Abstract
OBJECTIVE
To compare the analgesic efficacy of valdecoxib with placebo and naproxen sodium for relieving menstrual cramping and pain due to primary dysmenorrhea.
DESIGN
Single-center, double-blind study with a 4-period, 4-sequence crossover design. Patients assessed pain intensity and pain relief at regular intervals up to 12 hours following the initial dose.
SETTING
Privately owned outpatient clinic.
PATIENTS/PARTICIPANTS
One hundred twenty patients with moderate to severe menstrual cramping were randomized. Eighty-seven patients completed all treatment cycles.
INTERVENTIONS
Valdecoxib 20 mg or 40 mg, naproxen sodium 550 mg, or placebo twice a day as required for ≤3 days in a single menstrual cycle.
MEASUREMENTS AND MAIN RESULTS
Both doses of valdecoxib (20 and 40 mg) were comparable to naproxen sodium and superior to placebo at all time points assessed for each of the primary end points. Valdecoxib and naproxen sodium had comparable onset and duration of action. Although the study design allowed patients 2 doses per day, only 15% and 20% of patients in the valdecoxib 20 mg and valdecoxib 40 mg groups, respectively, required remedication within the first 12 hours. The incidence of adverse events was similar between active and placebo groups.
CONCLUSION
Valdecoxib provided a fast onset of analgesic action, a level of efficacy similar to naproxen sodium, and a high level of patient satisfaction in the relief of menstrual pain due to primary dysmenorrhea. Valdecoxib was effective and well tolerated and thus appears to be a viable treatment for menstrual pain due to primary dysmenorrhea.
Keywords: valdecoxib, naproxen sodium, primary dysmenorrhea, menstrual pain
Primary dysmenorrhea, or painful menstruation, can be an incapacitating problem, causing significant disruption in a woman's life each month. It is one of the most common gynecologic complaints, thought to affect approximately one half of all menstruating women, with 10% of women having symptoms severe enough to interfere with daily responsibilities.1
The underlying cause of primary dysmenorrhea is believed to be the excessive production of uterine prostaglandins primarily derived from cyclooxygenase (COX)-2 activity.2 Inhibition of COX-2 by nonspecific nonsteroidal anti-inflammatory drugs (NSAIDs) such as aspirin, ibuprofen, or naproxen exerts anti-inflammatory, antipyretic, and analgesic effects. However, nonspecific NSAIDs also inhibit COX-1 at therapeutic doses, the latter activity impairing platelet function and predisposing patients to gastrointestinal (GI) side effects.3,4 COX-2 specific inhibitors produce the analgesic and anti-inflammatory effects of nonspecific NSAIDs without COX-1-associated side effects and are effective in treating pain, including menstrual pain.3,5–8
Valdecoxib, a potent COX-2 specific inhibitor, is approximately 28,000-fold more selective in vitro for human recombinant COX-2 than for human recombinant COX-1.9 When administered after oral surgery, valdecoxib has demonstrated a fast onset of action (approximately 30 minutes) and a magnitude of efficacy comparable to 10 mg oxycodone/1,000 mg acetaminophen.10 Compared with nonspecific NSAIDs in controlled trials, valdecoxib exhibits improved GI and platelet safety.11,12
This study compared the analgesic efficacy of valdecoxib 20 mg or 40 mg with naproxen sodium and placebo in relieving moderate or severe menstrual cramping pain due to primary dysmenorrhea. Up to 2 doses could be taken daily, as required, for up to 3 days. Standard measures were used to evaluate analgesic efficacy.
METHODS
Patients
Women aged 18 to 35 years with a history of regular menstruation (28-day cycle ± 7 days) and primary dysmenorrhea (onset <3 years after menarche) were included. Menstrual cramping pain had to be of moderate or severe intensity for at least 4 of the 6 months immediately preceding study entry. Patients were in satisfactory health, as determined by medical history and physical examination; had a negative urine pregnancy test result at the screening and baseline visits and at each postcycle visit; and used an accepted means of birth control during the study. All patients provided written informed consent prior to enrollment.
Patients with dysmenorrhea secondary to organic pathology or a current condition with symptoms of abdominal pain that could confound study assessments were excluded. Use of contraceptive implants within 6 months or use of injectable or intrauterine contraceptive devices within 3 months prior to study entry was not permitted. Patients with active peptic ulcer or any GI disease associated with clinically significant blood loss within the last 2 years were also excluded.
Study Design
This was a single-center, double-blind, single- and multiple-dose, placebo- and active-controlled, randomized, complete block crossover study, which was approved by Quorum Review Inc. (Seattle, WA), an institutional review board. This study was conducted in accordance with the Declaration of Helsinki. Patients were randomized to treatment using a computer-generated randomization schedule produced by the study sponsor, in the order in which they were enrolled in the study.
Efficacy of valdecoxib 20 mg bid prn (twice daily, as needed) and valdecoxib 40 mg bid prn was compared with that of placebo and naproxen sodium 550 mg bid prn in relieving moderate to severe menstrual cramping pain due to primary dysmenorrhea. Study medications were provided in 2 bottles, which were sufficient for 3 days of treatment. Patients were instructed to take 2 tablets from bottle A and 2 capsules from bottle B for each dose of study medication (Table 1). All tablets were white and round and all capsules were size #00 opaque gray. All study treatments were prepared, packaged, and distributed to the investigator by the study sponsor. Study drug supplies were then inventoried by study monitors prior to dispensing. Each bottle was labeled with the study and patient information, along with a sealed tear-off portion that contained the same data plus the identity of the study medication. This sealed portion was removed from the bottle by the study staff member who dispensed the medication and attached to the patient's case report form.
Table 1.
Contents of Assigned Study Medication Bottles
| Treatment | Bottle A | Bottle B |
|---|---|---|
| Valdecoxib 20 mg bid prn | 14 valdecoxib 10 mg tablets | 14 placebo capsules |
| Valdecoxib 40 mg bid prn | 14 valdecoxib 20 mg tablets | 14 placebo capsules |
| Naproxen sodium 550 mg bid prn | 14 placebo tablets | 14 naproxen sodium 275 mg tablets, encapsulated |
| Placebo | 14 placebo tablets | 14 placebo capsules |
An individual dose of study medication consisted of 2 tablets plus 2 capsules.
Patients, investigators, and study coordinators were blinded to the identity of study medication throughout the entire study. If medically indicated in case of emergency, the investigator could break the blind. Otherwise, the study blind was not broken until the study was complete and the data were statistically analyzed.
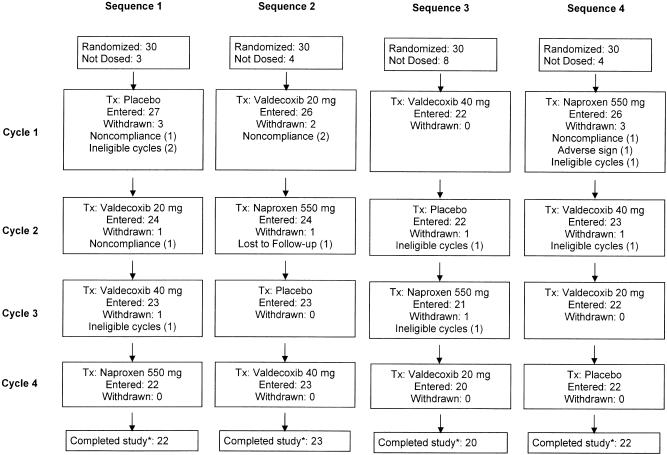
This study used a 4-sequence crossover design over 4 menstrual cycles (Fig. 1). After consulting the investigator's staff, who verified that patients' menstrual cramping pain was of moderate or severe intensity, patients took the first dose of study medication. Patients could remedicate with study drug every 8 to 12 hours as needed for pain (but no more than 2 doses within 24 hours). If a patient took rescue medication during any treatment cycle less than 1 hour after taking study medication, she was dropped from that particular cycle. Each cycle included a single-dose phase followed by a multiple-dose phase, meaning that a second dose of medication was taken between 8 and 12 hours following the initial dose. Patients were allowed to use their “usual medications” for rescue during the study period.
FIGURE 1.
Patient disposition. A total of 120 women entered the study and 101 received at least 1 dose of study medication; 87 patients in total completed the study*; 31 patients entered the multiple dose phase at some point of the study. *Completed the study means that patients were randomized, received treatment, and completed 1 evaluable menstrual cycle for each of 4 study medications (evaluable cycle=no rescue medication/remedication within 1 hour of first dose of study medication). Two of the 87 patients were not included in efficacy analyses; 1 took rescue medication prior to 1 hour following study medication, and 1 did not record time with pain assessments, both during Cycle 2.
Study Objectives and Hypothesis
The primary objective of this study was to compare the analgesic efficacy of an initial dose of valdecoxib 20 mg or 40 mg versus placebo in relieving moderate or severe menstrual cramping pain due to primary dysmenorrhea. The secondary objectives were to compare the analgesic efficacy of a single dose of valdecoxib 20 mg to that of valdecoxib 40 mg, to compare single valdecoxib 20 mg and 40 mg doses with a single dose of naproxen sodium 550 mg, and to assess safety and tolerability of the study medications.
The cramping pain associated with primary dysmenorrhea is believed to be a product of excessive COX-2-mediated production of uterine prostaglandins. This study was therefore designed to provide evidence that a single 20- or 40-mg dose of valdecoxib, a COX-2 specific inhibitor, is efficacious in relieving moderate to severe uterine cramping pain associated with primary dysmenorrhea.
Efficacy Assessments
Patients assessed pain intensity (PI) prior to taking the first dose of study medication in each treatment cycle (0 hour) and assessed PI and pain relief (PR) at regular intervals after the first dose (or immediately prior to rescue medication or remedication). Pain intensity was rated according to the following categorical scale, where 0=no pain, 1=mild pain, 2=moderate pain, and 3=severe pain. The level of PR was rated on a 5-point scale: 0=none (0% reduction), 1=a little (25% reduction), 2=some (50% reduction), 3=a lot (75% reduction), and 4=complete (100% reduction). Maximum PI and maximum PR were assessed 24 hours after the first dose on Day 2 and Day 3 or just before use of rescue medication. Pain intensity was assessed prior to and 2 hours after each dose on Day 2 and Day 3.
Time-specific PR and time-specific PI difference (PID) were analyzed at scheduled intervals over the 12-hour study period following administration of the first dose of study medication. Pain intensity difference was calculated by subtracting time-specific PI from baseline PI. The magnitude of analgesia was assessed by the time-weighted sum of PR (TOTPAR) and time-weighted sum of PI difference (SPID) at 8 and 12 hours after the first dose of study medication. TOTPAR and SPID over the 8- and 12-hour periods after the first dose of study medication were calculated from the area under the PR and PID curves through 8 and 12 hours, respectively. The time to rescue medication or first remedication, whichever came first, and the percentage of patients taking rescue medication before the second dose of study medication, were also monitored.
The patient's global evaluation of study medication was performed 12 hours after the first dose of study medication on Day 1 (or immediately prior to rescue medication or remedication) and 24 hours after the first dose on Day 2 and Day 3 or just before rescue medication use. Patients assessed their study medication on a 4-point scale, where 1=poor, 2=fair, 3=good, and 4=excellent.
Safety Assessments
Adverse events were monitored throughout the study. Clinical laboratory evaluations were performed at the screening visit (21 to 30 days prior to the anticipated first dose of study medication) and after treatment (within 5 to 9 days of the final treated menstrual cycle).
Statistical Analysis
The sample size calculation was based on 2 primary efficacy variables, SPID8 and TOTPAR8 (SPID and TOTPAR at 8 hours postdose). With 90% power and type I error of 0.025 (for a 2-sided test adjusted for 2 comparisons), a sample size of 93 patients per treatment was needed to detect a difference of ≥3 in the SPID8 scores between valdecoxib and placebo, with a maximum estimate of variability of 5.7 for SPID8. This sample size was also sufficient to detect a difference of ≥4.9 in the TOTPAR8 score between valdecoxib and placebo, with a maximum estimate of variability of 8.54 for the TOTPAR8 score with the same type I error and power. With the assumption that there would be a 20% dropout rate, a sample size of 116 patients per treatment was needed.
All efficacy analyses included randomized patients who had received study medication and had completed 1 evaluable cycle for each of the 4 study medications in the order of the sequence group in which they were assigned. A cycle was evaluable if patients treated their menstrual pain with study medication and did not take rescue medication or remedicate within 1 hour of the first dose of study medication. The last observation carried forward was applied only within each treatment period and was not used for the imputation of crossperiod missing values.
Baseline demographic variables were compared across treatment sequence using analysis of variance (ANOVA), with treatment sequence as the factor for continuous variables and a Fisher exact test for categorical variables. A Cochran-Mantel-Haenszel test adjusted for treatment sequence was used to compare baseline PI across treatments.
TOTPAR, SPID, and patient's global evaluation of study medication were analyzed using ANOVA with fixed effects for baseline PI, treatment, period, sequence, and a random effect for patient (sequence). The Fisher protected least significant difference multiple comparison procedure was used for pairwise comparisons of the model-adjusted treatment means. Because the crossover effect was found not to be significant for each of the continuous variables, the analyses were performed without including carryover effects in the model.
Patient's global evaluation of study medication was analyzed using a categorical data analysis method. The number of doses of medication taken and time between doses in the multiple-dose phase were summarized by treatment.
RESULTS
Patient Baseline Characteristics and Disposition
The study period commenced on September 22, 1999 and final follow-up was completed on August 14, 2000. Demographics and additional baseline characteristics are summarized in Table 2. All treatment sequences were comparable with respect to patients' mean age, weight, and race. Across all 4 treatment sequences, 92, 91, 93, and 94 patients received at least 1 dose of valdecoxib 20 mg, valdecoxib 40 mg, naproxen sodium 550 mg, or placebo, respectively. Of these, 87 patients completed 1 evaluable menstrual cycle on each of the 4 study medications. Two of the 87 patients were not included in efficacy analyses; 1 took rescue medication prior to 1 hour following study medication, and 1 did not record time with pain assessments, both during Cycle 2 (Fig. 1). Thirty-one patients entered the multiple dose assessment phase (6 valdecoxib 20 mg, 8 valdecoxib 40 mg, 12 naproxen sodium, and 5 placebo) and 3 took all doses of the study medication (2 valdecoxib 40 mg, and 1 naproxen sodium).
Table 2.
Patient Baseline Characteristics
| Treatment Sequence 1 | Treatment Sequence 2 | Treatment Sequence 3 | Treatment Sequence 4 | P Value | |
|---|---|---|---|---|---|
| (n = 30) | (n = 30) | (n = 30) | (n = 30) | ||
| Mean age, y (± SD) | 24.8 ± 4.8 | 24.7 ± 4.7 | 24.3 ± 5.0 | 23.1 ± 4.9 | .54 |
| Mean weight, kg (± SD) | 67.0 ± 15.7 | 65.2 ± 15.3 | 71.8 ± 17.1 | 63.8 ± 11.9 | .19 |
| Race, n (%) | .59 | ||||
| White | 22 (73) | 23 (77) | 26 (87) | 21 (70) | |
| Black | 2 (7) | 3 (10) | 2 (7) | 3 (10) | |
| Asian | 0 (0) | 2 (7) | 1 (3) | 1 (3) | |
| Hispanic | 6 (20) | 2 (7) | 1 (3) | 4 (13) | |
| Other | 0 (0) | 0 (0) | 0 (0) | 1 (3) | |
| Mean age, y, at onset of primary dysmenorrhea (± SD) | 13.0 ± 1.5 | 13.4 ± 1.9 | 13.5 ± 1.5 | 13.0 ± 1.5 | .42 |
| Mean number of days of menstrual cramping pain per cycle (± SD) | 3.0 ± 1.6 | 2.7 ± 1.0 | 2.7 ± 1.2 | 2.9 ± 1.1 | .65 |
| Mean average length, days, per cycle (± SD) | 27.6 ± 1.7 | 27.5 ± 2.3 | 27.7 ± 1.7 | 28.4 ± 1.6 | .18 |
SD, standard deviation.
Efficacy Outcomes
The treatment sequences were statistically indistinguishable with respect to baseline PI, with 76% to 85% of patients in each group experiencing moderate pain and 15% to 24% experiencing severe pain. Valdecoxib 20 mg and valdecoxib 40 mg provided analgesia that was significantly greater than that provided by placebo and similar to that provided by naproxen sodium after the initial dose. TOTPAR and SPID scores for the 3 active treatments were similar to and significantly higher than those for placebo at 8 and 12 hours, indicating greater reductions from baseline pain levels (Table 3).
Table 3.
Total Pain Relief and Sum of Pain Intensity Difference Scores for the First 8 and 12 Hours
| Placebo | Valdecoxib 20 mg | Valdecoxib 40 mg | Naproxen Sodium | |
|---|---|---|---|---|
| TOTPAR 8 hr | 13.5 | 19.3* | 20.4* | 20.3* |
| TOTPAR 12 hr | 21.1 | 30.0* | 32.1* | 31.2* |
| SPID 8 hr | 7.9 | 11.4* | 11.9* | 12.0* |
| SPID 12 hr | 12.5 | 17.8* | 18.7* | 18.4* |
P <.001 versus placebo.
TOTPAR, total pain relief; SPID, sum of pain intensity difference.
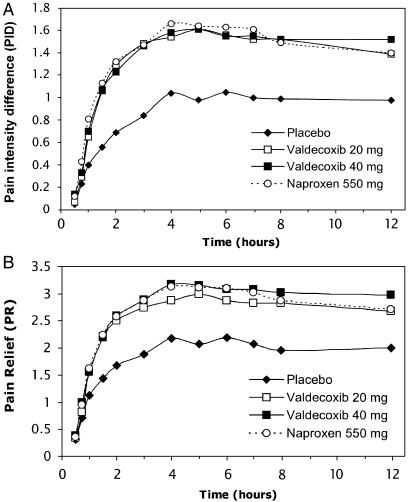
Single doses of valdecoxib 20 mg or 40 mg demonstrated a rapid onset of PR similar to naproxen sodium as shown by the PID and PR scores over the first 12-hour assessment period (Fig. 2). For each active treatment, the PID and PR scores were significantly greater than for placebo by 1 hour postdose and remained so through the 12-hour assessment (P <.001). There were no significant differences between either dose of valdecoxib and naproxen sodium at any time point. The percentage of patients requiring rescue medication or remedication over the 12-hour assessment period was highest with placebo and lower but similar among the active treatments (placebo, 33%; naproxen sodium, 20%; valdecoxib 40 mg, 20%; and valdecoxib 20 mg, 15%).
FIGURE 2.
A. Mean pain intensity difference from baseline. B. Mean pain relief scores. All active treatments were significantly different from placebo from 1 hour through 12 hours (P <.001)
The patients' global evaluation of study medication mean scores for the active treatments were similar to and significantly higher than the score for placebo (P <.001 vs placebo). Overall scores for the active treatments were similar: 62% of patients treated with valdecoxib 20 mg, 72% of patients treated with valdecoxib 40 mg, and 68% of patients treated with naproxen sodium rated their medication as good or excellent, compared with 40% of patients treated with placebo.
Safety Outcomes
The incidence of adverse events reported during valdecoxib treatment was similar to that for placebo and naproxen sodium. Adverse events were reported by 16% of patients who received placebo, 14.1% of patients who received valdecoxib 20 mg, 17.6% of patients who received valdecoxib 40 mg, and 12.9% of patients who received naproxen sodium. Adverse events with an incidence of ≥2% for any treatment group are summarized in Table 4. The most common adverse event was headache, which had the highest occurrence in the placebo group. Most adverse events were of mild or moderate severity. No differences in clinical laboratory values were observed among the groups.
Table 4.
Adverse Events with Incidence of ≥ 2% in Any Treatment Group
| Placebo N (%) | Valdecoxib 20 mg N (%) | Valdecoxib 40 mg N (%) | Naproxen Sodium N (%) | |
|---|---|---|---|---|
| Abdominal pain | 0 (0.0) | 0 (0.0) | 2 (2.2) | 0 (0.0) |
| Dizziness | 2 (2.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Headache | 10 (10.6) | 5 (5.4) | 6 (6.6) | 6 (6.5) |
| Nausea | 1 (1.1) | 2 (2.2) | 1 (1.1) | 0 (0.0) |
| Somnolence | 0 (0.0) | 1 (1.1) | 2 (2.2) | 2 (2.2) |
DISCUSSION
Primary dysmenorrhea, or cramping pain with the onset of menstruation, can be an incapacitating problem.1 The results of the present study demonstrate that a single 20-mg or 40-mg dose of valdecoxib, a COX-2 specific inhibitor, is effective in relieving moderate or severe menstrual cramping pain from primary dysmenorrhea. Both doses of valdecoxib were effective in treating menstrual pain, as evidenced by significant differences from placebo in the TOTPAR and SPID scores at 8 and 12 hours postdose. The results of this study confirm those of another recent study of valdecoxib 20 mg and 40 mg and naproxen 550 mg (all dosed bid prn) in primary dysmenorrhea.13 In the present study, significant differences in PID and PR scores from 1 hour through 12 hours show the rapid onset and long duration of the analgesic effect of each dose of valdecoxib. The onset, magnitude, and duration of action of valdecoxib and naproxen sodium were similar. In addition, the occurrence of adverse events was low and similar across all treatments. There was a high degree of compliance with the study, as 87 of 92 patients completed the study.
Significantly fewer patients required rescue medication or remedication following active treatment than following placebo, and additional medication was required significantly earlier following placebo than following active treatments. Although the study design allowed patients 2 doses per day, only 15% of patients in the valdecoxib 20 mg and 20% of patients in both the valdecoxib 40 mg group and the naproxen sodium 550 mg group required remedication within the first 12 hours. In addition, patient satisfaction with study medication was significantly higher for both doses of valdecoxib than placebo and was similar to that reported for naproxen sodium.
In this study, the occurrence of adverse events, ranging from 12.9% to 17.6% for active treatments and placebo, was not significantly different between groups. However, use of nonspecific NSAIDs can result in potentially significant side effects—particularly related to GI tolerability, GI ulcers, and reduced platelet aggregation.14 The results of several studies have demonstrated that these complications may arise even with short-term use. One study showed a increase in GI and operative site bleeding in just over 5 or more days of consistent use of nonspecific NSAIDs.15 Another study demonstrated a significant increase in incidence of gastroduodenal ulcers in patients who took naproxen 500 mg bid compared with those who took valdecoxib 40 mg bid or placebo after only 6.5 consecutive days.16 The combined effect on the GI mucosa and platelet function, resulting from COX-1 inhibition by nonspecific NSAIDs, can multiply the overall risk of excessive GI bleeding. Valdecoxib and other COX-2 specific inhibitors have demonstrated improved GI safety profiles compared with nonspecific NSAIDs due to their COX-1-sparing effects.11,17,18
Naproxen was chosen as the comparator in this study for several reasons. A 550-mg dose of naproxen sodium twice daily is indicated for treatment of primary dysmenorrhea, as is valdecoxib 20 mg twice daily (or 40 mg daily), and the duration of effect for naproxen is similar to the duration of effect for valdecoxib.13 These were important considerations for blinding. Naproxen has been shown to be at least as effective as ibuprofen in the treatment of menstrual cramping associated with primary dysmenorrhea. A study comparing naproxen with ibuprofen, acetaminophen, and placebo for treatment of primary dysmenorrhea in a total of 443 female patients revealed that naproxen provided significantly greater PR than ibuprofen at 6 hours postdose.19 More recently, COX-2 specific inhibitors have been demonstrated to be effective treatment for primary dysmenorrhea, suggesting that COX-2-derived prostanoids play a role in the pathophysiology of primary dysmenorrhea.7,13
This study was not designed to examine cost-effectiveness of valdecoxib use for primary dysmenorrhea but rather to strengthen the concept of strong analgesic efficacy via COX-2 inhibition. While no empirical research to this effect has been done, certain subgroups of patients would likely benefit from the use of valdecoxib rather than a nonspecific NSAID to treat primary dysmenorrhea. These subgroups would include patients with known GI risk factors such as active ulcer, patients being treated concomitantly with corticosteroids or coagulants, and those who take nonspecific NSAIDs chronically for other conditions. It has been observed that up to 81% of patients who were hospitalized with serious GI complications such as GI bleeding had no prior GI symptoms or warning signs,20 indicating that there is a level of risk associated with use of nonspecific NSAIDs even in patients who are not suspected to have any preexisting GI risk factors. There is evidence for slightly increased incidence of GI and operative site bleeding with 5 or fewer days of consecutive use of nonspecific NSAIDs.15 In light of this, increased risk of incidence of GI adverse events with even 1 dose of a nonspecific NSAID cannot be ruled out.
Those patients with menorrhagia who require multiple doses of anti-inflammatory medication can also avoid the potential risk of serious GI adverse events with nonspecific NSAIDs by taking a COX-2 specific inhibitor instead. This is particularly relevant to any patient who has a history of GI ulcers. It is estimated that impaired platelet aggregation is an underlying cause of menorrhagia in approximately 20% to 30% of patients.21 COX-2 specific inhibitors, in contrast to nonspecific NSAIDs, have been shown not to interfere with platelet aggregation,12,22 thus offering another potential benefit to menorrhagic patients.
COX-2 specific inhibitors have the ability to cross the blood-brain barrier and inhibit prostanoid production in the central nervous system (CNS).23,24 It has been postulated that general feelings of illness coinciding with the inflammatory response are due not to direct neural transmission of peripheral nerve impulses to the CNS, but to a signal molecule produced at the local site of inflammation, which crosses the blood-brain barrier and induces COX-2 expression in the CNS.25 Nonspecific NSAIDs are known to reduce peripheral inflammation, but it is unclear to what extent they do so in the CNS. If COX-2 specific inhibitors are more effective at reducing prostanoid production in the CNS, they may be more effective at reducing the general effects of inflammatory pain, such as fever, lethargy, and general muscle and joint pain.26
In conclusion, valdecoxib 20 mg and 40 mg doses were superior to placebo and similar to naproxen sodium 550 mg for all efficacy measures. Both doses were well tolerated and provided rapid and effective relief of menstrual pain with no significant differences between the 2 doses. Because menstrual pain tends to be transient, the rapid onset and long duration of action demonstrated in this study with valdecoxib are important characteristics for improving the management of pain associated with primary dysmenorrhea. On the basis of these results, valdecoxib appears to be a viable new therapeutic agent for the treatment of pain associated with primary dysmenorrhea.
Acknowledgments
This study was sponsored by Pfizer Incorporated and Pharmacia Corporation.
REFERENCES
- 1.Dawood MY. Nonsteroidal anti-inflammatory drugs and changing attitudes toward dysmenorrhea. Am J Med. 1988;84(suppl 5A):23–9. doi: 10.1016/0002-9343(88)90473-1. [DOI] [PubMed] [Google Scholar]
- 2.Rosenwaks Z, Seegar-Jones G. Menstrual pain: its origin and pathogenesis. J Reprod Med. 1980;25(4 suppl):207–12. [PubMed] [Google Scholar]
- 3.Masferrer JL, Zweifel BS, Manning PT, et al. Selective inhibition of inducible cyclooxygenase 2 in vivo is antiinflammatory and nonulcerogenic. Proc Natl Acad Sci USA. 1994;91:3228–32. doi: 10.1073/pnas.91.8.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.García Rodríguez LA, Jick H. Risk of upper gastrointestinal bleeding and perforation associated with individual non-steroidal anti-inflammatory drugs. Erratum in Lancet. 1994;343:1048. doi: 10.1016/s0140-6736(94)91843-0. 1994;343:769–72. [DOI] [PubMed] [Google Scholar]
- 5.Copeland RA, Williams JM, Giannaras J, et al. Mechanism of selective inhibition of the inducible isoform of prostaglandin G/H synthase. Proc Natl Acad Sci USA. 1994;91:11202–6. doi: 10.1073/pnas.91.23.11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gierse JK, McDonald JJ, Hauser SD, Rangwala SH, Koboldt CM, Seibert K. A single amino acid difference between cyclooxygenase-1 (COX-1) and -2 (COX-2) reverses the selectivity of COX-2 specific inhibitors. J Biol Chem. 1996;271:15810–4. doi: 10.1074/jbc.271.26.15810. [DOI] [PubMed] [Google Scholar]
- 7.Morrison BW, Daniels SE, Kotey P, Cantu N, Seidenberg B. Rofecoxib, a specific cyclooxygenase-2 inhibitor, in primary dysmenorrhea: a randomized controlled trial. Obstet Gynecol. 1999;94:504–8. doi: 10.1016/s0029-7844(99)00360-9. [DOI] [PubMed] [Google Scholar]
- 8.Brooks PM, Day RO. COX-2 inhibitors. New Engl J Med. 1991;324:1716–25. doi: 10.1056/NEJM199106133242407. [DOI] [PubMed] [Google Scholar]
- 9.Talley JJ, Brown DL, Carter JS, et al. 4-[5-methyl-3-phenylisoxazol-4-yl]-benzenesulfonamide, valdecoxib: a potent and selective inhibitor of COX-2. J Med Chem. 2000;43:775–7. doi: 10.1021/jm990577v. [DOI] [PubMed] [Google Scholar]
- 10.Daniels SE, Desjardins PJ, Talwalker S, Recker DP, Verburg KM. The analgesic efficacy of valdecoxib vs. oxycodone/acetaminophen after oral surgery. J Am Dent Assoc. 2002;133:611–21. doi: 10.14219/jada.archive.2002.0237. [DOI] [PubMed] [Google Scholar]
- 11.Sikes DH, Agrawal NM, Zhao WW, Kent JD, Recker DP, Verburg KM. Incidence of gastroduodenal ulcers associated with valdecoxib compared with that of ibuprofen and diclofenac in patients with osteoarthritis. Eur J Gastroenterol Hepatol. 2002;14:1101–11. doi: 10.1097/00042737-200210000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Leese PT, Talwalker S, Kent JD, Recker DP. Valdecoxib does not impair platelet function. Am J Emerg Med. 2002;20:275–81. doi: 10.1053/ajem.2002.32635. [DOI] [PubMed] [Google Scholar]
- 13.Daniels SE, Talwalker S, Torri S, Snabes MC, Recker DP, Verburg KM. Valdecoxib, a cyclooxygenase-2-specific inhibitor, is effective in treating primary dysmenorrhea. Obstet Gynecol. 2002;100:350–8. doi: 10.1016/s0029-7844(02)02085-9. [DOI] [PubMed] [Google Scholar]
- 14.Singh G, Rosen Ramey D. NSAID induced gastrointestinal complications: the ARAMIS perspective—1997. J Rheumatol. 1998;25(suppl 51):8–16. [PubMed] [Google Scholar]
- 15.Strom BL, Berlin JA, Kinman JL, et al. Parenteral ketorolac and risk of gastrointestinal and operative site bleeding. A postmarketing surveillance study. JAMA. 1996;275:376–82. [PubMed] [Google Scholar]
- 16.Goldstein JL, Kivitz AJ, Verburg KM, Recker DP, Palmer RC, Kent JD. A comparison of the upper gastrointestinal mucosal effects of valdecoxib, naproxen and placebo in healthy elderly subjects. Aliment Pharmacol Ther. 2003;18:125–32. doi: 10.1046/j.1365-2036.2003.01650.x. [DOI] [PubMed] [Google Scholar]
- 17.Hawkey CJ, Jackson L, Harper SE, Simon TJ, Mortensen E, Lines CR. Review article: the gastrointestinal safety profile of rofecoxib, a highly selective inhibitor of cyclooxygenase-2, in humans. Aliment Pharmacol Ther. 2001;15:1–9. doi: 10.1046/j.1365-2036.2001.00894.x. [DOI] [PubMed] [Google Scholar]
- 18.Ashcroft DM, Chapman SR, Clark WK, Millson DS. Upper gastroduodenal ulceration in arthritis patients treated with celecoxib. Ann Pharmacother. 2001;35:829–34. doi: 10.1345/aph.10382. [DOI] [PubMed] [Google Scholar]
- 19.Milsom I, Minic M, Dawood MY, et al. Comparison of the efficacy and safety of nonprescription doses of naproxen and naproxen sodium with ibuprofen, acetaminophen, and placebo in the treatment of primary dysmenorrhea: a pooled analysis of five studies. Clin Ther. 2002;24:1384–400. doi: 10.1016/s0149-2918(02)80043-1. [DOI] [PubMed] [Google Scholar]
- 20.Singh G, Rosen Ramey D, Morfeld D, Shi H, Hatoum HT, Fries JF. Gastrointestinal tract complications of nonsteroidal anti-inflammatory drug treatment in rheumatoid arthritis. Arch Intern Med. 1996;156:1530–6. [PubMed] [Google Scholar]
- 21.Kouides PA. Evaluation of abnormal bleeding in women. Curr Hematol Rep. 2002;1:11–8. [PubMed] [Google Scholar]
- 22.Leese PT, Recker DP, Kent JD. The COX-2 selective inhibitor, valdecoxib, does not impair platelet function in the elderly: results of a randomized controlled trial. J Clin Pharmacol. 2003;43:504–13. [PubMed] [Google Scholar]
- 23.Zhang Y, Isakson P, Rathmell J, Panchal S, Seiber K. Distribution of the COX-2 specific inhibitor valdecoxib into cerebrospinal fluid following oral administration. J Pain. 2003;4(suppl 1):A765. Abstract. [Google Scholar]
- 24.Zhang Y, Isakson P, Rathmell J, Panchal S, Seibert K. Reduction of hyperalgesia, PGE2 in paw exudates, and PGE2 in CSF after oral administration of valdecoxib or ketorolac. J Pain. 2003;4(suppl 1):A794. Abstract. [Google Scholar]
- 25.Samad TA, Sapirstein A, Woolf CJ. Prostanoids and pain: unraveling mechanisms and revealing therapeutic targets. Trends Mol Med. 2002;8:390–6. doi: 10.1016/s1471-4914(02)02383-3. [DOI] [PubMed] [Google Scholar]
- 26.Bartfai T. Telling the brain about pain. Nature. 2001;410:425–7. doi: 10.1038/35068663. [DOI] [PubMed] [Google Scholar]