bone marrow. Stem cell factor (SCF) is a major chemotactic factor for mast cells and their progenitors. SCF also elicits cell-cell and cell-substratum adhesion, facilitates the proliferation, and sustains the survival, differentiation, and maturation, of mast cells. Therefore, many aspects of mast cell biology can be understood as interactions of mast cells and their precursors with SCF and factors that modulate their responses to SCF and its signaling pathways. Numerous factors known to have such a capacity include cytokines that are secreted from activated T cells and other immune cells including mast cells themselves. Recent studies also demonstrated that monomeric IgE binding to FcεRI can enhance mast-cell survival. In this review we discuss the factors that regulate mast cell development, migration, and survival.
Keywords: mast cell, development, proliferation, migration, survival, apoptosis, SCF, Kit, IL-3, IgE
Introduction
Mast cells play a central role in allergic reactions through high-affinity IgE receptor (FcεRI)-mediated responses. Cross-linking of the FcεRI on mast cells activates multiple signaling pathways that lead to degranulation, de novo synthesis of arachidonic acid metabolites, and production of various cytokines and chemokines [1]. Beyond this classical role of mast cell activation in allergic reactions, recent studies have expanded our understanding of the involvement of mast cells in the defense against bacteria [2] and parasites [1, 2] and the pathogenesis of experimental allergic encephalomyelopathy [3], rheumatoid arthritis [4], and congestive heart failure [5].
The number of mast cells in inflamed tissue can be regulated by proliferation, migration, and survival (and apoptosis). The number of tissue mast cells in healthy individuals is stable, but this homeostasis is disturbed by a number of pathophysiologic conditions: their numbers increase in inflamed tissues in allergic diseases, such as allergic rhinitis [6] and allergic asthma [7]. Thus, our improved knowledge of the proliferation, migration, and survival (and apoptosis) of mast cells will provide a conceptual framework that may lead to the development of novel strategies for a better management of allergic diseases. In this review we focus on the factors that regulate mast cell development, migration, and survival.
Development of mast cells
1. Mast cells arise from multipotent hematopoietic progenitors in the bone marrow.
During the first 100 years after Paul Ehrlich discovered them, mast cells were believed to be a component of connective tissue that was derived from undifferentiated mesenchymal cells [8]. However, Kitamura and co-workers [9] demonstrated that mast cells arise from multipotent hematopoietic progenitors in bone marrow: WBB6F1-W/Wv mice are devoid of mast cells, but mast cells develop if the mice receive bone marrow cells from a normal littermate [9, 10]. Mast cells normally do not mature before leaving the bone marrow but circulate through the vascular system as immature progenitors that then complete their development peripherally within connective or mucosal tissues. A cell population was identified in murine fetal blood that fulfills the criteria of progenitor mastocytes [11]: the cells were defined by the surface phenotype of Thy-1loKithi, contained cytoplasmic granules, and expressed RNAs encoding mast cell-associated proteases but lacked expression of FcεRI. Thy-1loKithi cells generated functionally competent mast cells at high frequencies in vitro but lacked developmental potential for other hematopoietic lineages. When transferred, this population reconstituted the peritoneal mast cell compartment in W/Wv mice. More recently, Chen et al. a cell population, identified as Lin− Kit+Sca-1−Ly6c−FcεRIα−CD27−β7+T1/ST2+, as mast cell progenitors (MCPs) in adult mouse bone marrow [12]: these cells give rise to mast cells in culture and could reconstitute the mast cell compartment when transferred into mast cell-deficient mice. This study also suggests that these MCPs are derived from multipotential progenitors (MPPs), but not from common myeloid progenitors (CMPs) or granulocyte/macrophage progenitors (GMPs) (Figure 1A). The culture conditions used by these authors indicate that even hematopoietic stem cells (HSCs) can quickly develop into mast cells, suggesting the possibility that circulating HSCs may also serve as a source of recruited mast cell precursors in infection or other settings. However, Arinobu et al. identified a cell population (Lin−Kit+FcγRII/IIIhiβ7hi) as bipotent progenitors for the basophil and mast cell lineages (termed BMCPs) in mouse spleens, which can be generated mainly from GMPs in the bone marrow [13]. They also identified basophil progenitors (BaPs; Lin−CD34+FcεRIαhiKit−) in the bone marrow and mast cell progenitors (MCPs; CD45+Lin−CD34+β7hiFcεRIαlo) in the intestine. Importantly, CCAAT/enhancer-binding protein α (C/EBPα) was shown to play a critical role in the fate decision of BMCPs, being expressed in BaPs but not in MCPs. Therefore, this study established the close developmental relationship as well as the distinct difference in their relation between basophils and mast cells (Figure 1B). The relation between the MCPs described by these groups as well as CD34+CD13+Kit+ cells characterized by Jamur et al. [14] remain to be examined.
Figure 1.
Two models of mast cell-related hematopoiesis. (A) The model by Chen et al. [12] proposes that MCPs derives mainly from MPPs. (B) Another model described by Arinobu et al. [13] proposes that intgerin β7-expressing GMPs in the bone marrow are the major source of BMCPs, a population of bipotent progenitors for basophils and mast cells, which are supposed to go through the circulation to the spleen. BMCPs expressing C/EBPα will differentiate to basophils whereas BMCPs without C/EBPα expression become MCPs. In this scheme, lineal linkages between LT-HSCs and MPPs in the bone marrow are not shown. LT-HSCs, long-term hematopoietic stem cell; ST-HSC, short-term hematopoietic stem cell; MPP, multipotential progenitor; CLP, common lymphoid progenitor; CMP, common myeloid progenitor; MEP, megakaryocyte/erythrocyte progenitor; MK, megakaryocyte.
Tissue mast cells in humans also differentiate from committed progenitor cells that arise in the marrow compartment from pluripotent hematopoietic progenitors [15]. Human mast cell progenitors circulate as mononuclear leukocytes lacking characteristic secretory granules [16], express CD13, CD33, CD38, CD34, and Kit, but rarely HLA-DR [17-19]. Although our understanding of the developmental path of mast cells has been advanced substantially, a lot should be learned: for instance, how are fetal blood MCPs related with adult MCPs? Do we know all the stages from hematopoietic stem cells via MCPs to mature mast cells? How are the known progenitors related with subtypes of mast cells such as mucosal and connective tissue-type mast cells in mice and tryptase-positive vs. tryptase/chymase-positive mast cells in humans? Can we define mast cells at each developmental stage with a set of cell surface markers like T or B cell subsets?
2. Mast cell development and growth are crucially regulated by the survival and developmental factor, stem cell factor.
Stem cell factor (SCF, also known as Kit ligand) binds its receptor, Kit, on their target cells that has an intrinsic protein-tyrosine kinase domain in its cytoplasmic region. SCF and Kit signaling are essential for the development of murine mast cells: both the W and Sl mice, which have mutations in the chromosomal loci coding for Kit and SCF, respectively, have profoundly deficient numbers of tissue mast cells [20, 21]. SCF is also a pivotal growth factor that promotes the development of human mast cells. Cultures of single CD34+ cells have shown that SCF acts as a proliferative rather than a survival factor in mast cell development from human cord-blood hematopoietic progenitors [22].
3. SCF-mediated mast cell development is regulated by a variety of factors including cytokines and growth factors.
Table 1 lists the growth factors and cytokines that are involved in mast cell growth and differentiation in rodent and human systems. Mast cells can be developed at high efficiency by culturing mouse bone marrow cells in IL-3-containing media. The resulting cells, termed bone marrow-derived mast cells (BMMC), are usually more than 95% pure populations of immature mast cells and have been extensively used in the research of mouse mast cell biology. IL-3 also stimulates the proliferation of BMMCs and the survival of connective tissue mast cells [23, 24]. Although IL-3 and granulocyte-macrophage colony-stimulating factor (GM-CSF) share the common receptor β-chain (CD131), GM-CSF inhibits mast cell development in both mouse [25] and human systems [26]. By contrast, the role of IL-3 on the development of human mast cells is controversial. IL-3 is not required for the development of human cord blood-derived mast cells in the presence of low oxygen concentrations [27], but it can enhance SCF-dependent mast cell development at low cell densities (e.g., in methylcellulose culture) at normal oxygen concentrations [28]. IL-3 at 1 ng/ml can enhance the growth of mast cell colonies without inducing granulocyte or macrophage (GM) colonies. At the concentrations above 5 ng/ml, however, IL-3 induces a substantial number of GM colonies [28].
Table 1.
Factors that affects mast cell development
| Cytokine | Receptor | Effects on mouse MCs | Effects on human MCs |
|---|---|---|---|
| SCF | Kit |
|
|
| IL-3 | IL-3R | ||
| IL-4 | IL-4R |
|
Depends on the MC subtype or cytokine milieu [30-33, 135] |
| IL-5 | IL-5R | Not clearly characterized | Cofactor for proliferation [19, 37] |
| IL-6 | IL-6R | Induces MC development in combination with TNF-α [43, 44] | Cofactor for proliferation or inhibition [19, 27, 33, 37, 45] |
| IL-9 | IL-9R |
|
Cofactor for proliferation [37, 38] |
| IL-10 | IL-10R | Not clearly characterized | |
| IFN-γ | INF-γR | Inhibits proliferation [39] | Inhibits proliferation [37, 40] |
| NGF | NGFR |
|
Inhibit apoptosis in the presence of SCF [45, 47] |
| TGF-β | TGF-βR | Inhibits proliferation [48] | Inhibits proliferation [44] |
| GM-CSF | GM-CSFR | Inhibits proliferation [25] | Inhibits proliferation [26] |
| TPO | TPOR | Not done | Induces MC development [22] |
Numerous studies have been carried out on the effects of Th2 versus Th1 cytokines on mast cells because of the notion that mast cells are the prominent effector cells of allergy and allergic pathology is mainly controlled by Th2 T cells and Th2 cytokines. IL-4 possesses mast cell growth factor activity in mice, and can promote phenotype switching to connective tissue-type mast cells in the presence of IL-3 [29]. In contrast, IL-4 was reported to inhibit SCF-dependent differentiation of human mast cells [30-33], but other reports claimed that IL-4 and SCF synergistically enhance the proliferation of human intestinal mast cells [34] and have no effect on lung mast cells [33]. These results suggest that SCF-induced mast cell proliferative or developmental responses are differentially modulated by IL-4, depending on the microenvironment (probably a cytokine milieu) or the subtype of mast cells.
BMMC contain high steady-state levels of mouse mast cell protease (mMCP)-5 transcript but undetectable levels of mMCP-1, mMCP-2, or mMCP-4 transcripts. Although IL-9 alone has no effect on BMMC proliferation, IL-9 + SCF enhances the long-term viability of BMMC [35]. Furthermore, IL-9 + SCF stimulates mouse BMMC to undergo a phenotypic change by inducing accumulation of mMCP-1 and mMCP-2 transcripts, resulting in the mucosal phenotype. In contrast, in BMMC, IL-4 suppresses IL-9-induced accumulation of mMCP-1 and mMCP-2 transcripts, IL-10-induced accumulation of mMCP-1 and mMCP-2 transcripts, and SCF-induced accumulation of mMCP-4 transcript, but not IL-3-induced accumulation of mMCP-5 transcript. IL-3 also suppresses IL-9-induced accumulation of mMCP-1 and mMCP-2 transcripts. Because of their counter-regulatory actions on the steady-state levels of the transcripts that encode three late-expressed serine proteases (e.g., mMCP-1, mMCP-2, and mMCP-4) in mice, IL-4 and IL-3 both inhibit the final stages of differentiation and maturation of mast cells [35]. IL-10 alone fails to support the growth of mast cell lines and mast cell progenitors [36]. Nevertheless, it dramatically enhances their growth when combined with IL-3 or IL-4. Moreover, IL-4 + IL-10 support the proliferation of mast cells as well as IL-3 does. In the human system, Th2 cytokines, such as IL-5 [19, 37] and IL-9 [37, 38], stimulate SCF-dependent proliferation of mast cells from cord-blood cells, bone marrow, and peripheral blood cells.
In stark contrast to Th2 cytokines, the Th1 cytokine IFN-γ suppresses SCF-mediated differentiation of mast cell progenitors from murine [39] and human bone marrow [40], human peripheral blood [40], and human cord blood cells [37]. IFN-γ induces apoptosis in mouse mast cells in a Stat1-dependent manner [41]. IFN-γ hyperresponsiveness results in inflammatory disease and death in mice lacking the regulatory protein suppressor of cytokine signaling (SOCS)-1. Bone marrow cells from SOCS-1-deficient mice cannot give rise to viable mast cells in IL-3 + SCF, with profound apoptosis occurring as the cultures matured. However, bone marrow cells lacking both SOCS-1 and IFN-γ survive normally. Consistent with this in vitro defect, SOCS-1−/− mice demonstrated a 67% decrease in peritoneal mast cell numbers relative to wild-type mice, a deficiency that is reversed in SOCS-1/IFN-γ KO mice. Importantly, IFN-γ treatment of a patient with mastocytosis showed symptomatic improvement that abated as the patient developed anti-IFN-γ antibodies [42].
In the murine system, although TNF-α and IL-6 are not the mast cell growth factors, these cytokines can induce mast cell development [43]. The development of homogeneously pure metamastocytes from uncommitted mouse bone marrow cells has also been reported using a combination of SCF, IL-6, and IL-10 [44]. Regardless of the source of mast cell progenitors, e.g., bone-marrow, peripheral-blood, cord-blood or fetal liver, appropriate culture conditions give rise to pure populations of human mast cells by 8 – 10 or more weeks. Both SCF and IL-6 are used as growth factors for such long-term cultures. IL-6 exhibit mast cell growth-promoting [19, 37] and anti-apoptotic [33, 45] activities in cultures of cord blood-derived mononuclear cells and CD34+ cells. IL-6 family cytokines, such as IL-11 and leukemia inhibitory factor (LIF), are capable of supporting the survival of hematopoietic stem cells by upregulating the signal transducers and activators of transcription (Stat) 3 expression via gp130 signaling molecule, but a study has demonstrated that IL-6 directly modulates SCF-dependent mast cell development of CD34+ cord blood cells via gp130 [27]. Although IL-6 inhibits mast cell growth and decreases Kit expression, it increases cell size, histamine content, and frequency of chymase-positive mast cells [27].
Nerve growth factor (NGF) stimulates the differentiation and proliferation of mouse BMMC in the presence of IL-3 [46]. By contrast, NGF has no effect on human mast cell survival [45] or mast-cell growth promoting activity [47] in the absence of SCF. However, NGF and SCF synergistically suppress human mast cell apoptosis [47].
TGF-β1 inhibits both IL-3-dependent growth of mouse mast cells [48] and SCF-dependent growth of human intestinal mast cells [49]. Thrombopoietin (TPO) can support the growth of hematopoietic progenitors, especially multipotent progenitors, in combination with other cytokines, including SCF, IL-3, and Flt3 ligand [50, 51]. TPO stimulates an early stage of mast cell development in concert with SCF [22].
4. Signaling molecules and transcriptional systems that are critical for mast cell development
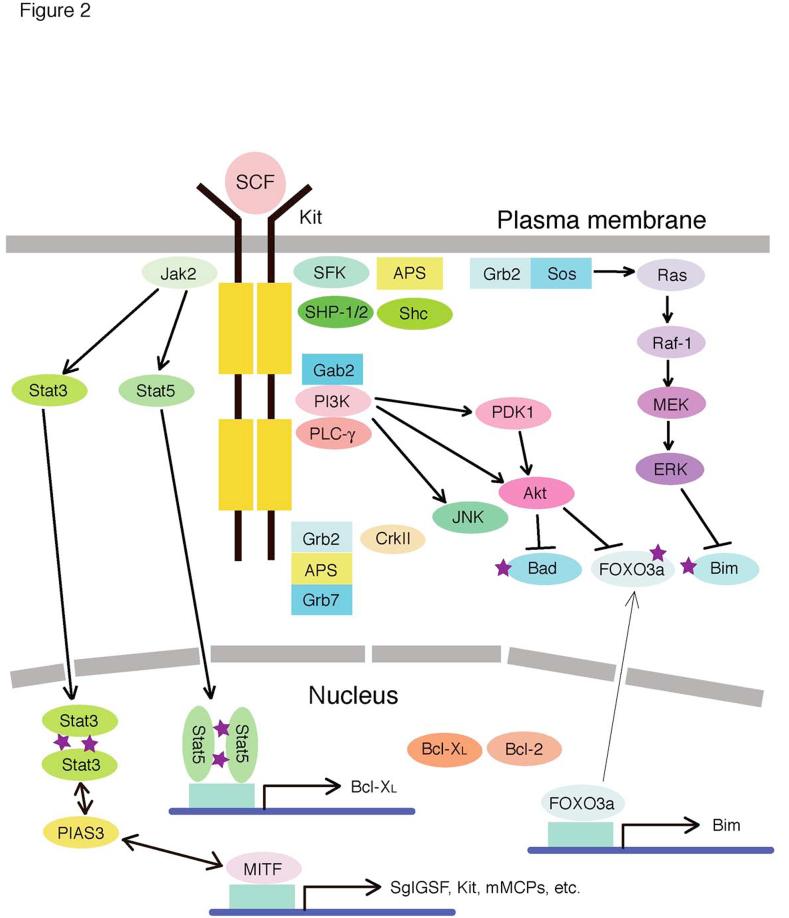
As described above, SCF and Kit signaling play a central role in the development of mast cells in both mice and humans. SCF binding to its receptor, Kit, induces the dimerization or oligomerization of Kit leading to the activation of its intrinsic kinase activity. Signaling molecules, including Grb2-associated binder 2 (Gab2) and phosphatidylinositol-3 kinase (PI3K), become tyrosine-phosphorylated and activated, and tyrosine-phosphorylated adaptors recruit interacting molecules to nucleate signaling complexes to activate multiple signaling pathways, including PI3K, phospholipase C, protein kinase C, the Ras-MAPK cascade, and the Jak-Stat pathway (Figure 2). For an in-depth insight SCF/Kit signaling, the reader is referred to the recent reviews [52, 53]. Mastocytosis is characterized by accumulation of mast cells in various organs and release of mast cell mediators. Abnormalities in SCF/Kit signaling have been implicated in mastocytosis: activating mutations in Kit have been reported in a variety of patients with mastocytosis. Direct and indirect evidence shows that these mutations promote ligand (i.e., SCF)-independent autophosphorylation of the mutant receptor [53].
Figure 2.
Kit signaling pathways in mast cells. This figure summarizes signaling proteins activated by Kit. Filled boxes of Kit indicate split kinase domains. Abbreviations: APS, adaptor containing PH and SH2 domains; Grb, growth factor receptor-bound protein; JAK, Janus kinase; JNK, c-Jun NH2-terminal kinase; MEK, mitogen-activated protein kinase kinase; ERK, extracellular regulated protein kinase; MITF, microphthalmia transcription factor; PI3K, phosphoinositide 3'-kinase; PLCγ, phospholipase Cγ; SCF, stem cell factor; SFK, Src family kinases; Shc, SH2-containing transforming protein C1; SHP, SH2 domain-containing phosphatase; Stat, signal transducers and activators of transcription.
A number of signaling molecules and transcription factors have been identified that appear to be critical for mediating the SCF/Kit signal for mast cell development. Members of the phosphoinositide-3 kinase (PI3K) family have important functions in the immune system. Studies of several gene-manipulated mice have shown that the development of gastrointestinal, but not dermal, mast cells is dependent on signaling mediated by class IA PI3Ks (reviewed in [54]). Class IA heteromeric PI3Ks consist of a catalytic subunit (p110α, p110β, p110δ) and a regulatory subunit (p85α, p85β, p55γ) [55, 56]. Mice lacking p85α mice [57], mice expressing a catalytically inactive form of p110δ, p110δD910A/D910A [58], mice with a mutation in the p85α-binding site of Kit [59, 60], and mice with Gab2 deficiency all lack gastrointestinal mast cells and have impaired bacterial clearance in acute septic peritonitis [57, 61]. However, whereas SCF-mediated signaling is affected by p85α or Gab2 deficiency, FcεRI-mediated signaling is not. On the contrary, p110δD910A/D910A mast cells are impaired in IgE+Ag-mediated degranulation and cytokine production [58]. In contrast to cells deficient in class IA enzymes, cells deficient in the class IB enzyme p110γ exhibit no developmental defects [62-64], although G protein-coupled receptor (GPCR)-mediated amplification of FcεRI-mediated degranulation is severely impaired. Stat5 is also required for normal mast cell development and survival both in vitro and in vivo [65]: Stat5−/− mice lack mast cells in skin, stomach, and spleen and have only few mast cells in the peritoneal cavity. Stat5 deficiency dramatically reduces survival and proliferation of developing mast cells (generated in SCF + IL-3 in vitro). Stat5−/− mast cells survive poorly in SCF or IL-3 alone and their apoptosis under such culture conditions is associated with reduced expression of Bcl-2 and Bcl-XL and mitochondrial damage. Further, Stat5−/− mast cells exhibit delayed G1-to-S cell cycle progression. Stat5 is the most ubiquitously activated member of the Stat family, used not only by SCF, but also by IL-2, IL-3, IL-4, IL-5, IL-7, IL-9, IL-15, GM-CSF, erythropoietin, TPO, prolactin, and growth hormone, some of which were discussed above as regulators of mast cell development. SCF stimulation results in activation of Jak2 in mast cells [66, 67]. Jak2 constitutively associates with Kit and becomes transiently activated in response to SCF [68]. After SCF stimulation, Stat1, Stat5A, Stat5B, and Stat6 associate with Kit and become tyrosine-phosphorylated [69, 70]. SCF also induces serine phosphorylation of Stat3 [69]. Interestingly, Jak3 was shown to be essential for IL-4- and IL-9-induced proliferation and survival of murine mast cells [71].
RasGRP4 also appears to be placed downstream of Kit. RasGRP4 is a recently identified guanine nucleotide exchange factor for the Ras family with a diacylglycerol-binding C1 domain and Ca2+-binding EF hand motif (reviewed in [72]). Importantly, this protein is specifically expressed in mast cells and their progenitors unlike other family members (RasGRP1-3). SCF-dependent Kit activation induces phospholipase C enzymes to hydrolyze phosphatidylinositol 4,5-bisphoaphate to DAG and inositol 1,4,5-trisphosphate. Binding of DAG to the C1 domain recruits RasGRP4 to the plasma membrane [73]. Then RasGRP4 activates Ras. On the other hand, Ca2+ binding to the EF hand motif inhibits the ability of RasGRP4 to activate H-Ras, therefore suggesting that increased Ca2+ levels and reduced DAG levels probably catalyzed by DAG kinases may dampen RasGRP4-mediated activation signals. Although RasGRP4 seems nonessential to the proliferation or survival of mast cells, it seems necessary for both mast cell granule development and expression of prostaglandin (PG) D2 synthase [74]. It is known that the Kit controls PGD2 expression in mast cells by regulating the levels of the synthase that converts PGH2 to PGD2 (see below for PGD2 synthase regulation by MITF).
A basic-helix-loop-helix leucine zipper transcription factor called microphthalmia transcription factor (MITF) is essential for mast cell development (reviewed in [75]). Although mast cell numbers in the skin are less severely defective in mi/mi (∼30% of control mice) than in W/WV or Sl/Sld (∼1% of control mice) mice, MITF mutations result in both intrinsic and extrinsic defects in mast cell development [76]: when bone marrow cells from MITF mutant (B6-Mitfmi-vga9/Mitfmi-vga9) mice were transplanted to irradiated WBB6F1-KitW/KitW-v mice that possessed normal tissue environment, mast cells of B6-Mitfmi-vga9/ Mitfmi-vga9 origin did not developed: when bone marrow cells from WBB6F1-+/+ mice were transplanted, the number of developing mast cells was significantly lower in B6-Mitfmi-vga9/ Mitfmi-vga9 than in WBB6F1-KitW/KitW-v recipients. MITF regulates the expression of Kit, granzyme B, several mast cell-specific proteases (mMCP-2, -4, -5, -6, -7, and -9) in mice as well as expression of PGD2 synthase [77]. MITF also controls the transcription of SgIGSF, a recently identified member of the immunoglobulin superfamily. SgIGSF mediates the adhesion of BMMC to NIH/3T3 fibroblasts. Transfection of SgIGSF can improve the survival of B6-Mitfmi-vga9/ Mitfmi-vga9 BMMC in the peritoneal cavity in B6-Mitfmi-vga9/ Mitfmi-vga9 and WBB6F1-KitW/KitW-v mice [78]. MITF-interacting proteins include PKC/Hint [79] and PIAS [80], repressors of MITF transcriptional activity. In NIH 3T3 cells stimulated via gp130 receptor, transfected MITF is phosphorylated at S409 [81]. Such phosphorylation of MITF leads to PIAS3 dissociation from MITF and its association with Stat3. Activation of mouse melanoma and mast cells through gp130 or Kit receptors induced the mobilization of PIAS3 from MITF to Stat3. Thus, the MITF-PIAS3-Stat3 network of interactions is mediated by IL-6 and SCF.
The zinc-finger transcription factor GATA-1 is expressed in erythroid cells, megakaryocytes, eosinophils, and mast cells, and is involved in their development as well as in cell type-specific gene expression [82]. GATA-1 protein is expressed in peritoneal mast cells and BMMCs after culture in the presence of IL-3 and SCF [83]. GATA-1-negative mast cells derived from fetal liver have histochemical staining profiles similar to those of wild-type mast cells [84], whereas targeted deletion of the GATA-1 gene promoter decreases the amount of heparin in connective tissue-type mast cells [83]. The phenotype of GATA-1low mice lacking the first enhancer (DNA hypersensitive site I) and the distal promoter of the GATA-1 gene shows defects in mast cell development, including the presence of morphologically abnormal alcian blue+ mast cells and apoptotic metachromatic mast cell precursors in connective tissue and peritoneal lavage fluid, and of numerous cells committed to erythroid, megakaryocytic, and mast cell developments in bone marrow and spleen [85]. These observations are consistent with the role of GATA-1 in mast cell maturation. Furthermore, GATA-1 recognizes critical elements in the promoters of mast cell carboxypeptidase A [86], FcεRI α-, and β-chain genes [87-89], and expression of the α- and β-chains are lower in mast cells in GATA-1 knockdown heterozygous mice [90]. GATA-2 protein is also expressed in mast cells [83]. GATA-2 is required for mast cell development but dispensable for the differentiation of erythroid cells and macrophages [91].
The Ets family transcription factor PU.1 is also essential for mast cell generation [92]. PU.1−/− hematopoietic progenitors can be expanded in IL-3 and differentiate into mast cells or macrophages upon restoration of PU.1 activity. However, in the absence of GATA-2, PU.1 promotes macrophage, but not mast cell, differentiation. Re-expression of GATA-2 in such progenitors enables mast cell generation. Therefore, PU.1 and GATA transcription factors antagonize each other's function in the development of distinct lineages of the hematopoietic system. Overexpression of PU.1 in mouse bone marrow hematopoietic progenitors [93] or differentiated BMMCs [94] induces monocyte-specific gene expression, causes monocyte-like morphological change, and confers T cell-stimulatory activity, indicating that differentiated mast cells still maintain potential to display monocytic features. These studies may point to the similar regulatory role for PU.1 in the distinct differentiation of MCPs versus BaPs as well as monocyte/macrophages versus granulocytes: high concentrations of PU.1 are required for macrophage differentiation whereas low concentrations of PU.1 promote granulocytic differentiation [95]. PU.1 activity is antagonized by C/EBPα, a transcription factor essential for neutrophil development [96]. C/EBPα expression is low in MCPs unlike BaPs [13]. Therefore, the relative PU.1 activity can be higher in MCPs and monocytes/macrophages than in BaPs and granulocytes.
The above discussions collectively indicate that Kit activation by SCF is critically important for mast cell development. This interaction initiates activation of tyrosine kinase activity of Kit and recruits downstream lineage-restricted effectors, such as RasGRP4 and MITF, as well as less restricted signaling molecules, such as Gab2 and PI3K. Several transcription factors are responsible for the transcriptional and/or post-transcriptional control of mast cell-specific genes and for the eventual differentiation of mast cells. Many factors that influence mast cell development discussed in the preceding section interact directly or indirectly with Kit signaling pathways.
Migration of mast cells
Critical signals for homing and recruitment of mast cells to various tissues are also provided by SCF binding to Kit. Under various experimental conditions, the membrane bound SCF and/or its soluble isoform is chemotactic for mast cells and their progenitors; SCF not only elicits adhesion of mast cells, but also facilitates their proliferation and sustains their survival, differentiation, and maturation.
Integrins are also involved in the accumulation of mast cells in inflamed mucosal tissues. In the mouse, large numbers of mast cell-committed progenitors reside in the small intestine and are constitutively recruited by a mechanism involving the α4β7 integrin [97], an adhesion molecule that is also crucial for T-cell homing to the gut. This constitutive homing mechanism may reflect a unique requirement for mast cells in intestinal mucosal immune responses, especially in the host defense against helminths. Additionally, human mast cell progenitors express α4β1, which mediates their adhesion to activated endothelial cells under flow conditions [98].
Chemokine receptors expressed by mast cell progenitors and mature tissue mast cells are most likely involved in directing the progenitors from the circulation into the tissue where they mature. Mature mast cells express a set of chemokine receptors that is somewhat different from those expressed by their progenitors, and their expression pattern also differs between mast cell subtypes. Human mast cell progenitors derived in vitro from cord blood express several chemokine receptors, including CXCR2, CCR3, CXCR4, and CCR5, and respond to the corresponding ligands in vitro [37]. A role for CCR3 in mast cell homing has been identified in CCR3-deficient mice [99]. Increased numbers of intraepithelial mast cells are found in the trachea of the mutant mice after sensitization and allergen challenge in allergic airway inflammation model, suggesting that CCR3 may be involved in the egress of mast cells, but not in the recruitment of their progenitors. Mast cell localization within the airway smooth muscle bundle is an important determinant of the asthmatic phenotype. This can be explained by the function of another chemokine receptor, CXCR3, the most abundantly expressed chemokine receptor on human lung mast cells in the airway smooth muscle in asthma: it is expressed by 100% of these mast cells as opposed to only 47% of mast cells in the submucosa [100]. Migration of human lung mast cells is induced by airway smooth muscle cultures predominantly through activation of CXCR3. Importantly, CXCL10 (a ligand of CXCR3) is preferentially expressed by asthmatic airway smooth muscles in bronchial biopsy specimens and ex vivo cells compared with airway smooth muscles from healthy control subjects [100].
Antigen works not only as a stimulant for the release of allergic mediators from IgE-sensitized mast cells, but also as a chemotactic factor for the mast cells [101]. Both p38 mitogen-activated protein kinase (MAPK) activation and Rho-dependent activation of Rho-associated coiled-coil-forming protein kinase (ROCK) may be required for Fc∊RI-mediated cell migration. Cross-linking of Fc∊RI (with IgE+Ag) activates sphingosine kinases 1 and 2 (SphK1 and SphK2), leading to the production and secretion of sphingosine 1-phosphate (S1P) [102]. S1P activates its receptors S1P1 and S1P2, the only S1P receptors expressed in mast cells. Transactivation of S1P1 and Gi signaling are important for cytoskeletal rearrangement and migration of mast cells toward antigen, while S1P2, whose expression is upregulated by Fc∊RI cross-linking, is required for maximal degranulation and inhibition of mast cell migration toward antigen [103]. SphK1 interacts with Lyn and is recruited to the Fc∊RI on the plasma membrane via this interaction [104]. More recently, Olivera et al. showed that Fyn is required for Fc∊RI-induced activation of SphK1 and SphK2, subsequent S1P formation, and migration toward antigen and SCF [105]. The defect in migration in Fyn-deficient BMMCs toward antigen or highly cytokinergic (HC) IgEs (see below) was consistent with an earlier study by Kitaura et al. [106]. Inhibition or downregulation of SphK1 impairs motility and degranulation. Surprisingly, overexpression of SphK1 in rat basophilic leukemia (RBL)-2H3 mast cells also impairs degranulation and migration toward antigen [103]. This appears to be due to restricted formation of S1P at the plasma membrane, resulting in activation, subsequent internalization, and desensitization of S1P receptors. Serum starvation, which significantly reduces membrane-associated SphK1 activity, restores S1P receptor functions.
Highly cytokinergic (HC, see below) IgE molecules can efficiently activate mast cells in the absence of antigen. In addition to antigen that can attract IgE-bound mast cells, HC IgEs alone can promote the migration of mast cells in the absence of antigen [106]. IgE− and IgE+Ag-mediated migration involves an autocrine/paracrine secretion of soluble factors including adenosine, leukotriene B4, and several chemokines [106]. Secretion of these factors depends on two tyrosine kinases, Lyn and Syk, and they are agonists of G-protein-coupled receptors and signal through PI3K-γ, leading to mast cell migration [106]. Therefore, the homing and recruitment of mast cells to various tissues may be exquisitely regulated by a variety of factors, including integrins, chemokines, and chemokine receptors.
Survival of mast cells
1. SCF is crucial to sustain mast cell survival.
Bcl-2 family proteins are critical for the regulation of survival and death in a variety of cell types including mast cells. The reader is referred to recent reviews on these proteins [107, 108]. They can be classified as prosurvival (or antiapoptotic) and proapoptotic members: the prosurvival members include Bcl-2, Bcl-XL, Bcl-w, Mcl-1, and A1/Bfl-1, while the proapoptotic members include Bax, Bak, and Bok, which share 3 regions of homology (BH1-BH3) with their prosurvival relatives; BH3–only proteins, including Bad, Hrk, Bim, Bid, Puma, Noxa, and Bmf, share only the BH3 domain. Analysis of Bax-deficient BMMCs indicates tha Bax is involved in the induction of mast cell apoptosis [109]. BH3 domain-only molecules activate multidomain proapoptotic members (Bax and Bak) to trigger a mitochondrial pathway, in which mitochondria releases cytochrome c [110, 111]. Bcl-2 and Bcl-XL can sequester BH3-only molecules in stable complexes, preventing the activation of Bax and Bak [110].
Mast cell treatment with SCF increases the levels of the prosurvival members Bcl-2 and Bcl-XL [112, 113]. Whereas SCF does not affect the expression of A1/Bfl-1, this prosurvival protein is crucial for Fc∊RI activation-induced mast cell survival [114]. Following SCF stimulation, Kit signaling results in the activation of PI3K and its downstream target Akt (also known as protein kinase B). Akt promotes cell survival through phosphorylation-mediated inactivation of the BH3-only protein Bad [115-117], but the relevance of this process to the survival of hematopoietic cells remains unclear. Thus, bad−/− mice have normal numbers of immune cells [118]. Another Akt target involved in survival/apoptosis is Forkhead family transcription factors [119, 120]. When Forkhead proteins, such as FOXO1a/FKHR, FOXO3a/FKHRL1, and FOXO4/AFX, are phosphorylated by active Akt, they are exported from the nucleus, and thus transcription of their target genes is inhibited [119, 121]. One of the transcriptional targets of FOXO3a is Bim [122]. Bim is essential for growth factor deprivation-induced mast cell apoptosis [123]. Bim deficiency or Bcl-2 overexpression delays or even prevents growth factor deprivation-induced mast cell apoptosis. SCF prevents mast cell apoptosis induced by growth factor withdrawal by actively preventing Bim expression via phosphorylation /inactivation of FOXO1a and FOXO3a. SCF also promotes phosphorylation of Bim by PI3K and mitogen-activated protein kinase/extracellular regulated protein kinase (MEK/ERK)-dependent pathways. Phosphorylation of BimEL on Ser69 through the MEK/ERK pathway has recently been described as promoting proteasome-dependent degradation of Bim [124, 125], thus representing an additional mechanism by which SCF promotes protection from Bim-mediated apoptosis in mast cells.
2. The fate of mast cells upon Fc∊RI activation depends on the relative levels of pro- and anti-apoptotic Bcl-2 family members.
Stimulation of IgE-sensitized mouse mast cells with antigen can promote their survival under certain conditions [126]. The reader is referred to a recent review on Fc∊RI stimulation effects on mast cell survival [127]. Cross-linking of Fc∊RI with IgE+Ag prevents apoptosis of MC9 mast cells by an autocrine mechanism, producing IL-3, IL-4, and GM-CSF [128]. Although secretion of endogenous IL-3 and GM-CSF is not sufficient for MC9 survival, IL-4 renders the cells reactive to these cytokines. IgE+Ag can induce enhanced expression of FLIP, a caspase-8 inhibitor, and consequently a resistance to Fas-induced apoptosis [129]. Among other factors, the signal strength via the Fc∊RI seems most critical for determining the cell fate: for instance, 1-10 ng/ml of DNP21-BSA, which is suboptimal for histamine release and IL-6 production, can promote the survival of anti-DNP IgE-sensitized cells, but 100-1000 ng/ml of the same antigen cannot [106]. On the other hand, 10-100 ng/ml of DNP3-BSA can promote the cell survival [106]. Therefore, weak stimulation via the Fc∊RI, i.e., IgE + low antigen concentrations, IgE + anti-IgE, and monomeric IgE (see below), can promote survival. Activation of mast cells through the Fc∊RI results in strong induction of A1 mRNA and protein [114]. A1-deficient mast cells release granule mediators similar to the wild-type control, but the mutant cells do not survive allergic activation, revealing the importance of A1 for mast cell survival. Furthermore, A1-deficient mice that had been sensitized and provoked with allergen contained a lower number of mast cells than littermate controls [114]. On the other hand, Bim is essential for growth factor deprivation-induced mast cell apoptosis [123]. Bcl-XL and Bim are both induced upon Fc∊RI activation. Taken together, these findings suggest that the fate of mast cells upon Fc∊RI activation depends on the relative levels of pro- and anti-apoptotic Bcl-2 family members.
3. Monomeric IgE binding to Fc∊RI enhances mast cell survival mainly by an autocrine production of IL-3.
Mouse IgE molecules display a wide spectrum of heterogeneity in the ability to induce the production and secretion of IL-6 and TNF-α, with HC IgEs at one extreme end and poorly cytokinergic (PC) IgEs at the other [130]. Anisotropy data suggest that more extensive receptor aggregation occurs with HC IgEs than with PC IgEs [130]. Mouse mast cell survival and growth are promoted by the binding of both HC and PC IgEs to Fc∊RI [131, 132]. HC IgE-mediated survival is predominantly mediated by high production of IL-3, as evidenced by severe impairment of survival by IL-3 neutralization or deficiency. The upregulation of Bcl-XL and Bcl-2 by IgE is abrogated in IL-3−/− BMMCs [133]. Downstream of IL-3R signaling, Stat5 was shown to be important for mast cell survival [65]. These results indicate that IL-3 plays a crucial role in HC IgE-induced mast cell survival (and other functions) in an autocrine manner by inducing the Bcl-XL and Bcl-2 via Stat5. Taken together, these results suggest that IgE-mediated gene expression in mast cells is regulated by at least two mechanisms: IL-3-dependent as well as IL-3-independent mechanisms. The relative importance of the IL-3-dependent versus the IL-3-independent mechanism appears to depend on the IgE phenotype; i.e., HC and PC IgEs. In addition to Stat5-dependent expression of Bcl-XL and Bcl-2, sustained ERK activation seems important for mast cell survival [134]. Sustained ERK activation by active MEK induces BMMC survival. HC IgE-induced survival requires Lyn and Syk, whereas Fyn, Gab2, and the PI3K-Akt pathway are dispensable.
Conclusions
SCF is the crucial factor for the development, proliferation, and maturation of mast cells, and many factors modulate SCF-mediated mast cell development. In addition to the SCF/Kit system, some integrins and chemokine receptors expressed in mast cells regulate mast cell homing and recruitment. Whilst SCF is crucial for mast cell survival, FcεRI aggregation induced by monomeric IgE or IgE+Ag can also enhance mast cell survival. The mechanism of mast cell development, migration, and survival is not fully understood, but recent advances in research in this field have led to the discovery of several molecules that regulate these processes that can be considered as potential therapeutic targets. Further basic and translational research will lead to better understanding and hopefully better treatment of allergic and other diseases.
Acknowledgements
We thank Yuko Kawakami for careful reading of the manuscript. The study in the Kawakami laboratory is supported in part by the National Institutes of Health grants (AI50209 and AI38348).
References
- 1.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77(4):1033–79. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 2.Galli SJ, Maurer M, Lantz CS. Mast cells as sentinels of innate immunity. Curr Opin Immunol. 1999;11(1):53–9. doi: 10.1016/s0952-7915(99)80010-7. [DOI] [PubMed] [Google Scholar]
- 3.Secor VH, Secor WE, Gutekunst CA, Brown MA. Mast cells are essential for early onset and severe disease in a murine model of multiple sclerosis. J Exp Med. 2000;191(5):813–22. doi: 10.1084/jem.191.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee DM, Friend DS, Gurish MF, Benoist C, Mathis D, Brenner MB. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science. 2002;297(5587):1689–92. doi: 10.1126/science.1073176. [DOI] [PubMed] [Google Scholar]
- 5.Hara M, Ono K, Hwang MW, Iwasaki A, Okada M, Nakatani K, Sasayama S, Matsumori A. Evidence for a role of mast cells in the evolution to congestive heart failure. J Exp Med. 2002;195(3):375–81. doi: 10.1084/jem.20002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Viegas M, Gomez E, Brooks J, Davies RJ. Changes in nasal mast cell numbers in and out of the pollen season. Int Arch Allergy Appl Immunol. 1987;82(34):275–6. doi: 10.1159/000234205. [DOI] [PubMed] [Google Scholar]
- 7.Gibson PG, Allen CJ, Yang JP, Wong BJ, Dolovich J, Denburg J, Hargreave FE. Intraepithelial mast cells in allergic and nonallergic asthma. Assessment using bronchial brushings. Am Rev Respir Dis. 1993;148(1):80–6. doi: 10.1164/ajrccm/148.1.80. [DOI] [PubMed] [Google Scholar]
- 8.Kitamura Y, Ito A. Mast cell-committed progenitors. Proc Natl Acad Sci U S A. 2005;102(32):11129–30. doi: 10.1073/pnas.0505073102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitamura Y, Yokoyama M, Matsuda H, Ohno T, Mori KJ. Spleen colony-forming cell as common precursor for tissue mast cells and granulocytes. Nature. 1981;291(5811):159–60. doi: 10.1038/291159a0. [DOI] [PubMed] [Google Scholar]
- 10.Kitamura Y, Go S, Hatanaka K. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood. 1978;52(2):447–52. [PubMed] [Google Scholar]
- 11.Rodewald HR, Dessing M, Dvorak AM, Galli SJ. Identification of a committed precursor for the mast cell lineage. Science. 1996;271(5250):818–22. doi: 10.1126/science.271.5250.818. [DOI] [PubMed] [Google Scholar]
- 12.Chen CC, Grimbaldeston MA, Tsai M, Weissman IL, Galli SJ. Identification of mast cell progenitors in adult mice. Proc Natl Acad Sci U S A. 2005;102(32):11408–13. doi: 10.1073/pnas.0504197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arinobu Y, Iwasaki H, Gurish MF, et al. Developmental checkpoints of the basophil/mast cell lineages in adult murine hematopoiesis. Proc Natl Acad Sci U S A. 2005;102(50):18105–10. doi: 10.1073/pnas.0509148102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jamur MC, Grodzki AC, Berenstein EH, Hamawy MM, Siraganian RP, Oliver C. Identification and characterization of undifferentiated mast cells in mouse bone marrow. Blood. 2005;105(11):4282–9. doi: 10.1182/blood-2004-02-0756. [DOI] [PubMed] [Google Scholar]
- 15.Kirshenbaum AS, Kessler SW, Goff JP, Metcalfe DD. Demonstration of the origin of human mast cells from CD34+ bone marrow progenitor cells. J Immunol. 1991;146(5):1410–5. [PubMed] [Google Scholar]
- 16.Castells MC, Friend DS, Bunnell CA, Hu X, Kraus M, Osteen RT, Austen KF. The presence of membrane-bound stem cell factor on highly immature nonmetachromatic mast cells in the peripheral blood of a patient with aggressive systemic mastocytosis. J Allergy Clin Immunol. 1996;98(4):831–40. doi: 10.1016/s0091-6749(96)70133-1. [DOI] [PubMed] [Google Scholar]
- 17.Rottem M, Okada T, Goff JP, Metcalfe DD. Mast cells cultured from the peripheral blood of normal donors and patients with mastocytosis originate from a CD34+/FcεRI− cell population. Blood. 1994;84(8):2489–96. [PubMed] [Google Scholar]
- 18.Kempuraj D, Saito H, Kaneko A, et al. Characterization of mast cell-committed progenitors present in human umbilical cord blood. Blood. 1999;93(10):3338–46. [PubMed] [Google Scholar]
- 19.Kirshenbaum AS, Goff JP, Semere T, Foster B, Scott LM, Metcalfe DD. Demonstration that human mast cells arise from a progenitor cell population that is CD34(+), c-kit(+), and expresses aminopeptidase N (CD13) Blood. 1999;94(7):2333–42. [PubMed] [Google Scholar]
- 20.Chabot B, Stephenson DA, Chapman VM, Besmer P, Bernstein A. The protooncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature. 1988;335(6185):88–9. doi: 10.1038/335088a0. [DOI] [PubMed] [Google Scholar]
- 21.Copeland NG, Gilbert DJ, Cho BC, et al. Mast cell growth factor maps near the steel locus on mouse chromosome 10 and is deleted in a number of steel alleles. Cell. 1990;63(1):175–83. doi: 10.1016/0092-8674(90)90298-s. [DOI] [PubMed] [Google Scholar]
- 22.Sawai N, Koike K, Mwamtemi HH, et al. Thrombopoietin augments stem cell factor-dependent growth of human mast cells from bone marrow multipotential hematopoietic progenitors. Blood. 1999;93(11):3703–12. [PubMed] [Google Scholar]
- 23.Nakahata T, Kobayashi T, Ishiguro A, et al. Extensive proliferation of mature connective-tissue type mast cells in vitro. Nature. 1986;324(6092):65–7. doi: 10.1038/324065a0. [DOI] [PubMed] [Google Scholar]
- 24.K Tsuji, Nakahata T, Takagi M, et al. Effects of interleukin-3 and interleukin-4 on the development of “connective tissue-type” mast cells: interleukin-3 supports their survival and interleukin-4 triggers and supports their proliferation synergistically with interleukin-3. Blood. 1990;75(2):421–7. [PubMed] [Google Scholar]
- 25.Bressler RB, Thompson HL, Keffer JM, Metcalfe DD. Inhibition of the growth of IL-3-dependent mast cells from murine bone marrow by recombinant granulocyte macrophage-colony-stimulating factor. J Immunol. 1989;143(1):135–9. [PubMed] [Google Scholar]
- 26.Saito H, Ebisawa M, Tachimoto H, et al. Selective growth of human mast cells induced by Steel factor, IL-6, and prostaglandin E2 from cord blood mononuclear cells. J Immunol. 1996;157(1):343–50. [PubMed] [Google Scholar]
- 27.Kinoshita T, Sawai N, Hidaka E, Yamashita T, Koike K. Interleukin-6 directly modulates stem cell factor-dependent development of human mast cells derived from CD34(+) cord blood cells. Blood. 1999;94(2):496–508. [PubMed] [Google Scholar]
- 28.Saito H. Culture of human mast cells from hemopoietic progenitors. Methods Mol Biol. 2005;315:113–22. doi: 10.1385/1-59259-967-2:113. [DOI] [PubMed] [Google Scholar]
- 29.Hamaguchi Y, Kanakura Y, Fujita J, Takeda S, Nakano T, Tarui S, Honjo T, Kitamura Y. Interleukin 4 as an essential factor for in vitro clonal growth of murine connective tissue-type mast cells. J Exp Med. 1987;165(1):268–73. doi: 10.1084/jem.165.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nilsson G, Miettinen U, Ishizaka T, Ashman LK, Irani AM, Schwartz LB. Interleukin-4 inhibits the expression of Kit and tryptase during stem cell factor-dependent development of human mast cells from fetal liver cells. Blood. 1994;84(5):1519–27. [PubMed] [Google Scholar]
- 31.Sillaber C, Sperr WR, Agis H, Spanblochl E, Lechner K, Valent P. Inhibition of stem cell factor dependent formation of human mast cells by interleukin-3 and interleukin-4. Int Arch Allergy Immunol. 1994;105(3):264–8. doi: 10.1159/000236767. [DOI] [PubMed] [Google Scholar]
- 32.Xia HZ, Du Z, Craig S, et al. Effect of recombinant human IL-4 on tryptase, chymase, and Fcε receptor type I expression in recombinant human stem cell factor-dependent fetal liver-derived human mast cells. J Immunol. 1997;159(6):2911–21. [PubMed] [Google Scholar]
- 33.Oskeritzian CA, Wang Z, Kochan JP, Grimes M, Du Z, Chang HW, Grant S, Schwartz LB. Recombinant human (rh)IL-4-mediated apoptosis and recombinant human IL-6-mediated protection of recombinant human stem cell factor-dependent human mast cells derived from cord blood mononuclear cell progenitors. J Immunol. 1999;163(9):5105–15. [PubMed] [Google Scholar]
- 34.Bischoff SC, Sellge G, Lorentz A, Sebald W, Raab R, Manns MP. IL-4 enhances proliferation and mediator release in mature human mast cells. Proc Natl Acad Sci U S A. 1999;96(14):8080–5. doi: 10.1073/pnas.96.14.8080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eklund KK, Ghildyal N, Austen KF, Stevens RL. Induction by IL-9 and suppression by IL-3 and IL-4 of the levels of chromosome 14-derived transcripts that encode late-expressed mouse mast cell proteases. J Immunol. 1993;151(8):4266–73. [PubMed] [Google Scholar]
- 36.Thompson-Snipes L, Dhar V, Bond MW, Mosmann TR, Moore KW, Rennick DM. Interleukin 10: a novel stimulatory factor for mast cells and their progenitors. J Exp Med. 1991;173(2):507–10. doi: 10.1084/jem.173.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ochi H, Hirani WM, Yuan Q, Friend DS, Austen KF, Boyce JA. T helper cell type 2 cytokine-mediated comitogenic responses and CCR3 expression during differentiation of human mast cells in vitro. J Exp Med. 1999;190(2):267–80. doi: 10.1084/jem.190.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsuzawa S, Sakashita K, Kinoshita T, Ito S, Yamashita T, Koike K. IL-9 enhances the growth of human mast cell progenitors under stimulation with stem cell factor. J Immunol. 2003;170(7):3461–7. doi: 10.4049/jimmunol.170.7.3461. [DOI] [PubMed] [Google Scholar]
- 39.Takagi M, Koike K, Nakahata T. Antiproliferative effect of IFN-γ on proliferation of mouse connective tissue-type mast cells. J Immunol. 1990;145(6):1880–4. [PubMed] [Google Scholar]
- 40.Kirshenbaum AS, Worobec AS, Davis TA, Goff JP, Semere T, Metcalfe DD. Inhibition of human mast cell growth and differentiation by interferon γ-1b. Exp Hematol. 1998;26(3):245–51. [PubMed] [Google Scholar]
- 41.Mann-Chandler MN, Kashyap M, Wright HV, Norozian F, Barnstein BO, Gingras S, Parganas E, Ryan JJ. IFN-γ induces apoptosis in developing mast cells. J Immunol. 2005;175(5):3000–5. doi: 10.4049/jimmunol.175.5.3000. [DOI] [PubMed] [Google Scholar]
- 42.Fiehn C, Prummer O, Gallati H, Heilig B, Hunstein W. Treatment of systemic mastocytosis with interferon-γ: failure after appearance of anti-IFN-γ antibodies. Eur J Clin Invest. 1995;25(8):615–8. doi: 10.1111/j.1365-2362.1995.tb01754.x. [DOI] [PubMed] [Google Scholar]
- 43.Hu ZQ, Kobayashi K, Zenda N, Shimamura T. Tumor necrosis factor-α- and interleukin-6-triggered mast cell development from mouse spleen cells. Blood. 1997;89(2):526–33. [PubMed] [Google Scholar]
- 44.Yuan Q, Gurish MF, Friend DS, Austen KF, Boyce JA. Generation of a novel stem cell factor-dependent mast cell progenitor. J Immunol. 1998;161(10):5143–6. [PubMed] [Google Scholar]
- 45.Yanagida M, Fukamachi H, Ohgami K, et al. Effects of T-helper 2-type cytokines, interleukin-3 (IL-3), IL-4, IL-5, and IL-6 on the survival of cultured human mast cells. Blood. 1995;86(10):3705–14. [PubMed] [Google Scholar]
- 46.Matsuda H, Kannan Y, Ushio H, Kiso Y, Kanemoto T, Suzuki H, Kitamura Y. Nerve growth factor induces development of connective tissue-type mast cells in vitro from murine bone marrow cells. J Exp Med. 1991;174(1):7–14. doi: 10.1084/jem.174.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kanbe N, Kurosawa M, Miyachi Y, Kanbe M, Saitoh H, Matsuda H. Nerve growth factor prevents apoptosis of cord blood-derived human cultured mast cells synergistically with stem cell factor. Clin Exp Allergy. 2000;30(8):1113–20. doi: 10.1046/j.1365-2222.2000.00866.x. [DOI] [PubMed] [Google Scholar]
- 48.Broide DH, Wasserman SI, Alvaro-Gracia J, Zvaifler NJ, Firestein GS. Transforming growth factor-β 1 selectively inhibits IL-3-dependent mast cell proliferation without affecting mast cell function or differentiation. J Immunol. 1989;143(5):1591–7. [PubMed] [Google Scholar]
- 49.Gebhardt T, Lorentz A, Detmer F, Trautwein C, Bektas H, Manns MP, Bischoff SC. Growth, phenotype, and function of human intestinal mast cells are tightly regulated by transforming growth factor β1. Gut. 2005;54(7):928–34. doi: 10.1136/gut.2004.054650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kobayashi M, Laver JH, Kato T, Miyazaki H, Ogawa M. Thrombopoietin supports proliferation of human primitive hematopoietic cells in synergy with steel factor and/or interleukin-3. Blood. 1996;88(2):429–36. [PubMed] [Google Scholar]
- 51.Kobayashi M, Laver JH, Lyman SD, Kato T, Miyazaki H, Ogawa M. Thrombopoietin, steel factor and the ligand for flt3/flk2 interact to stimulate the proliferation of human hematopoietic progenitors in culture. Int J Hematol. 1997;66(4):423–34. doi: 10.1016/s0925-5710(97)00066-2. [DOI] [PubMed] [Google Scholar]
- 52.Roskoski R., Jr Signaling by Kit protein-tyrosine kinase--the stem cell factor receptor. Biochem Biophys Res Commun. 2005;337(1):1–13. doi: 10.1016/j.bbrc.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 53.Lennartsson J, Jelacic T, Linnekin D, Shivakrupa R. Normal and oncogenic forms of the receptor tyrosine kinase kit. Stem Cells. 2005;23(1):16–43. doi: 10.1634/stemcells.2004-0117. [DOI] [PubMed] [Google Scholar]
- 54.Koyasu S, Minowa A, Terauchi Y, Kadowaki T, Matsuda S. John Wiley & Sons Ltd; Chichster, England: 2005. The role of phosphoinositide-3-kinase in mast cell homing to the gastrointestinal tract; pp. 152–165. [PubMed] [Google Scholar]
- 55.Koyasu S. The role of PI3K in immune cells. Nat Immunol. 2003;4(4):313–9. doi: 10.1038/ni0403-313. [DOI] [PubMed] [Google Scholar]
- 56.Okkenhaug K, Vanhaesebroeck B. PI3K in lymphocyte development, differentiation and activation. Nat Rev Immunol. 2003;3(4):317–30. doi: 10.1038/nri1056. [DOI] [PubMed] [Google Scholar]
- 57.Fukao T, Yamada T, Tanabe M, et al. Selective loss of gastrointestinal mast cells and impaired immunity in PI3K-deficient mice. Nat Immunol. 2002;3(3):295–304. doi: 10.1038/ni768. [DOI] [PubMed] [Google Scholar]
- 58.Ali K, Bilancio A, Thomas M, et al. Essential role for the p110δ phosphoinositide 3-kinase in the allergic response. Nature. 2004;431(7011):1007–11. doi: 10.1038/nature02991. [DOI] [PubMed] [Google Scholar]
- 59.Agosti V, Corbacioglu S, Ehlers I, et al. Critical role for Kit-mediated Src kinase but not PI 3-kinase signaling in pro T and pro B cell development. J Exp Med. 2004;199(6):867–78. doi: 10.1084/jem.20031983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kissel H, Timokhina I, Hardy MP, et al. Point mutation in kit receptor tyrosine kinase reveals essential roles for kit signaling in spermatogenesis and oogenesis without affecting other kit responses. Embo J. 2000;19(6):1312–26. doi: 10.1093/emboj/19.6.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gu H, Saito K, Klaman LD, et al. Essential role for Gab2 in the allergic response. Nature. 2001;412(6843):186–90. doi: 10.1038/35084076. [DOI] [PubMed] [Google Scholar]
- 62.Hirsch E, Katanaev VL, Garlandav C, et al. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science. 2000;287(5455):1049–53. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- 63.Li Z, Jiang H, Xie W, Zhang Z, Smrcka AV, Wu D. Roles of PLC-β2 and -β3 and PI3Kγ in chemoattractant-mediated signal transduction. Science. 2000;287(5455):1046–9. doi: 10.1126/science.287.5455.1046. [DOI] [PubMed] [Google Scholar]
- 64.Sasaki T, Irie-Sasaki J, Jones RG, et al. Function of PI3Kγ in thymocyte development, T cell activation, and neutrophil migration. Science. 2000;287(5455):1040–6. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- 65.Shelburne CP, McCoy ME, Piekorz R, et al. Stat5 expression is critical for mast cell development and survival. Blood. 2003;102(4):1290–7. doi: 10.1182/blood-2002-11-3490. [DOI] [PubMed] [Google Scholar]
- 66.Brizzi MF, Zini MG, Aronica MG, Blechman JM, Yarden Y, Pegoraro L. Convergence of signaling by interleukin-3, granulocyte-macrophage colony-stimulating factor, and mast cell growth factor on JAK2 tyrosine kinase. J Biol Chem. 1994;269(50):31680–4. [PubMed] [Google Scholar]
- 67.Weiler SR, Mou S, DeBerry CS, Keller JR, Ruscetti FW, Ferris DK, Longo DL, Linnekin D. JAK2 is associated with the c-kit proto-oncogene product and is phosphorylated in response to stem cell factor. Blood. 1996;87(9):3688–93. [PubMed] [Google Scholar]
- 68.Linnekin D, Weiler SR, Mou S, DeBerry CS, Keller JR, Ruscetti FW, Ferris DK, Longo DL. JAK2 is constitutively associated with c-Kit and is phosphorylated in response to stem cell factor. Acta Haematol. 1996;95(34):224–8. doi: 10.1159/000203882. [DOI] [PubMed] [Google Scholar]
- 69.Brizzi MF, Dentelli P, Rosso A, Yarden Y, Pegoraro L. STAT protein recruitment and activation in c-Kit deletion mutants. J Biol Chem. 1999;274(24):16965–72. doi: 10.1074/jbc.274.24.16965. [DOI] [PubMed] [Google Scholar]
- 70.Hundley TR, Gilfillan AM, Tkaczyk C, Andrade MV, Metcalfe DD, Beaven MA. Kit and FcεRI mediate unique and convergent signals for release of inflammatory mediators from human mast cells. Blood. 2004;104(8):2410–7. doi: 10.1182/blood-2004-02-0631. [DOI] [PubMed] [Google Scholar]
- 71.Suzuki K, Nakajima H, Watanabe N, Kagami S, Suto A, Saito Y, Saito T, Iwamoto I. Role of common cytokine receptor gamma chain (γc)- and Jak3-dependent signaling in the proliferation and survival of murine mast cells. Blood. 2000;96(6):2172–8. [PubMed] [Google Scholar]
- 72.Stevens RL, Morokawa N, Wang J, Krilis SA. John Wiley & Sons Ltd; Chichster, England: 2005. RasGRP4 in mast cell signalling and disease susceptibility; pp. 54–77. [PubMed] [Google Scholar]
- 73.Li L, Yang Y, Wong GW, Stevens RL. Mast cells in airway hyporesponsive C3H/HeJ mice express a unique isoform of the signaling protein Ras guanine nucleotide releasing protein 4 that is unresponsive to diacylglycerol and phorbol esters. J Immunol. 2003;171(1):390–7. doi: 10.4049/jimmunol.171.1.390. [DOI] [PubMed] [Google Scholar]
- 74.Li L, Yang Y, Stevens RL. RasGRP4 regulates the expression of prostaglandin D2 in human and rat mast cell lines. J Biol Chem. 2003;278(7):4725–9. doi: 10.1074/jbc.C200635200. [DOI] [PubMed] [Google Scholar]
- 75.Kirtamura Y. John Wiley & Sons Ltd; Chichster, England: 2005. MITF and SgIGSF: an esential transcrption factor and its target adhesion molecule for development and survival of mast cells; pp. 4–14. [PubMed] [Google Scholar]
- 76.Morii E, Oboki K, Ishihara K, Jippo T, Hirano T, Kitamura Y. Roles of MITF for development of mast cells in mice: effects on both precursors and tissue environments. Blood. 2004;104(6):1656–61. doi: 10.1182/blood-2004-01-0247. [DOI] [PubMed] [Google Scholar]
- 77.Morii E, Oboki K. MITF is necessary for generation of prostaglandin D2 in mouse mast cells. J Biol Chem. 2004;279(47):48923–9. doi: 10.1074/jbc.M407026200. [DOI] [PubMed] [Google Scholar]
- 78.Ito A, Jippo T, Wakayama T, et al. SgIGSF: a new mast-cell adhesion molecule used for attachment to fibroblasts and transcriptionally regulated by MITF. Blood. 2003;101(7):2601–8. doi: 10.1182/blood-2002-07-2265. [DOI] [PubMed] [Google Scholar]
- 79.Razin E, Zhang ZC, Nechushtan H, Frenkel S, Lee YN, Arudchandran R, Rivera J. Suppression of microphthalmia transcriptional activity by its association with protein kinase C-interacting protein 1 in mast cells. J Biol Chem. 1999;274(48):34272–6. doi: 10.1074/jbc.274.48.34272. [DOI] [PubMed] [Google Scholar]
- 80.Levy C, Nechushtan H, Razin E. A new role for the STAT3 inhibitor, PIAS3: a repressor of microphthalmia transcription factor. J Biol Chem. 2002;277(3):1962–6. doi: 10.1074/jbc.M109236200. [DOI] [PubMed] [Google Scholar]
- 81.Sonnenblick A, Levy C, Razin E. Interplay between MITF, PIAS3, and STAT3 in mast cells and melanocytes. Mol Cell Biol. 2004;24(24):10584–92. doi: 10.1128/MCB.24.24.10584-10592.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yamamoto M, Takahashi S, Onodera K, Muraosa Y, Engel JD. Upstream and downstream of erythroid transcription factor GATA-1. Genes Cells. 1997;2(2):107–15. doi: 10.1046/j.1365-2443.1997.1080305.x. [DOI] [PubMed] [Google Scholar]
- 83.Harigae H, Takahashi S, Suwabe N, et al. Differential roles of GATA-1 and GATA-2 in growth and differentiation of mast cells. Genes Cells. 1998;3(1):39–50. doi: 10.1046/j.1365-2443.1998.00166.x. [DOI] [PubMed] [Google Scholar]
- 84.Pevny L, Lin CS, D'Agati V, Simon MC, Orkin SH, Costantini F. Development of hematopoietic cells lacking transcription factor GATA-1. Development. 1995;121(1):163–72. doi: 10.1242/dev.121.1.163. [DOI] [PubMed] [Google Scholar]
- 85.Migliaccio AR, Rana RA, Sanchez M, et al. GATA-1 as a regulator of mast cell differentiation revealed by the phenotype of the GATA-1low mouse mutant. J Exp Med. 2003;197(3):281–96. doi: 10.1084/jem.20021149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zon LI, Gurish MF, Stevens RL, Mather C, Reynolds DS, Austen KF, Orkin SH. GATA-binding transcription factors in mast cells regulate the promoter of the mast cell carboxypeptidase A gene. J Biol Chem. 1991;266(34):22948–53. [PubMed] [Google Scholar]
- 87.Nishiyama C, Yokota T, Okumura K, Ra C. The transcription factors Elf-1 and GATA-1 bind to cell-specific enhancer elements of human high-affinity IgE receptor α-chain gene. J Immunol. 1999;163(2):623–30. [PubMed] [Google Scholar]
- 88.Nishiyama C, Hasegawa M, Nishiyama M, et al. Regulation of human FcεRI α-chain gene expression by multiple transcription factors. J Immunol. 2002;168(9):4546–52. doi: 10.4049/jimmunol.168.9.4546. [DOI] [PubMed] [Google Scholar]
- 89.Maeda K, Nishiyama C, Tokura T, Akizawa Y, Nishiyama M, Ogawa H, Okumura K, Ra C. Regulation of cell type-specific mouse FcεRI β-chain gene expression by GATA-1 via four GATA motifs in the promoter. J Immunol. 2003;170(1):334–40. doi: 10.4049/jimmunol.170.1.334. [DOI] [PubMed] [Google Scholar]
- 90.Nishiyama C, Ito T, Nishiyama M, et al. GATA-1 is required for expression of FcεRI on mast cells: analysis of mast cells derived from GATA-1 knockdown mouse bone marrow. Int Immunol. 2005;17(7):847–56. doi: 10.1093/intimm/dxh278. [DOI] [PubMed] [Google Scholar]
- 91.Tsai FY, Orkin SH. Transcription factor GATA-2 is required for proliferation/survival of early hematopoietic cells and mast cell formation, but not for erythroid and myeloid terminal differentiation. Blood. 1997;89(10):3636–43. [PubMed] [Google Scholar]
- 92.Walsh JC, DeKoter RP, Lee HJ, et al. Cooperative and antagonistic interplay between PU.1 and GATA-2 in the specification of myeloid cell fates. Immunity. 2002;17(5):665–76. doi: 10.1016/s1074-7613(02)00452-1. [DOI] [PubMed] [Google Scholar]
- 93.Nishiyama C, Nishiyama M, Ito T, et al. Overproduction of PU.1 in mast cell progenitors: its effect on monocyte- and mast cell-specific gene expression. Biochem Biophys Res Commun. 2004;313(3):516–21. doi: 10.1016/j.bbrc.2003.11.145. [DOI] [PubMed] [Google Scholar]
- 94.Ito T, Nishiyama C, Nishiyama M, et al. Mast cells acquire monocyte-specific gene expression and monocyte-like morphology by overproduction of PU.1. J Immunol. 2005;174(1):376–83. doi: 10.4049/jimmunol.174.1.376. [DOI] [PubMed] [Google Scholar]
- 95.Dahl R, Walsh JC, Lancki D, Laslo P, Iyer SR, Singh H, Simon MC. Regulation of macrophage and neutrophil cell fates by the PU.1:C/EBPα ratio and granulocyte colony-stimulating factor. Nat Immunol. 2003;4(10):1029–36. doi: 10.1038/ni973. [DOI] [PubMed] [Google Scholar]
- 96.Zhang DE, Zhang P, Wang ND, Hetherington CJ, Darlington GJ, Tenen DG. Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein α-deficient mice. Proc Natl Acad Sci U S A. 1997;94(2):569–74. doi: 10.1073/pnas.94.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gurish MF, Tao H, Abonia JP, Arya A, Friend DS, Parker CM, Austen KF. Intestinal mast cell progenitors require CD49dβ7 (α4β7 integrin) for tissue-specific homing. J Exp Med. 2001;194(9):1243–52. doi: 10.1084/jem.194.9.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Boyce JA, Mellor EA, Perkins B, Lim YC, Luscinskas FW. Human mast cell progenitors use α4-integrin, VCAM-1, and PSGL-1 E-selectin for adhesive interactions with human vascular endothelium under flow conditions. Blood. 2002;99(8):2890–6. doi: 10.1182/blood.v99.8.2890. [DOI] [PubMed] [Google Scholar]
- 99.Humbles AA, Lu B, Friend DS, et al. The murine CCR3 receptor regulates both the role of eosinophils and mast cells in allergen-induced airway inflammation and hyperresponsiveness. Proc Natl Acad Sci U S A. 2002;99(3):1479–84. doi: 10.1073/pnas.261462598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brightling CE, Ammit AJ, Kaur D, Black JL, Wardlaw AJ, Hughes JM, Bradding P. The CXCL10/CXCR3 axis mediates human lung mast cell migration to asthmatic airway smooth muscle. Am J Respir Crit Care Med. 2005;171(10):1103–8. doi: 10.1164/rccm.200409-1220OC. [DOI] [PubMed] [Google Scholar]
- 101.Ishizuka T, Okajima F, Ishiwara M, et al. Sensitized mast cells migrate toward the antigen: a response regulated by p38 mitogen-activated protein kinase and Rho-associated coiled-coil-forming protein kinase. J Immunol. 2001;167(4):2298–304. doi: 10.4049/jimmunol.167.4.2298. [DOI] [PubMed] [Google Scholar]
- 102.Olivera A, Rivera J. Sphingolipids and the balancing of immune cell function: lessons from the mast cell. J Immunol. 2005;174(3):1153–8. doi: 10.4049/jimmunol.174.3.1153. [DOI] [PubMed] [Google Scholar]
- 103.Jolly PS, Bektas M, Olivera A, Gonzalez-Espinosa C, Proia RL, Rivera J, Milstien S, Spiegel S. Transactivation of sphingosine-1-phosphate receptors by FcεRI triggering is required for normal mast cell degranulation and chemotaxis. J Exp Med. 2004;199(7):959–70. doi: 10.1084/jem.20030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Urtz N, Olivera A, Bofill-Cardona E, et al. Early activation of sphingosine kinase in mast cells and recruitment to FcεRI are mediated by its interaction with Lyn kinase. Mol Cell Biol. 2004;24(19):8765–77. doi: 10.1128/MCB.24.19.8765-8777.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Olivera A, Urtz N, Mizugishi K, et al. IgE-dependent activation of spingosine kinase 1 and 2 and secretion of sphingosine-1-phosphate requires FYN kinase and contributes to mast cell responses. J Biol Chem. 2005 doi: 10.1074/jbc.M508931200. [DOI] [PubMed] [Google Scholar]
- 106.Kitaura J, Kinoshita T, Matsumoto M, et al. IgE- and IgE+Ag-mediated mast cell migration in an autocrine/paracrine fashion. Blood. 2005;105(8):3222–9. doi: 10.1182/blood-2004-11-4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Droin NM, Green DR. Role of Bcl-2 family members in immunity and disease. Biochim Biophys Acta. 2004;1644(23):179–88. doi: 10.1016/j.bbamcr.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 108.Kuwana T, Newmeyer DD. Bcl-2-family proteins and the role of mitochondria in apoptosis. Curr Opin Cell Biol. 2003;15(6):691–9. doi: 10.1016/j.ceb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 109.Maurer M, Tsai M, Metz M, Fish S, Korsmeyer SJ, Galli SJ. A role for Bax in the regulation of apoptosis in mouse mast cells. J Invest Dermatol. 2000;114(6):1205–6. doi: 10.1046/j.1523-1747.2000.00005.x. [DOI] [PubMed] [Google Scholar]
- 110.Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, Korsmeyer SJ. BCL-2, BCL-XL sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8(3):705–11. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- 111.Zong WX, Lindsten T, Ross AJ, MacGregor GR, Thompson CB. BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev. 2001;15(12):1481–6. doi: 10.1101/gad.897601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mekori YA, Gilfillan AM, Akin C, Hartmann K, Metcalfe DD. Human mast cell apoptosis is regulated through Bcl-2 and Bcl-XL. J Clin Immunol. 2001;21(3):171–4. doi: 10.1023/a:1011083031272. [DOI] [PubMed] [Google Scholar]
- 113.Baghestanian M, Jordan JH, Kiener HP, et al. Activation of human mast cells through stem cell factor receptor (KIT) is associated with expression of bcl-2. Int Arch Allergy Immunol. 2002;129(3):228–36. doi: 10.1159/000066773. [DOI] [PubMed] [Google Scholar]
- 114.Xiang Z, Ahmed AA, Moller C, Nakayama K, Hatakeyama S, Nilsson G. Essential role of the prosurvival bcl-2 homologue A1 in mast cell survival after allergic activation. J Exp Med. 2001;194(11):1561–69. doi: 10.1084/jem.194.11.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91(2):231–41. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 116.del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278(5338):687–9. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 117.Blume-Jensen P, Janknecht R, Hunter T. The kit receptor promotes cell survival via activation of PI 3-kinase and subsequent Akt-mediated phosphorylation of Bad on Ser136. Curr Biol. 1998;8(13):779–82. doi: 10.1016/s0960-9822(98)70302-1. [DOI] [PubMed] [Google Scholar]
- 118.Ranger AM, Zha J, Harada H, et al. Bad-deficient mice develop diffuse large B cell lymphoma. Proc Natl Acad Sci U S A. 2003;100(16):9324–9. doi: 10.1073/pnas.1533446100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Brunet A, Bonni A, Zigmond MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96(6):857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 120.Rena G, Guo S, Cichy SC, Unterman TG, Cohen P. Phosphorylation of the transcription factor forkhead family member FKHR by protein kinase B. J Biol Chem. 1999;274(24):17179–83. doi: 10.1074/jbc.274.24.17179. [DOI] [PubMed] [Google Scholar]
- 121.Dijkers PF, Birkenkamp KU, Lam EW, Thomas NS, Lammers JW, Koenderman L, Coffer PJ. FKHR-L1 can act as a critical effector of cell death induced by cytokine withdrawal: protein kinase B-enhanced cell survival through maintenance of mitochondrial integrity. J Cell Biol. 2002;156(3):531–42. doi: 10.1083/jcb.200108084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dijkers PF, Medema RH, Lammers JW, Koenderman L, Coffer PJ. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr Biol. 2000;10(19):1201–4. doi: 10.1016/s0960-9822(00)00728-4. [DOI] [PubMed] [Google Scholar]
- 123.Alfredsson J, Puthalakath H, Martin H, Strasser A, Nilsson G. Proapoptotic Bcl-2 family member Bim is involved in the control of mast cell survival and is induced together with Bcl-XL upon IgE-receptor activation. Cell Death Differ. 2005;12(2):136–44. doi: 10.1038/sj.cdd.4401537. [DOI] [PubMed] [Google Scholar]
- 124.Ley R, Balmanno K, Hadfield K, Weston C, Cook SJ. Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein, Bim. J Biol Chem. 2003;278(21):18811–6. doi: 10.1074/jbc.M301010200. [DOI] [PubMed] [Google Scholar]
- 125.Luciano F, Jacquel A, Colosetti P, Herrant M, Cagnol S, Pages G, Auberger P. Phosphorylation of Bim-EL by Erk1/2 on serine 69 promotes its degradation via the proteasome pathway and regulates its proapoptotic function. Oncogene. 2003;22(43):6785–93. doi: 10.1038/sj.onc.1206792. [DOI] [PubMed] [Google Scholar]
- 126.Kitaura J, Xiao W, Maeda-Yamamoto M, Kawakami Y, Lowell CA, Kawakami T. Early divergence of Fcε receptor I signals for receptor up-regulation and internalization from degranulation, cytokine production, and survival. J Immunol. 2004;173(7):4317–23. doi: 10.4049/jimmunol.173.7.4317. [DOI] [PubMed] [Google Scholar]
- 127.Kawakami T, Kitaura J. Mast cell survival and activation by IgE in the absence of antigen: a consideration of the biologic mechanisms and relevance. J Immunol. 2005;175(7):4167–73. doi: 10.4049/jimmunol.175.7.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yoshikawa H, Nakajima Y, Tasaka K. Glucocorticoid suppresses autocrine survival of mast cells by inhibiting IL-4 production and ICAM-1 expression. J Immunol. 1999;162(10):6162–70. [PubMed] [Google Scholar]
- 129.Yoshikawa H, Nakajima Y, Tasaka K. Enhanced expression of Fas-associated death domain-like IL-1-converting enzyme (FLICE)-inhibitory protein induces resistance to Fas-mediated apoptosis in activated mast cells. J Immunol. 2000;165(11):6262–9. doi: 10.4049/jimmunol.165.11.6262. [DOI] [PubMed] [Google Scholar]
- 130.Kitaura J, Song J, Tsai M, et al. Evidence that IgE molecules mediate a spectrum of effects on mast cell survival and activation via aggregation of the FcεRI. Proc Natl Acad Sci U S A. 2003;100(22):12911–6. doi: 10.1073/pnas.1735525100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Asai K, Kitaura J, Kawakami Y, et al. Regulation of mast cell survival by IgE. Immunity. 2001;14(6):791–800. doi: 10.1016/s1074-7613(01)00157-1. [DOI] [PubMed] [Google Scholar]
- 132.Kalesnikoff J, Huber M, Lam V, Damen JE, Zhang J, Siraganian RP, Krystal G. Monomeric IgE stimulates signaling pathways in mast cells that lead to cytokine production and cell survival. Immunity. 2001;14(6):801–11. doi: 10.1016/s1074-7613(01)00159-5. [DOI] [PubMed] [Google Scholar]
- 133.Kohno M, Yamasaki S, Tybulewicz VL, Saito T. Rapid and large amount of autocrine IL-3 production is responsible for mast cell survival by IgE in the absence of antigen. Blood. 2005;105(5):2059–65. doi: 10.1182/blood-2004-07-2639. [DOI] [PubMed] [Google Scholar]
- 134.Yamasaki S, Ishikawa E, Kohno M, Saito T. The quantity and duration of FcRγ signals determine mast cell degranulation and survival. Blood. 2004;103(8):3093–101. doi: 10.1182/blood-2003-08-2944. [DOI] [PubMed] [Google Scholar]
- 135.Bischoff SC, Sellge G, Manns MP, Lorentz A. Interleukin-4 induces a switch of human intestinal mast cells from proinflammatory cells to Th2-type cells. Int Arch Allergy Immunol. 2001;124(13):151–4. doi: 10.1159/000053695. [DOI] [PubMed] [Google Scholar]