Abstract
The in vitro susceptibilities to garenoxacin (BMS-284756), an investigational des-fluoroquinolone, and eight other agents were determined for 63 Mycoplasma pneumoniae, 45 Mycoplasma hominis, 15 Mycoplasma fermentans, and 68 Ureaplasma sp. isolates. Garenoxacin was the most active quinolone, inhibiting all isolates at ≤1 μg/ml. The garenoxacin MIC at which 90% of isolates are inhibited (MIC90s; ≤0.008 μg/ml) was at least 4-fold less than those of moxifloxacin and clindamycin, 8-fold less than that of sparfloxacin, and 64-fold less than those of levofloxacin and ciprofloxacin for M. pneumoniae. For M. hominis, the garenoxacin MIC90 (≤0.008 μg/ml) was 4-fold less than those of clindamycin and moxifloxacin, 8-fold less than that of sparfloxacin, and 64-fold less than those of levofloxacin and ciprofloxacin. All 15 M. fermentans isolates were inhibited by garenoxacin at concentrations ≤0.008 μg/ml, making it the most active drug tested against this organism. For Ureaplasma spp., the garenoxacin MIC90 (0.25 μg/ml) was equivalent to those of moxifloxacin and doxycycline, 4-fold less than those of levofloxacin and sparfloxacin, 8-fold less than that of azithromycin, and 32-fold less than that of ciprofloxacin. Garenoxacin and the other fluoroquinolones tested were demonstrated to have bactericidal activities against M. pneumoniae and M. hominis by measurement of minimal bactericidal activities and by time-kill studies. Further study of garenoxacin is required, as it has great potential for use in the treatment of infections due to mycoplasmas and ureaplasmas.
Garenoxacin (also known as BMS 284756 and T-3811ME) is a new des-F(6)-quinolone that differs from other quinolones approved for use in the United States in that it lacks fluorine at the C-6 position (8). It has a broad spectrum of activity against a wide array of gram-positive and gram-negative bacteria, anaerobes, and fastidious organisms such as Haemophilus influenzae, legionellae, and chlamydiae (6, 8, 18, 19). Earlier studies have also shown promising results with respect to the activity of garenoxacin against mycoplasmas and ureaplasmas (8, 21; C. M. Bébéar et al., Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., p. 179, abstr. E-725, 2001). We present here the results of the largest evaluation to date of garenoxacin against mycoplasmas and ureaplasmas obtained from humans, comparing the MIC of garenoxacin with those of other fluoroquinolones, macrolides, clindamycin, and doxycycline. The bactericidal activities of these agents for a subgroup of organisms were determined by measuring minimal bactericidal concentrations (MBCs) for a single isolate of Mycoplasma hominis and Mycoplasma pneumoniae by time-kill studies.
(This work was presented at the 102nd General Meeting of the American Society for Microbiology, Salt Lake City, Utah, May 2002.)
MATERIALS AND METHODS
Organisms.
The M. pneumoniae isolates studied included 63 strains collected from the respiratory tracts of individuals with proven respiratory disease from six different countries between 1987 and 1999. Several of the isolates were obtained from outbreaks of M. pneumoniae respiratory tract disease in the United States and have been passaged only a few times in artificial media. The Mycoplasma fermentans isolates tested (n = 15) either were clinical strains or were derived from the mycoplasma collection at the National Institutes of Health. The M. hominis isolates tested (n = 45) were derived from clinical specimens of the urogenital tract or cultures of wound specimens. Six M. hominis isolates were known to be resistant to doxycycline (MICs, ≥8 μg/ml). Ureaplasma isolates (n = 68) representing both biovars, now designated separate species (U. urealyticum and U. parvum), were derived from cultures of the urogenital tracts of adults or the lower respiratory tracts of neonates.
Antimicrobial agents.
All antimicrobial powders except doxycycline and clindamycin were obtained from their respective manufacturers. Doxycycline and clindamycin were purchased from Sigma Chemical Co., St. Louis, Mo. The following agents were tested: garenoxacin, ciprofloxacin, levofloxacin, moxifloxacin, sparfloxacin, clarithromycin, erythromycin, azithromycin, doxycycline, and clindamycin. The powders were dissolved according to the recommendations of the manufacturers. Stock solutions of each drug were prepared fresh on the day on which each assay was performed. Clindamycin was tested only against M. hominis and M. fermentans. Clarithromycin and azithromycin were tested only against M. pneumoniae and Ureaplasma spp.
MIC determination.
A broth microdilution method was performed as described previously to determine the MICs (7, 26). Serial twofold dilutions of antimicrobials in 10 B broth for Ureaplasma spp. and SP 4 broth for Mycoplasma spp. were performed. An inoculum of 104 to 105 color-changing units of each isolate tested per ml was obtained by inoculation of organisms from a frozen stock culture of known concentration into prewarmed broth and incubation for 2 h at 37°C. Following inoculation, the microtiter plates were sealed and incubated aerobically at 37°C. The plates were examined daily until a color change was detected in the drug-free growth control. The MIC was defined as the lowest concentration of a drug in which the metabolism of the organism was inhibited, as evidenced by a lack of a color change in the medium at the time that the drug-free control first showed a color change For Ureaplasma spp. growth is usually evident in the positive control after 24 h, and for M. hominis growth is usually evident in the positive control after 48 h, whereas M. pneumoniae typically requires 4 to 8 days of incubation. Quality control procedures consisted of verification of the number of color-changing units of each isolate inoculated into the MIC system by using serial dilutions and plate counts on A 8 agar for ureaplasmas and SP 4 agar for mycoplasmas (26). In addition, a well-characterized low-passage clinical isolate of each species for which the previously established MIC ranges of the comparator drugs were within ±1 twofold dilution was tested by each assay. The results were considered valid only if the MICs for the control isolates were within the previously established ranges.
MBC determination.
A subgroup of isolates consisting of 11 M. pneumoniae, 1 M. hominis, and 2 Ureaplasma sp. isolates was tested to determine the MBCs of garenoxacin in comparison to those of the other agents. MBC testing was performed directly from the microtiter plates used to determine the MICs. Aliquots (30 μl) from each well that had not changed color at the time that the MIC was read were added to 2.97 ml of the corresponding broth (1:100 dilution) to make certain that the drug is diluted below the inhibitory concentration and therefore allow any living organisms to grow to detectable levels. An aliquot from the growth control was also subcultured to ensure the presence of viable organisms in the absence of antimicrobial. Broths were incubated at 37°C for various time periods, depending on the species and the rates of growth. The MBC was defined as the concentration of antimicrobial in which no growth was detected, as evidenced by a lack of a color change in broth after prolonged incubation (for M. pneumoniae, 30 days; for M. hominis, 14 days; for Ureaplasma spp., 7 days). The MBCs of drugs whose MICs were less than or equal to the lowest concentration tested were not measured because the actual endpoint MIC, which is necessary for interpretation of the results and determination of the dilution required to reduce the concentration of antimicrobial below the inhibitory levels sufficient to permit growth in subculture, could not be determined. A bactericidal effect was identified when the MBC was one to four times the MIC.
Time-kill studies.
Garenoxacin powder was dissolved as described earlier for MIC testing, diluted, and added to each of four tubes with SP 4 broth at concentrations equivalent to one, two, four, and eight times the MICs that had previously been determined for individual isolates of M. pneumoniae and M. hominis. The final volume in the tubes of broth was 4.5 ml. The isolate of M. pneumoniae chosen was thawed and incubated aerobically at 37°C for 5 days to allow the organisms to begin active growth and reach a concentration of approximately 106 CFU/ml. The isolate of M. hominis was grown for 48 h in the same manner. A 0.5-ml volume of the M. pneumoniae broth culture was added to each of the four tubes containing the antimicrobials at the four different concentrations. A fifth tube containing 5 ml of SP 4 broth was used as a growth control to which no antimicrobial was added. A 0.5-ml volume of the M. hominis culture was added to four tubes containing 4.5 ml of SP 4 broth and antimicrobials at the different concentrations and to a fifth control tube in the same manner. All tubes were then incubated aerobically at 37°C. For M. pneumoniae, 0.1-ml samples were immediately removed from each tube; and after 24, 48, 72, 96, 120, and 144 h of incubation, each sample was vigorously mixed on a vortex mixer and then serially diluted in 0.9 ml of SP 4 broth to 10−3. A volume of 0.2 ml of each dilution was plated in duplicate on SP 4 agar. For M. hominis, subcultures were processed in a similar manner, except that samples were obtained after 0, 3, 6, 12, 24, and 48 h of incubation. Agar plates were incubated in room air plus 5% CO2 at 37°C. Colony counts were performed by using a stereomicroscope for plated dilutions yielding 30 to 300 discrete colonies as soon as growth was detectable, unless killing occurred to the extent that only smaller numbers of colonies were present in the plates with lower dilutions. The number of colonies was averaged for each time point, and the results were recorded. A bactericidal effect was defined as a reduction of ≥3 log10 CFU (99.9%) from the original inoculum.
RESULTS
MIC results.
The microtiter susceptibility testing method used for the present study allowed the excellent reproducibility of MIC results within the simultaneous duplicate runs and between assays done on different days. Summaries of the in vitro activities of garenoxacin and the other antimicrobials tested against mycoplasmas and ureaplasmas are shown in Table 1. Garenoxacin was the most active quinolone tested, with the MICs for all isolates being ≤1 μg/ml. The garenoxacin MIC at which 90% of isolates are inhibited (MIC90) for M. pneumoniae (0.031 μg/ml) was 4-fold less than that of moxifloxacin (0.125 μg/ml), 8-fold less than that of sparfloxacin (0.25 μg/ml), 16-fold less than that of doxycycline (0.5 μg/ml), 32-fold less than that of levofloxacin (1 μg/ml), and 64-fold less than that of ciprofloxacin (2 μg/ml). Azithromycin and clarithromycin showed the greatest in vitro activities, inhibiting all 63 M. pneumoniae isolates at ≤0.004 μg/ml (MIC90s, ≤0.001 μg/ml).
TABLE 1.
Broth microdilution MICs of garenoxacin and the other antimicrobial agents tested for mycoplasmas and ureaplasmas
| Organism and drug | MIC (μg/ml)
|
||
|---|---|---|---|
| Range | 50% | 90% | |
| M. pneumoniae (n = 63) | |||
| Garenoxacin | 0.008-0.031 | 0.016 | 0.031 |
| Ciprofloxacin | 0.5-4.0 | 2.0 | 2.0 |
| Levofloxacin | 0.063-2.0 | 0.5 | 1.0 |
| Sparfloxacin | 0.031-0.25 | 0.125 | 0.25 |
| Moxifloxacin | 0.016-0.125 | 0.063 | 0.125 |
| Azithromycin | ≤0.001 | ≤0.001 | ≤0.001 |
| Clarithromycin | ≤0.001-0.004 | ≤0.001 | ≤0.001 |
| Doxycycline | 0.016-0.5 | 0.25 | 0.5 |
| M. fermentans (n = 15) | |||
| Garenoxacin | ≤0.008 | ≤0.008 | ≤0.008 |
| Ciprofloxacin | ≤0.008-0.063 | 0.031 | 0.063 |
| Levofloxacin | ≤0.008-0.063 | 0.031 | 0.063 |
| Sparfloxacin | ≤0.008-0.25 | ≤0.008 | 0.25 |
| Moxifloxacin | ≤0.008-0.016 | ≤0.008 | 0.016 |
| Doxycycline | ≤0.008-1.0 | 0.063 | 1.0 |
| Clindamycin | ≤0.008-0.063 | 0.016 | 0.031 |
| M. hominis (n = 45) | |||
| Garenoxacin | ≤0.008-0.063 | ≤0.008 | ≤0.008 |
| Ciprofloxacin | 0.016-2.0 | 0.25 | 0.5 |
| Levofloxacin | 0.016-2.0 | 0.125 | 0.5 |
| Sparfloxacin | ≤0.008-0.125 | 0.016 | 0.063 |
| Moxifloxacin | ≤0.008-0.063 | 0.016 | 0.031 |
| Doxycycline | ≤0.008-32 | 1.0 | 8.0 |
| Clindamycin | ≤0.008-0.125 | 0.016 | 0.031 |
| Ureaplasma spp. (n = 68) | |||
| Garenoxacin | 0.016-1.0 | 0.063 | 0.25 |
| Ciprofloxacin | 1.0-16.0 | 4.0 | 8.0 |
| Levofloxacin | 0.25-2.0 | 0.5 | 1.0 |
| Sparfloxacin | 0.063-2.0 | 0.5 | 1.0 |
| Moxifloxacin | 0.031-0.5 | 0.125 | 0.25 |
| Doxycycline | 0.016-0.5 | 0.125 | 0.25 |
| Azithromcyin | 0.125-4.0 | 1.0 | 2.0 |
| Clarithromcycin | ≤0.008-0.5 | 0.063 | 0.125 |
| Erythromycin | 0.125-2.0 | 0.5 | 1.0 |
All 15 isolates of M. fermentans were inhibited by garenoxacin at concentrations ≤0.008 μg/ml, making it the most active drug tested against this organism. The garenoxacin MIC90 (≤0.008 μg/ml) was at least 2-fold less than that of moxifloxacin (0.016 μg/ml), 4-fold less than that of clindamycin (0.031 μg/ml), 8-fold less than that of sparfloxacin (0.063 μg/ml), and 64-fold less than those of levofloxacin and ciprofloxacin (0.5 μg/ml).
All isolates of M. hominis were inhibited by garenoxacin at concentrations ≤0.063 μg/ml, making it the most active drug tested against this organism. The garenoxacin MIC90 (≤0.008 μg/ml) was at least 4-fold less than those of moxifloxacin and clindamycin (0.031 μg/ml), 8-fold less than that of sparfloxacin (0.063 μg/ml), and 64-fold less than those of levofloxacin and ciprofloxacin (0.5 μg/ml). Garenoxacin and the other quinolones tested were as active against doxycycline-resistant M. hominis isolates as they were against doxycycline-susceptible isolates.
In general, quinolones were somewhat less active against Ureaplasma spp. than against Mycoplasma spp. The garenoxacin MIC90 (0.25 μg/ml) was equivalent to those of moxifloxacin and doxycycline, 4-fold less than those of levofloxacin and sparfloxacin (1.0 μg/ml), 8-fold less than that of azithromycin (2 μg/ml), and 32-fold less than that of ciprofloxacin (8 μg/ml). The only agent more active than garenoxacin against Ureaplasma spp. was clarithromycin (MIC90, 0.125 μg/ml).
Table 2 shows the MIC90s of garenoxacin in relation to the published ranges of MIC90s of nine fluoroquinolones that represent the most widely used agents of this class approved for use in the United States and an investigational agent, gemifloxacin. On the basis of the MIC90s, garenoxacin was the most active quinolone tested against mycoplasmas and ureaplasmas of human origin.
TABLE 2.
In vitro activities of quinolones against mycoplasmas and ureaplasmas
| Organism and drug | MIC90 (μg/ml)
|
References | |
|---|---|---|---|
| Present study | Range from previous studiesa | ||
| M. pneumoniae | |||
| Garenoxacin | 0.031 | 0.031-0.06 | 8, 21; Bébéar et al., 41st ICAAC |
| Ciprofloxacin | 2 | 1-5 | 1, 2, 4, 8, 9, 15, 21, 24; Bébéar et al., 41st ICAAC |
| Ofloxacin | Not done | 1-2 | 2, 3, 4, 8, 15; Bébéar et al., 41st ICAAC |
| Levofloxacin | 1 | 0.5-2.5 | 3, 7, 8, 9, 17, 21; Bébéar et al., 41st ICAAC |
| Sparfloxacin | 0.25 | 0.125-1.25 | 1, 2, 4, 9, 15, 16, 17, 24 |
| Trovafloxacin | Not done | 0.25-0.5 | 4, 7, 8, 16, 17, 21 |
| Grepafloxacin | Not done | 0.125 | 2, 7 |
| Gatifloxacin | Not done | 0.125-0.25 | 21; Bébéar et al., 41st ICAAC |
| Moxifloxacin | 0.125 | 0.12-0.3 | 1, 8, 9, 17, 21; Bébéar et al., 41st ICAAC |
| Gemifloxacin | Not done | 0.125-0.25 | 7, 21 |
| M. hominis | |||
| Garenoxacin | ≤0.008 | ≤0.015 | 8, 21; Bébéar et al., 41st ICAAC |
| Ciprofloxacin | 0.5 | 0.5-4 | 2, 4, 8, 12, 17, 20, 22, 24, 25; Bébéar et al., 41st ICAAC |
| Ofloxacin | Not done | 0.5-8 | 2, 3, 4, 8, 14, 20, 22; Bébéar et al., 41st ICAAC |
| Levofloxacin | 0.5 | 0.25-2 | 3, 7, 8, 17, 22, 25; Bébéar et al., 41st ICAAC |
| Sparfloxacin | 0.063 | 0.03-0.063 | 1, 2, 4, 14, 16, 17, 20, 24, 25 |
| Trovafloxacin | Not done | 0.031-0.06 | 2, 4, 7, 8, 16, 17, 25 |
| Grepafloxacin | Not done | 0.016-0.25 | 2, 7, 17 |
| Gatifloxacin | Not done | 0.12-0.5 | 17; Bébéar et al., 41st ICAAC |
| Moxifloxacin | 0.031 | 0.06-0.031 | 1, 8, 17, 25; Bébéar et al., 41st ICAAC |
| Gemifloxacin | Not done | ≤0.008 | 7 |
| Ureaplasma spp. | |||
| Garenoxacin | 0.25 | 0.25 | 8, 21; Bébéar et al., 41st ICAAC |
| Ciprofloxacin | 8 | 4-16 | 2, 4, 12, 8, 20, 22, 24; Bébéar et al., 41st ICAAC |
| Ofloxacin | Not done | 2-4 | 2, 3, 4, 8, 14, 17, 20, 22; Bébéar et al., 41st ICAAC |
| Levofloxacin | 1 | 0.5-4 | 3, 78, 17, 22; Bébéar et al., 41st ICAAC |
| Sparfloxacin | 1 | 0.25-1 | 1, 2, 4, 14, 16, 17, 20, 24 |
| Trovafloxacin | Not done | 0.125-0.5 | 4, 7, 8, 16 |
| Grepafloxacin | Not done | 1 | 2, 7 |
| Gatifloxacin | Not done | 0.5-1 | 17; Bébéar et al., 41st ICAAC |
| Moxifloxacin | 0.25 | 0.25-0.5 | 1, 8, 17; Bébéar et al., 41st ICAAC |
| Gemifloxacin | Not done | 0.25 | 7 |
Data are limited to those for M. pneumoniae, M. hominis, and Ureaplasma spp. MICs were determined by agar- or broth-based methods in the cited references.
MBC results.
Table 3 summarizes the MBC data for 11 isolates of M. pneumoniae. Among the five quinolones tested, the antimicrobial with MBCs fourfold or less higher than the corresponding MICs for the largest number of isolates (10 of 11 isolates) was moxifloxacin, followed by garenoxacin (9 of 11 isolates), levofloxacin (7 of 11 isolates), ciprofloxacin (6 of 11 isolates), and sparfloxacin (4 of 11 isolates). Doxycycline showed a bacteriostatic effect against M. pneumoniae, with the MBCs for all isolates being eight or more times the MIC. No data on the MBCs of clarithromycin and azithromycin for M. pneumoniae were available because the MICs for all isolates were ≤0.001 μg/ml, the lowest concentration tested.
TABLE 3.
MBCs of garenoxacin and other antimicrobial agents for 11 isolates of M. pneumoniae
| Drug | No. of isolates for which MBC was:
|
|||
|---|---|---|---|---|
| 1× MIC | 2× MIC | 4× MIC | ≥8× MIC | |
| Garenoxacin | 0 | 4 | 5 | 2 |
| Ciprofloxacin | 0 | 1 | 5 | 5 |
| Levofloxacin | 0 | 3 | 4 | 4 |
| Sparfloxacin | 0 | 1 | 3 | 7 |
| Moxifloxacin | 0 | 7 | 3 | 1 |
| Doxycycline | 0 | 0 | 0 | 11 |
For two isolates of M. hominis tested, the MBCs of ciprofloxacin, levofloxacin, moxifloxacin, and sparfloxacin were one- to fourfold the MICs. The MBCs of garenoxacin were two- and eightfold higher than the corresponding MICs. The MBCs of doxycycline and clindamycin were ≥16 times the MICs for M. hominis. The MBCs of all drugs tested for two isolates of Ureaplasma spp. were ≥16 times the MICs, except that the MBC of ciprofloxacin for a single isolate was 2 times the MIC. The MBCs for M. fermentans were not determined since the MICs of garenoxacin for all 15 isolates were ≤0.008 μg/ml, the lowest concentration tested.
Time-kill assay results.
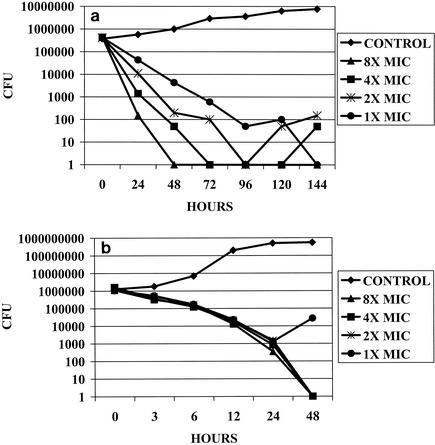
Data for the time-kill assays for the single M. pneumoniae and M. hominis isolates tested are shown in Fig. 1a and b, respectively. Garenoxacin demonstrated a bactericidal effect against M. pneumoniae at eight times the MIC after 24 h of incubation; at two, four, and eight times the MIC after 48 h of incubation; and at all concentrations tested after 96 h of incubation. After 120 and 144 h of incubation, regrowth of ≤2 log10 CFU was observed in the presence of some of the lower antimicrobial concentrations tested. A bactericidal effect against M. hominis was demonstrated in the presence of four to eight times the MIC after 24 h of incubation and two times the MIC after 48 h of incubation. Regrowth of 1 log10 CFU was observed after 48 h of incubation in the presence of the concentration equal to the MIC.
FIG. 1.
(a) Time-kill graph for an isolate of M. pneumoniae (MIC, 0.032 μg/ml) against which the activities of garenoxacin at concentrations one to eight times the MIC were tested. Bactericidal effects were demonstrated at eight times the MIC after 24 h of incubation; at two, four, and eight times the MIC after 48 h of incubation; and at all concentrations after 96 h of incubation. Regrowth of ≤2 log10 CFU was observed in the presence of some of the lower concentrations of antimicrobials after 120 and 144 h of incubation. (b) Time-kill graph for an isolate of M. hominis (MIC, 0.008 μg/ml) against which the activities of garenoxacin at concentrations one to eight times the MIC were tested. Bactericidal effects were demonstrated at four and eight times the MIC after 24 h of incubation; and bactericidal effects were demonstrated at two, four, and eight times the MIC after 48 h of incubation. Regrowth of 1 log10 CFU was observed after 48 h of incubation at the concentration equal to the MIC.
DISCUSSION
Quinolones have become important therapeutic alternatives for treatment for adults with community-acquired respiratory diseases as well as urogenital infections due to a variety of microorganisms. The advantages of the newer agents in this class include a broad spectrum of antimicrobial activity, long half-lives that allow once-daily dosing, generally good tolerability with few side effects for most agents, and the availability of most agents in both oral and parenteral formulations. An additional attractive feature for this growing class of antimicrobials that has contributed to its popularity includes the preservation of antibacterial action in the presence of genetic factors in various bacteria that confer resistance to other drug classes, including tetracyclines, macrolides, and beta-lactams.
Mycoplasmas and ureaplasmas may cause a variety of clinical infections, predominantly in the respiratory and/or urogenital tract. M. pneumoniae is a primary pathogen of the respiratory tract, commonly causing tracheobronchitis and as many as 20 to 50% of cases of community-acquired pneumonia (27). M. hominis, Ureaplasma spp., and the less common organism M. fermentans are generally considered opportunistic pathogens that can cause systemic disease, especially in the setting of impaired host immunity (27). Despite the frequency with which mycoplasmas and ureaplasmas may be causal or associated with human diseases, their presence in clinical specimens is seldom documented directly because of the limited availability of culture-based tests, the slow growth of organisms such as M. pneumoniae, and widespread reliance on indirect means of detection such as serology in the case of M. pneumoniae. A lack of rapid and reliable laboratory tests for detection of mycoplasmas and other atypical pathogens such as chlamydiae and legionellae, coupled with increasing drug resistance in other respiratory pathogens such as Streptococcus pneumoniae, has been responsible to a great extent for the increasing popularity of the newer quinolones for empirical treatment of a variety of infections that may be due to any of these types of organisms. Thus, the in vitro activities of quinolones against mycoplasmas and ureaplasmas have been the subject of numerous studies over the past several years, many of which have evaluated the same drugs by agar- and/or broth-based techniques (1-5, 7-17, 20-25).
We have evaluated the activities of garenoxacin against a large number of well-characterized clinical isolates of mycoplasmas and ureaplasmas and have shown that it is the most active quinolone tested against this group of microorganisms, results that were also apparent in three other in vitro studies that included smaller numbers of clinical isolates (8, 21; Bébéar et al., 41st ICAAC). When our data are considered in relation to the in vitro results of previously published studies that encompassed all of the newer fluoroquinolones that have enhanced potencies against gram-positive bacteria, as well as older agents such as ciprofloxacin and ofloxacin, garenoxacin is still the most active quinolone in vitro.
Prior to the availability of quinolones, treatment of mycoplasmal and ureaplasmal infections was limited to drugs such as macrolides, lincosamides, and tetracyclines that are primarily bacteriostatic. Use of bactericidal agents may be beneficial in some cases, especially in the setting of impaired host defenses, making potentially bactericidal quinolones the focus of considerable interest with respect to their in vitro activities against mycoplasmas and ureaplasmas. Previous investigations have assessed the bactericidal activities of quinolones against mycoplasmas and ureaplasmas and have shown that the newer agents have bactericidal effects and enhanced potencies according to their MICs, even though the numbers of isolates tested has been very small (1-4, 13) and the actual multiples of the MICs necessary to achieve the MBCs were variable. We were able to demonstrate that garenoxacin has cidal activities, with MBCs one to four times the MICs for several isolates of M. pneumoniae and one isolate of M. hominis, with complete eradication of viable organisms, as detected by a lack of a color change in broth. However, with the exception of a single test with ciprofloxacin, we were unable to demonstrate consistently an MBC within eight times the MIC of any of the quinolones tested for Ureaplasma spp. This is in contrast to other reports (1-4; Bébéar et al., 41st ICAAC). We are not certain why this apparent discrepancy occurred, but it may have to do with how the test was performed and, specifically, when the subcultures from the MIC systems were obtained for determination of MBCs. The rapid growth and subsequent death of ureaplasmas in liquid culture make tests of this nature very complex and difficult to interpret. It is also noteworthy that only two different Ureaplasma isolates were tested in our study. The studies of Bébéar et al. (1-4; Bébéar et al., 41st ICAAC) each involved one to five isolates.
In addition to determining the MBCs for a subgroup of organisms, we sought to evaluate the dynamics of killing by garenoxacin of a representative isolate each of M. pneumoniae and M. hominis, since the MBCs had shown that garenoxacin had bactericidal effects against these organisms. Due to the slower growth rate of mycoplasmas, especially M. pneumoniae, with a generation time of 6 h, the usual 24-h duration of time-kill studies had to be lengthened in order to demonstrate an effect. We were able to demonstrate that garenoxacin has concentration-dependent bactericidal activity against M. pneumoniae after 24 to 96 h of incubation. Garenoxacin also demonstrated bactericidal activity against M. hominis after 24 h of incubation at four to eight times the MIC and after 48 h of incubation at two times the MIC. The regrowth of ≤2 log10 CFU observed in the presence of some of the lower concentrations of garenoxacin could have been due to a very small population of viable organisms that survived and that were allowed to propagate over time, perhaps aided by the degradation and inactivation of garenoxacin after prolonged incubation for several days in the case of M. pneumoniae. This is the first demonstration of the bactericidal effects of an antimicrobial agent against M. pneumoniae by time-kill studies modified from those commonly used to evaluate the effects of antimicrobials against other bacteria. The present study has shown that garenoxacin is a promising drug for the treatment of infections caused by Mycoplasma and Ureaplasma species. Further clinical evaluation should be pursued.
Acknowledgments
This work was supported by a grant from Bristol-Myers Squibb Co., Wallingford, Conn.
REFERENCES
- 1.Bébéar, C. M., H. Renaudin, A. Boudjadja, and C. Bébéar. 1998. In vitro activity of BAY 12-8039, a new fluoroquinolone, against mycoplasmas. Antimicrob. Agents Chemother. 42:703-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bébéar, C. M., H. Renaudin, T. Shaeverbeke, F. LeBlanc, and C. Bébéar. 1999. In vitro activity of grepafloxacin, a new fluoroquinolone, against mycoplasmas. J. Antimicrob. Chemother. 43:711-714. [DOI] [PubMed] [Google Scholar]
- 3.Bébéar, C. M., H. Renaudin, A. Bryskier, and C. Bébéar. 2000. Comparative activities of telithromycin (HMR 3647), levofloxacin, and other antimicrobial agents against human mycoplasmas. Antimicrob. Agents Chemother. 44:1980-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bébéar, C. M., H. Renaudin, A. Charron, D. Gruson, M. Lefrancois, and C. Bébéar. 2000. In vitro activity of trovafloxacin compared to those of five antimicrobials against mycoplasmas including Mycoplasma hominis and Ureaplasma urealyticum fluoroquinolone-resistant isolates that have been genetically characterized. Antimicrob. Agents Chemother. 44:2557-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen, M. A., and M. D. Huband. 1997. In vitro susceptibilities of Mycoplasma pneumoniae, Mycoplasma hominis and Ureaplasma urealyticum to clinafloxacin, PD 131628, ciprofloxacin and comparator drugs. J. Antimicrob. Chemother. 40:308-309. [DOI] [PubMed] [Google Scholar]
- 6.Dubois, J., and C. St-Pierre. 2001. In vitro susceptibility of BMS-284756 against Legionella species. Diagn. Microbiol. Infect. Dis. 41:79-82. [DOI] [PubMed] [Google Scholar]
- 7.Duffy, L. B., D. Crabb, K. Searcey, and M. J. Kempf. 2000. Comparative potency of gemifloxacin, new quinolones, macrolides, tetracycline and clindamycin against Mycoplasma spp. J. Antimicrob. Chemother. 45(Suppl. S1):29-33. [DOI] [PubMed] [Google Scholar]
- 8.Fung-Tomc, J. C., B. Minassian, B. Kolem, E. Huczko, L. Aleksunes, T. Stickle, T. Washo E. Gradelski, L. Velera, and D. Bonner. 2000. Antibacterial spectrum of a novel des-fluoro(6) quinolone, BMS-284756. Antimicrob. Agents Chemother. 44:3351-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamamoto, K., T. Shimizu, N. Fujimoto, Y. Zhang, and S. Arai. 2001. In vitro activities of moxifloxacin and other fluoroquinolones against Mycoplasma pneumoniae. Antimicrob. Agents Chemother. 45:1908-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hannan, P. C. T., and G. Woodnutt. 2000. In vitro activity of gemifloxacin (SB 265805;LB20304a) against human mycoplasmas. J. Antimicrob. Chemother. 45:367-369. [DOI] [PubMed] [Google Scholar]
- 11.Kaku, M., K. Ishida, K. Irifune, R. Mizukane, H. Takemura, R. Yoshida, H. Tanaka, T. Usui, K. Tomono, N. Suyama, H. Koga, S. Kohno, and K. Hara. 1994. In vitro and in vivo activities of sparfloxacin against Mycoplasma pneumoniae. Antimicrob. Agents Chemother. 38:738-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kenny, G. E., T. M. Hooton, M. C. Roberts, F. D. Cartwright, and J. Hoyt. 1989. Susceptibilities of genital mycoplasmas to the newer quinolones as determined by the agar dilution method. Antimicrob. Agents Chemother. 33:103-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kenny, G. E., and F. D. Cartwright. 1990. Mycoplasmacidal activity of quinolones on genital mycoplasmas. Zentbl. Bakteriol. Parasitenkd. Infektkrankh. Hyg. Abt. 1 Orig. Suppl. 20:98-102. [Google Scholar]
- 14.Kenny, G. E., and F. D. Cartwright. 1991. Susceptibilities of Mycoplasma hominis and Ureaplasma urealyticum to two new quinolones, sparfloxacin and WIN 57273. Antimicrob. Agents Chemother. 35:1515-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kenny, G. E., and F. D. Cartwright. 1991. Susceptibility of Mycoplasma pneumoniae to several new quinolones, tetracycline, and erythromycin. Antimicrob. Agents Chemother. 35:587-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kenny, G. E., and F. D. Cartwright. 1996. Susceptibilities of Mycoplasma pneumoniae, Mycoplasma hominis, and Ureaplasma urealyticum to a new quinolone, trovafloxacin (CP-99,219). Antimicrob. Agents Chemother. 40:1048-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kenny, G. E., and F. D. Cartwright. 2001. Susceptibilities of Mycoplasma hominis, M. pneumoniae, and Ureaplasma urealyticum to GAR-936, dalfopristin, dirithromycin, evernimicin, gatifloxacin, linezolid, moxifloxacin, quinupristin-dalfopristin, and telithromycin compared to their susceptibilities to reference macrolides, tetracyclines, and quinolones. Antimicrob. Agents Chemother. 45:2604-2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malay, S., P. M. Roblin, T. Reznik, A. Kutin, and M. R. Hammerschlag. 2002. In vitro activities of BMS-284756 against Chlamydia trachomatis and recent clinical isolates of Chlamydia pneumoniae. Antimicrob. Agents Chemother. 46:517-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pankuch, G. A., K. Nagai, T. A. Davies, M. R. Jacobs, and P. C. Appelbaum. 2002. Antipneumococcal activity of BMS 284756 compared to those of six other agents. Antimicrob. Agents Chemother. 46:251-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perea, E. J., J. Aznar, M. C. Garcia-Iglesias, and A. Pascual. 1996. Comparative in-vitro activity of sparfloxacin against genital pathogens. J. Antimicrob. Chemother. 37(Suppl. A):19-25. [DOI] [PubMed] [Google Scholar]
- 21.Takahata, M., M. Shimakura, R. Hori, K. Kizawa, Y. Todo, S. Minami, Y. Watanabe, and H. Narita. 2001. In vitro and in vivo efficacies of T-3811ME (BMS-284756) against Mycoplasma pneumoniae. Antimicrob. Agents Chemother. 45:312-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ullmann, U., S. Schubert, and R. Krausse. 1999. Comparative in vitro activity of levofloxacin, other fluoroquinolones, doxycycline, and erythromycin against Ureaplasma urealyticum and Mycoplasma hominis. J. Antimicrob. Chemother. 43(Suppl. C):33-36. [DOI] [PubMed] [Google Scholar]
- 23.Waites, K. B., G. H. Casssell, K. C. Canupp, and P. B. Fernandes. 1988. In vitro susceptibilities of mycoplasmas and ureaplasmas to new macrolides and aryl-fluoroquinolones. Antimicrob. Agents Chemother. 32:1500-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waites, K. B., L. B. Duffy, T. Schmid, D. Crabb, M. S. Pate, and G. H. Cassell. 1991. In vitro susceptibilities of Mycoplasma pneumoniae, Mycoplasma hominis, and Ureaplasma urealyticum to sparfloxacin and PD 127391. Antimicrob. Agents Chemother. 35:1181-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waites, K. B., K. C. Canupp, and G. E. Kenny. 1999. In vitro susceptibilities of Mycoplasma hominis to six fluoroquinolones as determined by E test. Antimicrob. Agents Chemother. 43:2571-2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waites, K. B., C. M. Bébéar, J. A. Robertson, D. F. Talkington, and G. E. Kenny. 2001. Cumitech 34, Laboratory diagnosis of mycoplasmal infections. Coordinating ed., F. S. Nolte. American Society for Microbiology, Washington, D.C.
- 27.Waites, K. B., and D. Taylor-Robinson. 1999. Mycoplasma and Ureaplasma, p. 782-794. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. American Society for Microbiology, Washington, D.C.