Abstract
VanD type Enterococcus faecium 10/96A is constitutively resistant to vancomycin and to low levels of teicoplanin by nearly exclusive synthesis of peptidoglycan precursors terminating in d-alanyl-d-lactate (L. M. Dalla Costa, P. E. Reynolds, H. A. Souza, D. C. Souza, M. F. Palepou, and N. Woodford, Antimicrob. Agents Chemother. 44:3444-3446, 2000). A G184S mutation adjacent to the serine involved in the binding of d-Ala1 in the d-alanine:d-alanine ligase (Ddl) led to production of an impaired Ddl and accounts for the lack of d-alanyl-d-alanine-containing peptidoglycan precursors. The sequence of the vanD gene cluster revealed eight open reading frames. The organization of this operon, assigned to a chromosomal location, was similar to those in other VanD type strains. The distal part encoded the VanHD dehydrogenase, the VanD ligase, and the VanXD dipeptidase, which were homologous to the corresponding proteins in VanD-type strains. Upstream from the structural genes for these proteins was the vanYD gene; a frameshift mutation in this gene resulted in premature termination of the encoded protein and accounted for the lack of penicillin-susceptible d,d-carboxypeptidase activity. Analysis of the translated sequence downstream from the stop codon, but in a different reading frame because of the frameshift mutation, indicated homology with penicillin binding proteins (PBPs) with a high degree of identity with VanYD from VanD-type strains. The 5′ end of the gene cluster contained the vanRD-vanSD genes for a putative two-component regulatory system. Insertion of ISEfa4 in the vanSD gene led to constitutive expression of vancomycin resistance. This new insertion belonged to the IS605 family and was composed of two open reading frames encoding putative transposases of two unrelated insertion sequence elements, IS200 and IS1341.
In susceptible bacteria, the glycopeptide antibiotics vancomycin and teicoplanin form a complex with the d-alanyl-d-alanine (d-Ala-d-Ala) terminus of peptidoglycan precursors at the cell surface, leading to inhibition of transglycosylation and transpeptidation reactions in the cell wall layer (47). Three types, VanA, VanB, and VanD, of acquired resistance to glycopeptides by production of peptidoglycan precursors ending in the depsipeptide d-Ala-d-lactate (d-Ala-d-Lac) instead of the dipeptide d-Ala-d-Ala have been characterized in enterococci (13, 44, 54). Substitution of the C-terminal d-Ala residue by d-Lac eliminates a hydrogen bond critical for binding antibiotics and leads to a ca. 1,000-fold reduction in the affinity of vancomycin for peptidoglycan precursors (10, 19).
The general organization of the vanD operon is similar to those present in VanA- and VanB-type strains (12, 21, 24). Three proteins are required for glycopeptide resistance: a dehydrogenase (VanH, VanHB, or VanHD) to reduce pyruvate to d-Lac, a ligase (VanA, VanB, or VanD) to synthesize the depsipeptide d-Ala-d-Lac, and a d,d-dipeptidase (VanX, VanXB, or VanXD) to hydrolyze the d-Ala-d-Ala dipeptide synthesized by the host d-Ala:d-Ala Ddl ligase and thereby limit synthesis of precursors containing the target for glycopeptides (3, 13, 20, 49). In VanA- and VanB-type strains, a penicillin-insensitive and Zn2+-dependent d,d-carboxypeptidase (VanY and VanYB) contributes to vancomycin resistance by hydrolyzing the C-terminal d-Ala residue of late peptidoglycan precursors, when elimination of d-Ala-d-Ala by VanX is incomplete (3, 8, 9). Certain PBPs which function as d,d-carboxypeptidases preferentially cleave depsipeptide substrates (46), whereas the Zn2+-dependent VanY d,d-carboxypeptidase exhibits a higher catalytic efficiency for hydrolysis of substrates ending in d-Ala-d-Ala (3). The VanYD d,d-carboxypeptidase is distinct from VanY and VanYB since it displays substantial identity with some penicillin-binding proteins (17, 21). These catalytic-serine d,d-carboxypeptidases are susceptible to benzylpenicillin (48). VanZ, which confers teicoplanin resistance by an unknown mechanism, and VanW, with an unknown function, encoded by the vanA and vanB clusters, respectively, do not have counterparts in the vanD cluster (6, 24).
Synthesis of the resistance proteins is regulated at the transcriptional level by two-component regulatory systems (VanR-VanS and VanRB-VanSB) (11, 24). VanS is a putative membrane-associated sensor that controls the level of phosphorylation of VanR (55). Phosphorylation of the VanR and VanRB response regulators enhances the affinity of the proteins for the regulatory regions of the PR, PRB and PH, PYB promoters, and allows transcription of the regulatory (vanRS and vanRBSB) and resistance (vanHAX and vanHBBXB) genes, respectively (4, 5, 24, 28, 30). The VanR-VanS system activates the PH promoter for cotranscription of the vanH, vanA, and vanX genes in response to the presence of vancomycin or teicoplanin in the culture medium (8, 11). In contrast, the VanRB-VanSB system mediates activation of the PYB promoter only in the presence of vancomycin, and lack of induction by teicoplanin accounts for susceptibility of VanB-type strains to this antibiotic (8, 24). Low-level resistance to vancomycin in VanB strains results from a limited capacity to synthesize d-Ala-d-Lac and to hydrolyze d-Ala-d-Ala, leading to coproduction of d-Ala and d-Lac-ending peptidoglycan precursors (8).
Four VanD-type strains of Enterococcus faecium have been reported so far, and clinical isolates BM4339 and BM4416 (also designated N97-330) have been extensively studied (17, 22, 39, 43, 45). These two VanD-type strains are characterized by constitutively expressed resistance to moderate levels of vancomycin (MIC, 16 to 256 μg/ml) and teicoplanin (MIC, 2 to 64 μg/ml) despite the presence of the vanRD and vanSD genes expressed from the PRD promoter (22, 43, 45).
Strain 10/96A is resistant to vancomycin (MIC, 256 μg/ml) and to low levels of teicoplanin (MIC, 4 μg/ml) (23). A PCR product, obtained using the d-Ala:d-X ligase universal degenerate primers V1 and V2, was sequenced and revealed 83 to 85% identity with structural genes for VanD ligases (17, 21, 39). The vanD cluster of strain 10/96A was sequenced partially and found to contain two open reading frames (ORFs) encoding a dehydrogenase, VanHD, and a d-Ala:d-Lac ligase, VanD (23). The operon, which is not transferable, confers resistance by constitutive synthesis of peptidoglycan precursors ending in d-Ala-d-Lac, which represent the main components of cell wall cytoplasmic precursors. In contrast to the VanYD activities in strains BM4339 and BM4416, the VanYD d,d-carboxypeptidase activity in membrane fractions of strain 10/96A was not inhibited by penicillin G (23, 43, 45).
We report the organization of the vanD gene cluster in E. faecium 10/96A and the regulation of expression of the resistance genes. We also show that a single mutation in the chromosomal ddl gene accounts for the lack of precursors terminating in d-Ala-d-Ala in this strain and that an insertion sequence in the vanSD gene is likely to be responsible for constitutive expression of resistance.
MATERIALS AND METHODS
Strains, plasmids, and growth conditions.
The origin and characteristics of the bacterial strains and plasmids used in this study are listed in Table 1. E. faecium 10/96A was isolated in 1996 from the blood of a 9-year-old girl with aplastic anemia (23). Escherichia coli Top10 (Invitrogen, Groningen, The Netherlands) was used as a host for recombinant plasmids. Enterococcus faecalis JH2-2 is a derivative of strain JH2 that is resistant to fusidic acid and rifampin (31). Kanamycin (50 μg/ml) was used as a selective agent for cloning PCR products into the pCR-Blunt vector (Invitrogen). Spectinomycin (60 μg/ml) was added to the medium to prevent the loss of plasmids derived from pAT79 (11). Strains were grown in brain heart infusion (BHI) broth or agar (Difco Laboratories, Detroit, Mich.) at 37°C. The MICs of glycopeptides were determined by the method of Steers et al., with 105 CFU per spot on BHI agar after 24 h of incubation at 37°C (52).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant properties | Reference or source |
|---|---|---|
| Strains | ||
| E. coli Top10 | F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 deoR recA1 araD139 Δ(ara-leu) 7697 galU galK rpsL endA1 nupG | Invitrogen |
| E. faecalis JH2-2 | Fusr Rifr | 32 |
| E. faecium | ||
| 10/96A | Vmr Ter (VanD type) | 23 |
| BM4416 (N97-330) | Vmr Ter (VanD type) | 17, 43 |
| BM4339 | Vmr Ter (VanD type) | 45 |
| BM4409 | BM4339/pAT662 (P2ddlcat) containing ddl gene of BM4147 | 21 |
| BM4512 | BM4339/pAT640 (P2ddlG184Scat) containing ddl gene of 10/96A | This study |
| Plasmids | ||
| pCR-Blunt | Kmr, Zeocinr, oriR from colE1, lacZα, ccdB | Invitrogen |
| pAT79 | oriR from pAMβ1, oriR from pUC, oriT from RK2, SprlacZα P2cat | 11 |
| pAT637 | 632-bp PCR fragment (vanD′ vanXD) of 10/96A cloned into pCR-Blunt | This study |
| pAT638 | 1,060-bp PCR fragment (vanSD′ vanYD′) of 10/96A cloned into pCR-Blunt | This study |
| pAT639 | 3,725-bp PCR fragment (vanRD SD′ ORFA ORFB vanSD′ vanYD′) of 10/96A cloned into pCR-Blunt | This study |
| pAT640 | 1,135-bp SacI-XbaI PCR fragment (ddlG184S) of 10/96A cloned into pAT79 | This study |
| pAT635 | 672-bp SacI-XbaI PCR fragment (vanXD) of BM4339 cloned into pAT79 | This study |
| pAT633 | 1,153-bp SacI-XbaI PCR fragment (vanYD) of BM4339 cloned into pAT79 | This study |
| pAT636 | 672-bp SacI-XbaI PCR fragment (vanXD) of 10/96A cloned into pAT79 | This study |
| pAT634 | 1,153-bp SacI-XbaI PCR fragment (vanYD) of 10/96A cloned into pAT79 | This study |
Recombinant DNA techniques.
Plasmid DNA isolation, digestion with restriction endonucleases (Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, England and Gibco BRL-life Technologies Inc.), amplification of DNA by PCR with Pfu DNA polymerase (Stratagene, La Jolla, Calif.), ligation of DNA fragments with T4 DNA ligase (Amersham Pharmacia Biotech), and transformation of E. coli Top10 with recombinant plasmid DNA were performed by standard methods (14). Total DNA from enterococci was prepared according to the method of Le Bouguénec et al. (33).
Plasmid construction.
The plasmids were constructed as follows (Fig. 1).
FIG. 1.
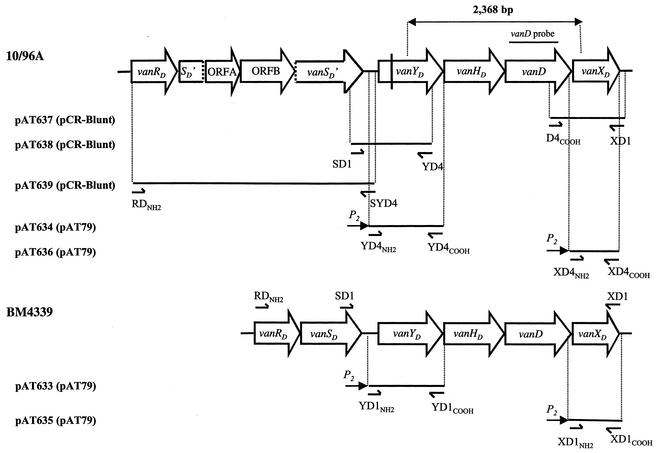
Schematic representation of vanD gene clusters and of recombinant plasmids. Open arrows represent coding sequences and indicate the direction of transcription. The double-headed arrow indicates the 2,368-bp PCR product previously sequenced (23). The PCR fragment internal to the vanD gene of strain 10/96A used as a probe in hybridization experiments is indicated above the corresponding region. Vertical dashed lines indicate separation of the vanSD gene of 10/96A into two parts SD′ and vanSD′. The vertical bar in the vanYD gene of 10/96A indicates the position of the frameshift mutation leading to a predicted truncated protein. The inserts in recombinant plasmids are represented by solid lines, and the vectors are indicated in parentheses. Arrowheads represent binding sites and orientation of oligodeoxynucleotides used for amplification.
(i) Plasmid pAT637.
To amplify the vanXD gene of 10/96A, the specific primer D4COOH (5′ ACTTTCACTTAGGAGGTAAC), complementary to a previously sequenced portion of the 3′ end of the vanD gene from this strain, was used in combination with specific primer XD1 (5′ CTAGGCAATGCAAAAATC), designed from comparative analysis of sequences downstream from the vanXD genes of E. faecium BM4339 and BM4416 (17, 21). The PCR product, with the expected size of 632 bp, obtained from 10/96A total DNA as a template, was cloned into pCR-Blunt, leading to plasmid pAT637.
(ii) Plasmid pAT638.
A strategy similar to that used for construction of pAT637 was followed to clone the vanYD gene of strain 10/96A. A sequence deduced from the alignment of the 3′ end of the vanSD genes of BM4339 and BM4416 was used to design primer SD1 (5′ GTTTTGAGGTTACATTGC). YD4 (5′ GGTAATAGGGACTGTTCGGAT) contained 21 bases complementary to the sequence of the 3′ end of the vanYD gene from strain 10/96A. These primers, used with total DNA from 10/96A as a template, yielded a product with the expected size of 1,060 bp that was cloned into pCR-Blunt generating plasmid pAT638.
(iii) Plasmid pAT639.
To complete the sequence of the vanD operon of strain 10/96A, the portion upstream from vanYD was amplified using primers RDNH2 (5′ ATGAATGAAAAAATCTTAGTGG) and SYD4 (5′ TTACGATTTTCCTACGG) and total DNA as a template. Alignment of the vanRD genes from BM4339 and BM4416 was used to design the RDNH2 primer, complementary to a sequence conserved at the 5′ end of these genes. When combined with primer SYD4, specific for the intergenic region upstream from the vanYD gene of 10/96A, RDNH2 led to amplification of a 3,725-bp fragment. This PCR fragment, with an unexpectedly large size, was cloned into pCR-Blunt, generating plasmid pAT639, and was sequenced.
(iv) Plasmids pAT635 and pAT633.
For construction of pAT635(P2vanXDcat) and pAT633(P2vanYDcat), the vanXD and vanYD genes of BM4339 were amplified using primer pairs XD1NH2-XD1COOH and YD1NH2-YD1COOH, respectively, and BM4339 total DNA as a template. Oligodeoxynucleotides XD1NH2 (5′ CAGTGAGCTCCGGTTTTACGCTTTCTG) and YD1NH2 (5′ CAGTGAGCTCGCGAAAACATAAATCGC) harbored a SacI restriction site (underlined) and 17 bases complementary to the sequence upstream from vanXD or vanYD of BM4339, respectively. Oligodeoxynucleotides XD1COOH (5′ AGTGTCTAGACTAGGCAATGCAAAAAT) and YD1COOH (5′ AGTGTCTAGATTACTGGGCTTTGATTT) contained an XbaI restriction site (underlined), the stop codon (italicized), and 14 bases complementary to the 3′ end sequence of vanXD or vanYD, respectively. The SacI and XbaI restriction sites allowed directional cloning of vanXD or vanYD upstream from the cat reporter gene of the shuttle vector pAT79 carrying the P2 promoter to generate pAT635(P2vanXDcat) and pAT633(P2vanYDcat). The 672-bp insert of plasmid pAT635(P2vanXDcat) corresponded to nucleotides 5057 to 5728 of the vanD operon of BM4339 and included the ribosome binding site (RBS), the initiation codon, the vanXD coding sequence, and the stop codon of the gene. The 1,153-bp insert of pAT633(P2vanYDcat) corresponded to nucleotides 1950 to 3102 of the vanD operon of BM4339 and consisted of the vanYD coding sequence with its RBS and initiation and stop codons.
(v) Plasmids pAT634 and pAT636.
To construct pAT636(P2vanXDcat) and pAT634(P2vanYDcat) from 10/96A, a strategy identical to that used for construction of pAT635(P2vanXDcat) and pAT633(P2vanYDcat) from BM4339 was followed. The vanXD and vanYD genes of strain 10/96A were amplified using primer pairs XD4NH2-XD4COOH and YD4NH2-YD4COOH, respectively, with 10/96A total DNA as a template. Primers XD4NH2 (5′ CAGTGAGCTCAGGGTTTACGCTTTCTG) and YD4NH2 (5′ CAGTGAGCTCGCGAAAAAATAAATCGC) harbored a SacI site (underlined) and 17 bases complementary to the sequence upstream from vanXD or vanYD of strain 10/96A, respectively. Primers XD4COOH (5′ AGTGTCTAGACTAGGCAATGCAAAAAT) and YD4COOH (5′ AGTGTCTAGATCACTGGGCCTTGATTT) contained an XbaI site (underlined), the stop codon (italicized), and 14 bases complementary to the 3′ end sequence of vanXD or vanYD of strain 10/96A, respectively. The vanXD and vanYD PCR products were digested with SacI and XbaI and cloned under the control of the P2 promoter of the shuttle vector pAT79, leading to plasmids pAT636(P2vanXDcat) and pAT634(P2vanYDcat), respectively (11). The 672- and 1,153-bp inserts of pAT636(P2vanXDcat) and pAT634(P2vanYDcat) corresponded, respectively, to nucleotides 6875 to 7546 and nucleotides 3765 to 4917 of strain 10/96A and consisted of the vanXD or vanYD coding sequences of 10/96A with their RBS and initiation and stop codons.
(vi) Plasmid pAT640.
The chromosomal ddl gene from E. faecium 10/96A with its RBS was amplified by PCR from total DNA with the previously described 4147-1 and 4147-2 oligodeoxynucleotides (26). Primers 4147-1 and 4147-2 contain, respectively, SacI and XbaI restriction sites that allow directional cloning of the ddl gene under the control of the constitutive P2 promoter and upstream from the cat reporter gene of the shuttle vector pAT79 (11). The 1,135-bp insert of the resulting pAT640(P2ddlG184Scat) plasmid contained the mutated ddl gene with the single G184S mutation and its own RBS.
The nucleotide sequences of the amplified fragments were redetermined.
Strain constructions.
Plasmids pAT635(P2vanXDcat) and pAT633(P2vanYDcat) from BM4339 and plasmids pAT636(P2vanXDcat) and pAT634(P2vanYDcat) from 10/96A were introduced into E. faecalis JH2-2 by electrotransformation. E. faecium BM4512 was obtained by introduction of plasmid pAT640(P2ddlG184Scat) into E. faecium BM4339 by electrotransformation (Table 1). Transformants selected on spectinomycin, 60 μg/ml for JH2-2 or 120 μg/ml for BM4339, were screened for resistance to chloramphenicol. Plasmid DNA from chloramphenicol-resistant clones was digested with EcoRI plus HindIII and compared to the restriction profiles of pAT635(P2vanXDcat) and pAT633(P2vanYDcat) and those of pAT636(P2vanXDcat), pAT634(P2vanYDcat), and pAT640(P2ddlG184Scat) purified from E. coli Top10 to screen for DNA rearrangements.
Nucleotide sequencing.
Plasmid DNA was extracted with the commercial Wizard Plus Minipreps DNA purification system (Promega, Madison, Wis.), labeled with a dye-labeled ddNTP Terminator cycle sequencing kit (Beckman Coulter UK Ltd., High Wycombe, United Kingdom), and the samples were sequenced and analyzed with a CEQ 2000 automated sequencer (Beckman).
Computer analysis of sequence data.
Determination of the degrees of identity and similarity with known proteins was carried out using BLASTN, BLASTX, and BLASTP (2) and FASTA (42) from the Genetics Computer Group suite of programs.
Contour-clamped homogeneous electric field gel electrophoresis.
Genomic DNA embedded in agarose plugs was digested for 3 h at 37°C with 0.01 U of I-CeuI, an intron-encoded endonuclease specific for rRNA genes (34). Fragments were separated on a 0.8% agarose gel using a CHEF-DRIII system (Bio-Rad Laboratories, Hercules, Calif.) under the following conditions: total migration, 24 h; initial pulse, 60 s; final pulse, 120 s; voltage, 6 V/cm; included angle, 120°; and temperature, 14°C. The DNA fragments were transferred to a nitrocellulose membrane and hybridized successively under stringent conditions at 68°C to an α-32P-labeled 16S rRNA (rrs) probe obtained by amplification of an internal portion of the rrs gene with primers RWO1 and DG74 (27) and to a vanD probe obtained by PCR with primers D4-1 and D4-2 and 10/96 total DNA as a template (Fig. 1). The amplification product used to generate the probe was labeled with [α-32P]dATP (3,000 Ci/mmol; Amersham Pharmacia Biotech) by Megaprime using a commercially available kit (Amersham).
Analysis of peptidoglycan precursors.
Extraction and analysis of peptidoglycan precursors was performed as described previously (37, 49). Enterococci were grown in BHI medium without or with vancomycin (4 μg/ml) to the mid-exponential phase (A600 = 1). Ramoplanin was added to inhibit peptidoglycan synthesis, and incubation was continued for 15 min to cause accumulation of peptidoglycan precursors. The bacteria were harvested, and the cytoplasmic precursors were extracted with 8% trichloroacetic acid (15 min, at 4°C), desalted, and analyzed by high-performance liquid chromatography. Results were expressed as the percentages of total late peptidoglycan precursors represented by UDP-MurNAc-tripeptide, UDP-MurNAc-tetrapeptide, UDP-MurNAc-pentapeptide, and UDP-MurNAc-pentadepsipeptide that were determined from the integrated peak areas.
d,d-Dipeptidase (VanX) and d,d-carboxypeptidase (VanY) activities.
The enzymatic activities in the supernatant and in the resuspended pellet fraction were assayed as described previously (8, 48). Strains were grown until the optical density at 600 nm reached 0.7 in the absence or presence of vancomycin at various concentrations (1, 8, and 64 μg/ml) for induction of 10/96A or with spectinomycin (60 μg/ml) to counterselect loss of derivatives of pAT79. Bacteria were then lysed by treatment with lysozyme (2 mg/ml) at 37°C, followed by sonication, and the membrane fraction was pelleted (100,000 × g, 45 min). The supernatant (S100) and resuspended pellet (C100) were collected and assayed for d,d-peptidase (VanX or VanY) activities by measuring the d-Ala released from substrate hydrolysis (d-Ala-d-Ala, 6.56 mM, or UDP-MurNAc-l-Ala-d-Glu-l-Lys-d-Ala-d-Ala, 5 mM) through coupled indicator reactions using d-amino acid oxidase and horseradish peroxidase (8, 48). Specific activity was defined as the number of nanomoles of product formed at 37°C per minute per milligram of protein contained in the extracts.
Preparation of membrane fragments and binding of benzyl[14C]penicillin.
The membrane fragments were prepared and labeling was carried out as described elsewhere (48). Briefly, a culture at an optical density at 600 nm of 1.0 was centrifuged, the pellet was washed in 50 mM Tris HCl (pH 7.2) and resuspended, and osmotic lysis was achieved in the presence of lysozyme (400 μg/ml) and muramidase (70 μg/ml) after incubation at 37°C. DNase (25 μg/ml) and MgCl2 (5 mM) were added, and after 3 min at 37°C, the suspension was cooled to 4°C, centrifuged, and washed. The membrane fraction was resuspended in 50 mM Tris HCl (pH 7.2) and incubated at 37°C with benzyl[14C]penicillin (1 μg/ml). After addition of unlabeled penicillin G (3 mg/ml) and sample buffer (New England Biolabs), the membrane proteins were solubilized by heating at 98°C. The labeled membrane proteins were run on a 12% polyacrylamide gel. The gel was stained, dried, and set up for phosphorimaging overnight to detect PBPs and determine the amount of penicilloyl-protein complex. Autoradiography was carried out for 5 weeks to reveal minor PBPs.
Nucleotide sequence accession number.
The 7,546-bp fragment containing the vanD gene cluster of strain 10/96A was submitted to GenBank and assigned accession no. AY082011.
RESULTS
E. faecium 10/96A produces a nonfunctional d-Ala:d-Ala ligase.
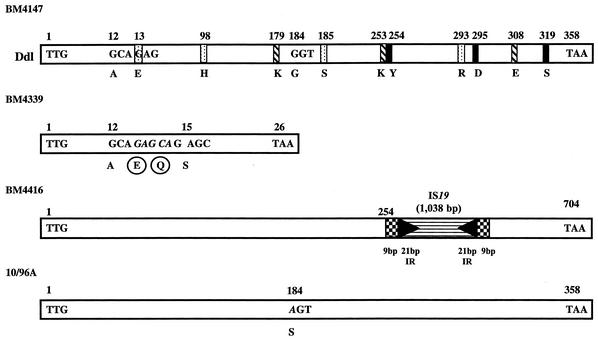
E. faecium 10/96A produced UDP-Mur-NAc-pentadepsipeptide almost exclusively (95%) when grown in the absence of vancomycin (Table 2). To elucidate the strategy adopted by this strain to prevent the susceptible chromosomal pathway of peptidoglycan synthesis, the entire chromosomal ddl gene for the d-Ala:d-Ala ligase was amplified and three independent PCR products with the expected length of 1,077 bp were sequenced. Comparative analysis revealed a point mutation in codon 184 of the ddl gene from 10/96A relative to that of E. faecium BM4147 (26), leading to a Gly-to-Ser substitution (Fig. 2). This mutation was located next to the serine involved in the binding of d-Ala1, presumably leading to a nonfunctional protein (Fig. 2). Strain BM4339 has an impaired Ddl, and introduction of an intact ddl gene under the control of a constitutive promoter restores its susceptibility to glycopeptides (MIC = 0.5 μg/ml) (21). The decrease in glycopeptide resistance is due to synthesis of the heterologous Ddl enzyme since BM4339 possesses only very weak VanX d,d-dipeptidase activity (45). To test if the G184S mutation was responsible for impairment of Ddl in 10/96A, plasmid pAT640 containing the ddlG184S gene and its RBS cloned under the control of the constitutive P2 promoter was electrotransformed into BM4339 (Table 1). The resulting transformant, BM4512, remained vancomycin resistant, confirming that the d-Ala:d-Ala ligase from 10/96A was not functional.
TABLE 2.
MICs of glycopeptides and cytoplasmic peptidoglycan precursors synthesized by VanD-type strains
| E. faecium strain | MIC (μg/ml)a
|
% of peptidoglycan precursorsb
|
||||
|---|---|---|---|---|---|---|
| VM | TE | UDP-MurNAc-tripeptide | UDP-MurNAc-tetrapeptide | UDP-MurNAc-pentapeptide | UDP-MurNAc-pentadepsipeptide | |
| BM4339 | 64 | 4 | 19 | 21 | 2 | 58 |
| BM4416 | 128 | 64 | 7 | 24 | <1 | 69 |
| 10/96A | 256 | 4 | 0 | 2 | 3 | 95 |
MICs were determined by the method of Steers et al. (52). Abbreviations: VM, vancomycin; TE, teicoplanin.
The bacteria were grown without vancomycin to the mid-exponential phase, and peptidoglycan synthesis was inhibited by addition of ramoplanin to the cultures for 15 min.
FIG. 2.
Schematic representation of d-Ala:d-Ala ligases from E. faecium BM4147 and from VanD-type enterococci. The positions of the amino acids implicated in the binding of d-Ala1, d-Ala2, and ATP are indicated by dotted, hatched, and black bars, respectively (25, 51). In strain BM4339, a 5-bp insertion (italics) at the position corresponding to amino acid 13 is responsible for a frameshift mutation leading to the synthesis of a 26-amino-acid peptide instead of the putative 358-amino-acid Ddl (21). In BM4416, a copy of IS19 is inserted at position 762 of the ddl gene. Checkerboard boxes, 9-bp duplications of target DNA; black arrowheads, 21-bp perfect inverted repeat sequences; horizontally striped box, putative transposase (17, 43). In 10/96A, the single base difference with ddl from E. faecium BM4147 leading to a Gly-to-Ser substitution at position 184 is indicated in italics.
The two other VanD-type E. faecium strains previously studied, BM4339 and BM4416, are also presumed to lack d-Ala:d-Ala ligase activity as the result of different insertion events in the chromosomal ddl gene (Fig. 2). In BM4339, a 5-bp insertion in the 5′ end of the ddl gene is responsible for a frameshift mutation leading to the synthesis of a 26-amino-acid truncated ligase, whereas in BM4416, inactivation of the gene is due to insertion of IS19 (21, 45). Thus, in the three VanD-type strains subjected to detailed study, production of an impaired Ddl accounts for the lack of peptidoglycan precursors terminating in d-Ala-d-Ala (Table 2) (43, 45).
Characterization of the van genes in E. faecium 10/96A and of the deduced proteins.
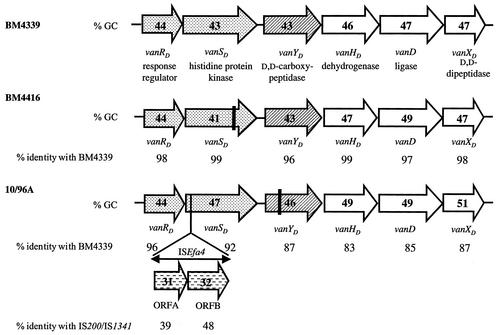
Plasmids pAT637(vanD′ vanXD), pAT638(vanSD′ vanYD′′) and pAT639 (vanRD, SD′, ORFA, ORFB, vanSD′) were obtained by cloning the 632-, 1,060-, and 3,725-bp PCR fragments obtained from total DNA of 10/96A into the pCR-Blunt vector (Table 1 and Fig. 1). Sequencing of both strands of the inserts in these plasmids revealed the presence of the structural genes for the d,d-peptidases (VanXD and VanYD) and also for the two-component regulatory system (VanRD-VanSD) which was interrupted by an insertion sequence composed of two ORFs in the same orientation (Fig. 3). The genes for the VanHD dehydrogenase and for the VanD d-Ala:d-Lac ligase have been characterized previously (23). The complete organization of the 7,546-bp vanD gene cluster composed of eight genes, of which six were found with the same organization as those of the vanD operons in E. faecium BM4339 and BM4416, is shown in Fig. 1 and 3.
FIG. 3.
Comparison of vanD gene clusters. Arrows represent coding sequences and indicate direction of transcription. The two-component regulatory systems are represented by dotted arrows, the d,d-carboxypeptidases are represented by hatched arrows, and the genes necessary for resistance are represented by open arrows. The guanosine-plus-cytosine content (% GC) is indicated in the arrows. The percentages of identity between the deduced proteins relative to those of BM4339 are indicated under the arrows. Insertion sequence ISEfa4 in 10/96A is indicated by a double-headed arrow, and horizontally dashed arrows correspond to ORFA and ORFB. The vertical bars in vanSD of BM4416 and vanYD of 10/96A indicate the positions of the frameshift mutations leading to predicted truncated proteins.
The deduced sequences of the proteins in 10/96A were compared with those from BM4339 and BM4416 (Fig. 3). The structural similarity between the VanHD dehydrogenases, the VanD ligases, and the VanXD d,d-dipeptidases was high, between 83 and 99%. Thus, closely related counterparts of the three enzymes required for VanA- and VanB-type resistance are present in a similar organization in VanD-type E. faecium. The VanXD protein, despite the very low d,d-dipeptidase activity in strain 10/96A (Tables 3 and 4), displayed the amino acid motifs YA, DXXR, SXHXXGXAXD, DXM, and EXXH, corresponding to active site residues that may be involved in Zn2+ binding and in catalysis (data not shown) (36).
TABLE 3.
d,d-Peptidase activities in extracts from E. faecium 10/96Ac
| Concn of VMa (μg/ml) | Mean d,d-dipeptidase activityb ± SD (nmol min−1 mg−1) | Mean d,d-carboxypeptidase activityd ± SD (nmol min−1 mg−1) in fraction
|
|
|---|---|---|---|
| Membrane | Cytoplasmic | ||
| 0 | 1.6 ± 0.5 | 24 ± 5.9 | 3.9 ± 0.3 |
| 1 | 2.4 ± 0.6 | 23 ± 6.5 | 5.2 ± 0.6 |
| 8 | 2.3 ± 0.6 | 26 ± 5.3 | 3.9 ± 1.2 |
| 64 | 1.3 ± 0.5 | 37 ± 6.8 | 3.8 ± 0.8 |
VM, vancomycin.
The activity was measured in the supernatant of lysed bacteria after centrifugation at 100,000 × g.
Results are obtained from a minimum of three independent extracts.
The activities were measured in the supernatant and in the resuspended pellet fraction after centrifugation of lysed bacteria at 100,000 × g for 45 min.
TABLE 4.
d,d-Peptidase activities in extracts from E. faecalis JH2-2 derivativesb
| Strain/plasmid | van gene (strain) | Mean d,d-dipeptidase activitya (nmol min−1 mg−1) | Mean d,d-carboxypeptidase activityc ± SD (nmol min−1 mg−1) in fraction
|
|
|---|---|---|---|---|
| Membrane | Cytoplasmic | |||
| JH2-2 | None | NDd | ND | ND |
| JH2-2/pAT635 | vanXD (BM4339) | 1.3 ± 0.5 | NAe | NA |
| JH2-2/pAT636 | vanXD (10/96A) | 0.8 ± 0.1 | NA | NA |
| JH2-2/pAT633 | vanYD (BM4339) | NA | 6.4 ± 1.5 | 1.9 ± 0.5 |
| JH2-2/pAT634 | vanYD (10/96A) | NA | ND | ND |
The activity was measured in the supernatant of lysed bacteria after centrifugation at 100,000 × g.
Results are obtained from a minimum of three independent extracts.
The activities were measured in the supernatant and in the resuspended pellet fraction after centrifugation of lysed bacteria at 100,000 × g for 45 min.
ND, not detectable.
NA, not applicable.
Analysis of the vanYD gene and its translation product indicated that, in comparison with VanYD from BM4339 and BM4416, the protein from 10/96A was terminated prematurely after amino acid 118, resulting in a polypeptide lacking the active sites of a PBP (Fig. 3). The hydropathic profile revealed that the truncated protein had a membrane-spanning portion at the N terminus (data not shown). Further investigation of one of the three reading frames downstream from the stop codon indicated 87% identity over a length of 237 amino acids with the C-terminal portion of VanYD from BM4339 and BM4416. This stretch contained the three motifs SXXK, SG(C/N), and KTG, which are characteristic of the penicillin binding domains of PBPs (Fig. 4) (40). It was concluded that a frameshift mutation had resulted in the presence of the stop codon and thus premature termination of what would otherwise have been a full-length protein corresponding to VanYD of BM4339 and BM4416. In spite of the mutation in vanYD, d,d-carboxypeptidase activity was detected in the membrane fraction of broken-cell preparations of 10/96A, and this activity was not inhibited by penicillin, as opposed to BM4339 and BM4416 (Table 3). The protein with this activity is unlikely to have been encoded by vanYD from 10/96A.
FIG. 4.
Partial alignment of the deduced sequences of d,d-carboxypeptidases in VanD-type E. faecium strains 10/96A, BM4339, and BM4416 and PBPs from Streptomyces sp. strain K15 (40), Bacillus subtilis MB24 (DacF) (56), and E. coli (PBP6) (18). Conserved motifs involved in the scaffolding of the active site are indicated in boldface type. The numbers of amino acids between the NH2 terminus and motif I, motifs I and II, motifs II and III, and motif III and the COOH terminus are indicated.
In plasmid pAT639 (vanRD, SD′, ORFA, ORFB, vanSD′) (Fig. 1), the deduced sequences of the VanRD and VanSD proteins exhibited structural similarity with the VanRD response regulators and VanSD histidine protein kinases of the two-component regulatory systems in strains BM4339 and BM4416 (17, 21). In addition, sequence analysis of the 3,725-bp insert in this plasmid revealed the presence of a 1,920-bp insertion sequence, designated ISEfa4, which was inserted in the vanSD gene of strain 10/96A (Fig. 1 and 3). Two ORFs, ORFA and ORFB, were identified in the insertion sequence that exhibited 39 and 48% identity with the genes (tnp) for the putative transposases of unrelated elements, IS200 from Helicobacter pylori (16), and IS1341 from the thermophilic bacterium PS3 (38), respectively (Fig. 3). ISEfa4 belongs to the IS605 family (32). Unlike IS605, ORFA and ORFB were in the same orientation and showed only 29 and 39% identity with the tnpA and tnpB genes of IS605, respectively.
The C-terminal portion of VanSD in strain 10/96A contained the five blocks of conserved amino acids characteristic of transmitter modules in histidine protein kinases (Fig. 5). Histidine residue 140 of VanSD from strain 10/96A was aligned with histidine residues 166 of VanSD of BM4339 and BM4416, which are the putative sites of autophosphorylation of sensors (Fig. 5). The hydropathy profile of the N-terminal putative sensor domain of VanSD from strains BM4339 and BM4416 revealed the presence of two stretches of hydrophobic amino acids similar to those in VanS, VanSB, and EnvZ, suggesting a similar topology for these enzymes (data not shown). The ISEfa4 copy in vanSD of strain 10/96A not only removed one of the potential membrane spanning regions of VanSD, but the remaining larger portion of the protein would not have been produced. Lack of VanSD may lead to a high steady-state level of phosphorylated VanR and could thus account for the constitutive expression of the vanD operon in strain 10/96A.
FIG. 5.
Alignment of deduced amino acid sequences of VanSD sensors. Numbers at the left refer to the first amino acid in the corresponding sequence. Numbers at the right refer to the last amino acid in the corresponding line. Identical amino acids are indicated by asterisks, and the isofunctional amino acids are indicated by dots below the alignment. Conserved motifs H, N, G1, F, and G2 are indicated above the alignment by dashes lines (41). The histidine residue in boldface lettering is the putative autophosphorylation site. The proline (P) at position 173 putatively responsible for constitutive expression of resistance in BM4339 is indicated in boldface italic lettering. The amino acid sequence of VanSD from 10/96A starts after the insertion of ISEfa4.
The vanD gene cluster is chromosomally located in E. faecium 10/96A.
The location of the vanD gene cluster was determined by contour-clamped homogeneous electric field gel electrophoresis after digestion of genomic DNA from 10/96A with I-CeuI, an endonuclease specific for rRNA genes (34). The DNA fragments were transferred to a nitrocellulose membrane and hybridized successively with a 16S rRNA (rrs) probe (27) and a probe internal to vanD from strain 10/96A (Fig. 1). The rrs probe hybridized with four I-CeuI fragments, and the vanD probe cohybridized with a 450-kb fragment (data not shown). Consequently, the vanD resistance operon was assigned to a chromosomal location.
As shown previously, four and five I-CeuI fragments from BM4339 and BM4416, respectively, hybridized with the rrs probe, and a 330-kb fragment from BM4339 or a 380-kb fragment from BM4416 cohybridized with a vanHDDXD probe (43). The comparative analysis indicated that strain 10/96A was distinct from these two isolates
d,d-Peptidase activities in strain 10/96A.
d,d-Dipeptidase and d,d-carboxypeptidase activities in E. faecium 10/96A were assayed by determining the amount of d-Ala released from hydrolysis of the dipeptide d-Ala-d-Ala and of the pentapeptide UDP-Mur-NAc-l-Ala-γ-d-Glu-l-Lys-d-Ala-d-Ala, respectively (Table 3). The d,d-dipeptidase activity was measured in the supernatant of the lysed bacteria (after centrifugation at 100,000 × g) that had been grown in the presence of various concentrations of vancomycin (1, 8, and 64 μg/ml) as an inducer. As in BM4339, weak d,d-dipeptidase activity (VanXD) was found in the cytoplasmic extracts from induced or uninduced 10/96A (Table 3) (45). Since this strain has an impaired d-Ala:d-Ala ligase, it does not require an active VanX type d,d-dipeptidase for resistance.
The level of d,d-carboxypeptidase activity in the cytoplasmic fraction of 10/96A was low. However, the membrane preparation of this strain contained substantial activity, slightly weaker than that of membrane extracts of BM4339 and BM4416, in which the activity was inhibited by low concentrations of benzylpenicillin (Table 3) (48). In 10/96A the d,d-carboxypeptidase activity was not significantly induced by vancomycin, nor was it inhibited by benzylpenicillin (Table 3).
Comparison of d,d-peptidase activities from BM4339 and 10/96A in E. faecalis JH2-2.
Strains BM4339 and 10/96A do not produce d-Ala-d-Ala-containing peptidoglycan precursors following mutations in the chromosomal ddl gene (Table 2 and Fig. 2). Consequently, as mentioned previously no d,d-dipeptidase activity is required for glycopeptide resistance in this genetic background.
To test whether the vanXD and vanYD genes from BM4339 and 10/96A encode functional enzymes, the genes and their RBS were cloned under the control of the constitutive P2 promoter, leading to plasmids pAT635(P2vanXDcat) and pAT633(P2vanYDcat) from BM4339 and pAT636(P2vanXDcat) and pAT634(P2vanYDcat) from 10/96A, which were all electrotransformed into E. faecalis JH2-2 (Fig. 1). Although the deduced sequences of the two VanXD proteins do not contain mutations in the conserved residues known to be involved in zinc binding and catalysis (Fig. 4), only very weak hydrolysis of d-Ala-d-Ala was detected in cytoplasmic extracts from E. faecalis JH2-2 harboring pAT635(P2vanXDcat) and pAT636(P2vanXDcat) (Table 4). These results are in agreement with those obtained with crude extracts of BM4339 and 10/96A (Table 3).
No d,d-carboxypeptidase activity was detected in extracts from membrane or cytoplasmic fractions from JH2-2/pAT634(P2vanYDcat) harboring vanYD of 10/96A, whereas some activity was present in JH2-2/pAT633(P2vanYDcat) harboring vanYD of BM4339 (Table 4). Compared with the other VanD-type strains, E. faecium 10/96A produced almost exclusively UDP-MurNAc-pentadepsipeptide (95%), whereas UDP-MurNAc-tetrapeptide (2%) and UDP-MurNAc-tripeptide were present in insignificant amounts (Table 2). Despite the presence of the three conserved motifs in the same ORF corresponding to the cytoplasmic portion (Fig. 4), the frameshift mutation in the vanYD gene of strain 10/96A accounted for the lack of d,d-carboxypeptidase activity (Table 4). These results suggest that the truncated VanYD from 10/96A was not active due to loss of the domain containing the active site.
Analysis of PBPs from E. faecium 10/96A.
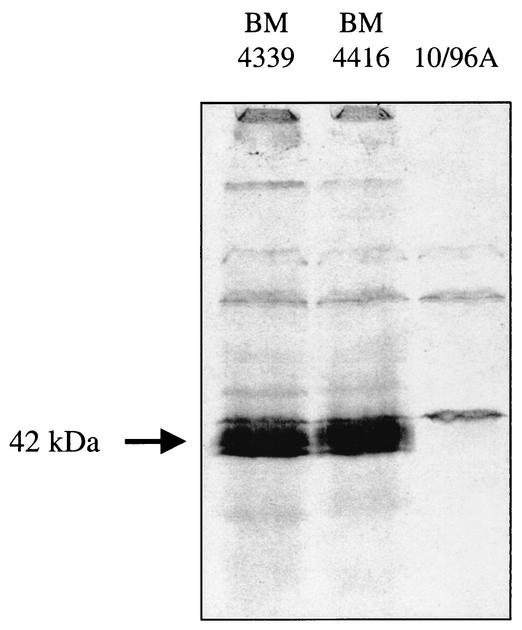
d,d-Carboxypeptidases from BM4339 and BM4416 belong to the PBP family of catalytic serine enzymes but are susceptible to benzylpenicillin (43, 45, 48). We have studied the binding of benzyl[14C]penicillin to membrane preparations of E. faecium 10/96A in comparison with the other VanD-type strains, BM4339 and BM4416 (Fig. 6). A PBP which migrated as a doublet on sodium dodecyl sulfate gel with an apparent molecular mass of 40 to 42 kDa was present in strains BM4339 and BM4416 but absent from membranes of E. faecium 10/96A (Fig. 6). This result supported the hypothesis that the defect in the vanYD gene in the latter strain results in a lack of inhibition by benzylpenicillin of the d,d-carboxypeptidase present, which is presumably encoded by a different gene.
FIG. 6.
Binding of benzyl[14C]penicillin to membrane proteins of VanD-type E. faecium. Membrane preparations of the strains indicated at the top were incubated with benzyl[14C]penicillin (1 μg/ml) for 5 min at 37°C; the proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on a 12% polyacrylamide gel; the gel was stained with Coomassie blue, destained, and dried; and the PBPs were revealed by autoradiography.
DISCUSSION
VanD-type resistance to glycopeptides is conferred on E. faecium 10/96A by the chromosomal vanD gene cluster, which includes at least eight ORFs (Fig. 3). The 5′ portion contains the vanRD and vanSD genes, which encode a two-component regulatory system although vanSD is disrupted by a copy of insertion sequence ISEfa4 (Fig. 1 and 3). The downstream vanYD gene encodes a polypeptide of only 118 amino acids rather than the expected 355 amino acids due to a mutation that disrupted the reading frame of the protein; if the mutation had not occurred, vanYD would have encoded a d,d-carboxypeptidase related to PBPs (Fig. 1 and 3). The 3′ portion of the vanD gene cluster contains the vanHD, vanD, and vanXD genes which encode a dehydrogenase, a d-Ala:d-Lac ligase, and a d,d-dipeptidase, respectively. Comparison of the vanD operon in 10/96A with those of BM4339 and BM4416 revealed 83 to 96% identity, and the deduced proteins are homologous to the corresponding enzymes in VanA- and VanB-type strains (Fig. 3). However, no genes homologous to vanZ and vanW from the vanA and vanB operons, respectively, were found. Strains of the VanD-type of acquired glycopeptide resistance share other characteristics that distinguish them from VanA- and VanB-type enterococci; in particular, resistance is constitutively expressed and is not transferable by conjugation to other enterococci (44).
Compared with the VanA- and VanB-type strains, VanD-type strains BM4339, BM4416 (also designated N97-330), and 10/96A have negligible d,d-dipeptidase activity, encoded by vanXD alleles, despite the presence of critical residues implicated in the binding of Zn2+ and in catalysis (Tables 3 and 4) (36, 43, 45). Lack of such an activity should result in a glycopeptide-susceptible phenotype, since bacteria are unable to remove peptidoglycan precursors ending in d-Ala-d-Ala, the target for glycopeptides. However, the chromosomal ddl gene is disrupted by a 5-bp insertion in BM4339; by insertion of an IS19 (also called ISEfm1) element in BM4416; and by a single mutation, G184S, next to the serine involved in the binding of d-Ala1 in strain 10/96A (Fig. 2) (17, 21, 43). Both insertions and the mutation lead to production of quasi exclusively peptidoglycan precursors terminating in d-lactate (Table 2). Enterococci containing a vancomycin resistance cluster but with an impaired d-Ala:d-Ala ligase can only grow in the presence of vancomycin if these strains are inducible for vancomycin resistance. Such strains rely entirely for growth on synthesis of peptidoglycan precursors containing d-Ala-d-Lac instead of d-Ala-d-Ala (7, 15, 50, 53). In the VanD-type strains studied, there were no qualitative differences between the peptidoglycan precursors produced by uninduced or induced cells, indicating that the vanD clusters were expressed constitutively, thus bypassing the requirement for glycopeptides (Table 2).
In VanA- and VanB-type strains, VanS or VanSB sensors act as a kinase in the presence of glycopeptides. VanS and VanSB also negatively control promoters PH, PYB and PR, PRB that mediate transcription of the resistance (vanHAX and vanHBBXB) and regulatory (vanRS and vanRBSB) genes, respectively, in the absence of glycopeptides (4, 5). Under noninducing conditions, the wild-type sensors are therefore considered to act as phosphatases preventing accumulation of the phosphorylated form of the response regulators. According to this model, a constitutive phenotype is associated with loss of the phosphatase activity of the kinase and expression of the resistance genes remains unaltered under noninducing or inducing conditions (4, 5, 15). In VanA-type strains, in the absence of VanS, dephosphorylation of VanR phosphorylated by an heterologous kinase is extremely slow compared to that of the related response regulators, leading to a high level of phospho-VanR and thus to constitutive high-level transcription of the resistance genes (5, 55). In VanB-type strains, constitutive expression of glycopeptide resistance is most probably due to an impaired dephosphorylation of VanRB by VanSB, since substitutions affecting homologous residues in related sensor kinases result in a defect of the phosphatase, but not of the kinase activity, of the proteins (1, 57). Constitutive expression of the vanB cluster is due to amino acid substitutions at two specific positions on either side of histidine 233, which corresponds to the putative autophosphorylation site of VanSB (15).
Alignment of the deduced amino acid sequences of the VanSD sensors from E. faecium BM4339, BM4416, and 10/96A revealed a mutation at position 173 in the sensor of BM4339, leading to a Pro-to-Ser substitution (Fig. 5). This substitution is in a critical region, since it alters a residue close to histidine 166, corresponding to the putative autophosphorylation site of VanSD, an observation which could account for the constitutive expression of the vanD cluster in BM4339 (Fig. 5). Previous comparison of the vanSD genes from BM4339 and N-97-330 (so called BM4416) showed that the latter strain had suffered a 1-bp deletion at position 670 (BM4339 numbering), which results in a frameshift mutation leading presumably to the synthesis of a 233-amino-acid truncated and nonfunctional sensor instead of a protein containing 381 amino acids as in BM4339 (17). Strain 10/96A contains a different type of mutational event which bypasses the requirement of glycopeptide for constitutive expression of the resistance genes. Insertion sequence ISEfa4 was found 45 bp downstream from the start site of the vanSD gene of strain 10/96A and it possessed the characteristics of the IS605-family (Fig. 3). IS605, detected in H. pylori, is unusual in that it contains, in opposite orientation, homologs of genes for the putative transposases of two other unrelated insertion sequence elements, IS200 from H. pylori and IS1341 from the thermophilic bacterium PS3 (32). ISEfa4 is characterized by (i) the absence of terminal inverted repeats, (ii) lack of duplication of target sequences, (iii) inserting with its left end next to 5′-TTTAAC, and (iv) two ORFs encoding putative transposases but in the same orientation.
To our knowledge, E. faecium 10/96A is only the third glycopeptide-resistant Enterococcus in which an insertion has been identified within a van gene but is the first in VanD-type strains. Disruption of vanY by IS1476 and insertion of IS1216V located towards the 3′ end of vanS have been reported in VanA-type strains (35; A. L. Darini, M. F. Palepou, D. James, and N. Woodford, Letter, Antimicrob Agents Chemother. 43:995-996, 1999). The latter insertion leads to the loss of 11 amino acids from the C terminus of the VanS sensor and their possible replacement by 10 amino acids resulting from read-through of the inserted IS1216V (Darini et al., letter). According to the authors, this change would not affect the function of the VanS sensor, because the critical residues remain intact. The disruption of vanY leads to a decrease of its activity but has no phenotypic consequence, since in VanA-type strains, VanY is not necessary for vancomycin resistance (3, 9, 35). In the case of ISEfa4, the insertion led to the production of a truncated VanSD, allowing strain 10/96A to grow in the absence of glycopeptide in the medium.
The frameshift mutation in the vanYD gene of strain 10/96A results in a truncated polypeptide of 118 amino acids lacking the active site of a d,d-carboxypeptidase as indicated by the lack of activity of the protein after cloning the complete gene in E. faecalis JH2-2 (Table 4). The mutation disrupted the reading frame of the VanYD protein of 10/96A, which would otherwise have contained the active site motifs of a PBP (Fig. 4). These motifs are present in VanYD of BM4339 and BM4416, the proteins bind benzylpenicillin, and the d,d-carboxypeptidase activity is inhibited by benzylpenicillin (Fig. 4) (48). The truncated product of the vanYD gene of 10/96A did not bind penicillin (Fig. 6), nor was there any d,d-carboxypeptidase activity in the cytoplasmic fraction, implying that reinitiation of the C-terminal portion of the protein was unlikely to have occurred, particularly as no potential RBS was identified upstream from possible start sites. Surprisingly, substantial d,d-carboxypeptidase activity was detected in the membrane fraction (Table 3). This activity was not inhibited by benzylpenicillin, was not induced by vancomycin, and was presumed to be catalyzed by a totally different protein. Further investigation will indicate whether strain 10/96A has acquired a gene, not present in the vanD gene cluster, which encodes a VanY- or VanYB type protein.
Consideration of the peptidoglycan precursors of the three VanD strains supports this hypothesis. When peptidoglycan synthesis was blocked by ramoplanin in BM4339 and BM4416, UDP-MurNAc-tetrapeptide was present (21 and 24%, respectively) in addition to UDP-MurNAc-pentadepsipeptide (Table 2). As the d-Ala:d-Ala ligase of both these strains is inactive, little if any UDP-MurNAc-pentapeptide would have been present; consequently, tetrapeptide is likely to have resulted from removal of d-lactate from UDP-MurNAc-pentadepsipeptide. PBPs that function as d,d-carboxypeptidases hydrolyze esters in addition to peptides (46); therefore, it is presumed that VanYD of BM4339 and BM4416, which are PBPs and whose activity was inhibited by low concentrations of benzylpenicillin, will hydrolyze UDP-MurNAc-pentadepsipeptide with the production of tetrapeptide. The peptidoglycan precursors accumulated in strain 10/96A were almost exclusively UDP-MurNAc-pentadepsipeptide (95%), and only 2% UDP-MurNAc-tetrapeptide was present. The d,d-carboxypeptidase activity present in VanA- and VanB-type strains and catalyzed by VanYΔ1-45 preferentially hydrolyzes peptidoglycan precursors terminating in acyl-d-Ala-d-Ala (3). If the d,d-carboxypeptidase in membranes of 10/96A has the same specificity as VanY or VanYB, it would account for the lack of UDP-MurNAc-tetrapeptide in the precursors accumulated in this strain, as virtually no UDP-MurNAc-pentapeptide was available as substrate (Table 2). The d,d-carboxypeptidase activity is not required for resistance, but it is possible that the gene encoding this enzyme was acquired prior to the mutation, leading to a defective d-Ala:d-Ala ligase.
We have shown previously that, in VanA-type strains, increased transcription of the vanHAX operon is associated with increased incorporation of d-Ala-d-Lac into peptidoglycan precursors, to the detriment of d-Ala-d-Ala, and with a gradual increase in vancomycin resistance levels (8). More-complete elimination of d-Ala-d-Ala-containing precursors is required for teicoplanin resistance (8). An unusual feature of the VanD-type strains is their susceptibility to teicoplanin (MIC = 4 μg/ml), despite constitutive production of peptidoglycan precursors that terminate essentially only in d-Ala-d-Lac. The small amount of pentapeptide could have been synthesized by the VanD ligase. Teicoplanin susceptibility has been associated with mutations in the VanS sensor of some VanA-type strains (29).
The nucleotide divergence between the vanD alleles (BM4339, BM4416, and 10/96A) and the geographical dispersion of the isolates (Canada, United States, and Brazil) lead to the hypothesis that the VanD-type strains represent independent introductions in enterococci of gene clusters from undefined donor species.
Acknowledgments
We thank P. Trieu-Cuot for helpful discussions.
This work was supported in part by a Bristol-Myers Squibb unrestricted biomedical research grant in infectious diseases.
REFERENCES
- 1.Aiba, H., F. Nakasai, S. Mizushima, and T. Mizuno. 1989. Phosphorylation of a bacterial activator protein, OmpR, by a protein kinase, EnvZ, results in stimulation of its DNA-binding ability. J. Biochem. 106:5-7. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arthur, M., F. Depardieu, L. Cabanié, P. Reynolds, and P. Courvalin. 1998. Requirement of the VanY and VanX D,D-peptidases for glycopeptide resistance in enterococci. Mol. Microbiol. 30:819-830. [DOI] [PubMed] [Google Scholar]
- 4.Arthur, M., F. Depardieu, and P. Courvalin. 1999. Regulated interactions between partner and non partner sensors and response regulators that control glycopeptide resistance gene expression in enterococci. Microbiology 145:1849-1858. [DOI] [PubMed] [Google Scholar]
- 5.Arthur, M., F. Depardieu, G. Gerbaud, M. Galimand, R. Leclercq, and P. Courvalin. 1997. The VanS sensor negatively controls VanR-mediated transcriptional activation of glycopeptide resistance genes of Tn1546 and related elements in the absence of induction. J. Bacteriol. 179:97-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arthur, M., F. Depardieu, C. Molinas, P. Reynolds, and P. Courvalin. 1995. The vanZ gene of Tn1546 from Enterococcus faecium BM4147 confers resistance to teicoplanin. Gene 154:87-92. [DOI] [PubMed] [Google Scholar]
- 7.Arthur, M., F. Depardieu, P. Reynolds, and P. Courvalin. 1999. Moderate-level resistance to glycopeptide LY333328 mediated by genes of the vanA and vanB clusters in enterococci. Antimicrob. Agents Chemother. 43:1875-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arthur, M., F. Depardieu, P. Reynolds, and P. Courvalin. 1996. Quantitative analysis of the metabolism of soluble cytoplasmic peptidoglycan precursors of glycopeptide-resistant enterococci. Mol. Microbiol. 21:33-44. [DOI] [PubMed] [Google Scholar]
- 9.Arthur, M., F. Depardieu, H. A. Snaith, P. E. Reynolds, and P. Courvalin. 1994. Contribution of VanY d,d-carboxypeptidase to glycopeptide resistance in Enterococcus faecalis by hydrolysis of peptidoglycan precursors. Antimicrob. Agents Chemother. 38:1899-1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arthur, M., C. Molinas, T. D. H. Bugg, G. D. Wright, C. T. Walsh, and P. Courvalin. 1992. Evidence for in vivo incorporation of d-lactate into peptidoglycan precursors of vancomycin-resistant enterococci. Antimicrob. Agents Chemother. 36:867-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arthur, M., C. Molinas, and P. Courvalin. 1992. The VanS-VanR two-component regulatory system controls synthesis of depsipeptide peptidoglycan precursors in Enterococcus faecium BM4147. J. Bacteriol. 174:2582-2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arthur, M., C. Molinas, F. Depardieu, and P. Courvalin. 1993. Characterization of Tn1546, a Tn3-related transposon conferring glycopeptide resistance by synthesis of depsipeptide peptidoglycan precursors in Enterococcus faecium BM4147. J. Bacteriol. 175:117-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arthur, M., P. Reynolds, and P. Courvalin. 1996. Glycopeptide resistance in enterococci. Trends Microbiol. 4:401-407. [DOI] [PubMed] [Google Scholar]
- 14.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1987. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 15.Baptista, M., F. Depardieu, P. Reynolds, P. Courvalin, and M. Arthur. 1997. Mutations leading to increased levels of resistance to glycopeptide antibiotics in VanB-type enterococci. Mol. Microbiol. 25:93-105. [DOI] [PubMed] [Google Scholar]
- 16.Beuzon, C. R., and J. Casadesus. 1997. Conserved structure of IS200 elements in Salmonella. Nucleic Acids Res. 25:1355-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyd, D. A., J. Conly, H. Dedier, G. Peters, L. Robertson, E. Slater, and M. R. Mulvey. 2000. Molecular characterization of the vanD gene cluster and a novel insertion element in a vancomycin-resistant enterococcus isolated in Canada. J. Clin. Microbiol. 38:2392-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Broome-Smith, J. K., I. Ioannidis, A. Edelman, and B. G. Spratt. 1988. Nucleotide sequences of the penicillin-binding protein 5 and 6 genes of Escherichia coli. Nucleic Acids Res. 16:1617.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bugg, T. D. H., S. Dutka-Malen, M. Arthur, P. Courvalin, and C. T. Walsh. 1991. Identification of vancomycin resistance protein VanA as a D-alanine:D-alanine ligase of altered substrate specificity. Biochemistry 30:2017-2021. [DOI] [PubMed] [Google Scholar]
- 20.Bugg, T. D. H., G. D. Wright, S. Dutka-Malen, M. Arthur, P. Courvalin, and C. T. Walsh. 1991. Molecular basis for vancomycin resistance in Enterococcus faecium BM4147: biosynthesis of a depsipeptide peptidoglycan precursor by vancomycin resistance proteins VanH and VanA. Biochemistry. 30:10408-10415. [DOI] [PubMed] [Google Scholar]
- 21.Casadewall, B., and P. Courvalin. 1999. Characterization of the vanD glycopeptide resistance gene cluster from Enterococcus faecium BM4339. J. Bacteriol. 181:3644-3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Casadewall, B., P. E. Reynolds, and P. Courvalin. 2001. Regulation of expression of the vanD glycopeptide resistance gene cluster from Enterococcus faecium BM4339. J. Bacteriol. 183:3436-3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dalla Costa, L. M., P. E. Reynolds, H. A. Souza, D. C. Souza, M. F. Palepou, and N. Woodford. 2000. Characterization of a divergent vanD-type resistance element from the first glycopeptide-resistant strain of Enterococcus faecium isolated in Brazil. Antimicrob. Agents Chemother. 44:3444-3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evers, S., and P. Courvalin. 1996. Regulation of VanB-type vancomycin resistance gene expression by the VanSB-VanRB two-component regulatory system in Enterococcus faecalis V583. J. Bacteriol. 178:1302-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan, C., P. C. Moews, C. T. Walsh, and J. R. Knox. 1994. Vancomycin resistance: Structure of D-alanine:D-alanine ligase at 2.3 Å resolution. Science 266:439-443. [DOI] [PubMed] [Google Scholar]
- 26.Gholizadeh, Y., M. Prevost, F. Van Bambeke, B. Casadewall, P. M. Tulkens, and P. Courvalin. 2001. Sequencing of the ddl gene and modeling of the mutated D-alanine:D-alanine ligase in glycopeptide-dependent strains of Enterococcus faecium. Protein Sci. 10:836-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greisen, K., M. Loeffelholz, A. Purohit, and D. Leong. 1994. PCR primers and probes for the 16S rRNA gene of most species of pathogenic bacteria, including bacteria found in cerebrospinal fluid. J. Clin. Microbiol. 32:335-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haldimann, A., S. L. Fisher, L. L. Daniels, C. T. Walsh, and B. L. Wanner. 1997. Transcriptional regulation of the Enterococcus faecium BM4147 vancomycin resistance gene cluster by the VanS-VanR two-component regulatory system in Escherichia coli K-12. J. Bacteriol. 179:5903-5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hashimoto, Y., K. Tanimoto, Y. Ozawa, T. Murata, and Y. Ike. 2000. Amino acid substitutions in the VanS sensor of the VanA-type vancomycin-resistant Enterococcus strains result in high-level vancomycin resistance and low-level teicoplanin resistance. FEMS Microbiol Lett. 185:247-254. [DOI] [PubMed] [Google Scholar]
- 30.Holman, T. R., Z. Wu, B. L. Wanner, and C. T. Walsh. 1994. Identification of the DNA-binding site for the phosphorylated VanR protein required for vancomycin resistance in Enterococcus faecium. Biochemistry 33:4625-4631. [DOI] [PubMed] [Google Scholar]
- 31.Jacob, A. E., and S. J. Hobbs. 1974. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J. Bacteriol. 117:360-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kersulyte, D., N. S. Akopyants, S. W. Clifton, B. A. Roe, and D. E. Berg. 1998. Novel sequence organization and insertion specificity of IS605 and IS606: chimaeric transposable elements of Helicobacter pylori. Gene 223:175-186. [DOI] [PubMed] [Google Scholar]
- 33.Le Bouguénec, C., G. de Cespédès, and T. Horaud. 1990. Presence of chromosomal elements resembling the composite structure Tn3701 in streptococci. J. Bacteriol. 172:727-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu, S. L., A. Hessel, and K. E. Sanderson. 1993. Genomic mapping with I-CeuI, an intron-encoded endonuclease specific for genes for ribosomal RNA, in Salmonella spp., Escherichia coli, and other bacteria. Proc. Natl. Acad. Sci. USA 90:6874-6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacKinnon, M. G., M. A. Drebot, and G. J. Tyrrell. 1997. Identification and characterization of IS1476, an insertion sequence-like element that disrupts VanY function in a vancomycin-resistant Enterococcus faecium strain. Antimicrob. Agents Chemother. 41:1805-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCafferty, D. G., I. A. Lessard, and C. T. Walsh. 1997. Mutational analysis of potential zinc-binding residues in the active site of the enterococcal D-Ala-D-Ala dipeptidase VanX. Biochemistry 36:10498-10505. [DOI] [PubMed] [Google Scholar]
- 37.Messer, J., and P. E. Reynolds. 1992. Modified peptidoglycan precursors produced by glycopeptide-resistant enterococci. FEMS Microbiol. Lett. 94:195-200. [DOI] [PubMed] [Google Scholar]
- 38.Murai, N., H. Kamata, Y. Nagashima, H. Yagisawa, and H. Hirata. 1995. A novel insertion sequence (IS)-like element of the thermophilic bacterium PS3 promotes expression of the alanine carrier protein-encoding gene. Gene 163:103-107. [DOI] [PubMed] [Google Scholar]
- 39.Ostrowsky, B. E., N. C. Clark, C. Thauvin-Eliopoulos, L. Venkataraman, M. H. Samore, F. C. Tenover, G. M. Eliopoulos, R. C. Moellering, Jr., and H. S. Gold. 1999. A cluster of VanD vancomycin-resistant Enterococcus faecium: molecular characterization and clinical epidemiology. J. Infect. Dis. 180:1177-1185. [DOI] [PubMed] [Google Scholar]
- 40.Palomeque-Messia, P., S. Englebert, M. Leyh-Bouille, M. Nguyen-Disteche, C. Duez, S. Houba, O. Dideberg, J. Van Beeumen, and J. M. Ghuysen. 1991. Amino acid sequence of the penicillin-binding protein/D,D-peptidase of Streptomyces K15. Predicted secondary structures of the low Mr penicillin-binding proteins of class A. Biochem. J. 279:223-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parkinson, J. S., and E. C. Kofoid. 1992. Communication modules in bacterial signaling proteins. Annu. Rev. Genet. 26:71-112. [DOI] [PubMed] [Google Scholar]
- 42.Pearson, W. R., and D. J. Lipman. 1988. Improved tools for biological sequence comparison. Proc. Natl. Acad. Sci. USA 85:2444-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perichon, B., B. Casadewall, P. Reynolds, and P. Courvalin. 2000. Glycopeptide-resistant Enterococcus faecium BM4416 is a VanD-type strain with an impaired d-alanine:d-alanine ligase. Antimicrob. Agents Chemother. 44:1346-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perichon, B., and P. Courvalin. 2000. Update on vancomycin resistance. Int. J. Clin. Pract. 54(Suppl. 115):88-93. [PubMed]
- 45.Perichon, B., P. E. Reynolds, and P. Courvalin. 1997. VanD type glycopeptide-resistant Enterococcus faecium BM4339. Antimicrob. Agents Chemother. 41:2016-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rasmussen, J. R., and J. L. Strominger. 1978. Utilization of a depsipeptide substrate for trapping acyl-enzyme intermediates of penicillin-sensitive D-alanine carboxypeptidases. Proc. Natl. Acad. Sci. USA 75:84-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reynolds, P. E. 1989. Structure, biochemistry and mechanism of action of glycopeptide antibiotics. Eur. J. Clin. Microbiol. Infect. Dis. 8:943-950. [DOI] [PubMed] [Google Scholar]
- 48.Reynolds, P. E., O. H. Ambur, B. Casadewall, and P. Courvalin. 2001. The VanYD D,D-carboxypeptidase of Enterococcus faecium BM4339 is a penicillin-binding protein. Microbiology 147:2571-2578. [DOI] [PubMed] [Google Scholar]
- 49.Reynolds, P. E., F. Depardieu, S. Dutka-Malen, M. Arthur, and P. Courvalin. 1994. Glycopeptide resistance mediated by enterococcal transposon Tn1546 requires production of VanX for hydrolysis of D-alanyl-D-alanine. Mol. Microbiol. 13:1065-1070. [DOI] [PubMed] [Google Scholar]
- 50.Sahm, D., J. Kissinger, M. S. Gilmore, P. R. Murray, R. Mulder, J. Solliday, and B. Clarke. 1989. In vitro susceptibility studies of vancomycin resistant Enterococcus faecalis. Antimicrob. Agents Chemother. 33:1588-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi, Y., and C. T. Walsh. 1995. Active site mapping of Escherichia coli D-Ala:D-Ala ligase by structure-based mutagenesis. Biochemistry 34:2768-2776. [DOI] [PubMed] [Google Scholar]
- 52.Steers, E., E. L. Foltz, B. S. Graves, and J. Riden. 1959. An inocula replicating apparatus for routine testing of bacterial susceptibility to antibiotics. Antibiot. Chemother. (Basel) 9:307-311. [PubMed] [Google Scholar]
- 53.Van Bambeke, F., M. Chauvel, P. E. Reynolds, H. S. Fraimow, and P. Courvalin. 1999. Vancomycin-dependent Enterococcus faecalis clinical isolates and revertant mutants. Antimicrob. Agents Chemother. 43:41-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Woodford, N. 2001. Epidemiology of the genetic elements responsible for acquired glycopeptide resistance in enterococci. Microb. Drug Resist. 7:229-236. [DOI] [PubMed] [Google Scholar]
- 55.Wright, G. D., T. R. Holman, and C. T. Walsh. 1993. Purification and characterization of VanR and the cytosolic domain of VanS: a two-component regulatory system required for vancomycin resistance in Enterococcus faecium BM4147. Biochemistry 32:5057-5063. [DOI] [PubMed] [Google Scholar]
- 56.Wu, J. J., R. Schuch, and P. J. Piggot. 1992. Characterization of a Bacillus subtilis sporulation operon that includes genes for an RNA polymerase sigma factor and for a putative d,d-carboxypeptidase. J. Bacteriol. 174:4885-4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamada, M., K. Makino, M. Amemura, H. Shinagawa, and A. Nakata. 1989. Regulation of the phosphate regulon of Escherichia coli: analysis of mutant phoB and phoR genes causing different phenotypes. J. Bacteriol. 171:5601-5606. [DOI] [PMC free article] [PubMed] [Google Scholar]