Abstract
The aim of this study was to develop a simple, field-practical, and effective in vitro method for determining the sensitivity of fresh erythrocytic Plasmodium vivax isolates to a range of antimalarials. The method used is a modification of the standard World Health Organization (WHO) microtest for determination of P. falciparum drug sensitivity. The WHO method was modified by removing leukocytes and using a growth medium supplemented with AB+ serum. We successfully carried out 34 in vitro drug assays on 39 P. vivax isolates collected from the Mae Sod malaria clinic, Tak Province, Thailand. The mean percentage of parasites maturing to schizonts (six or more merozoites) in control wells was 66.5% ± 5.9% (standard deviation). This level of growth in the control wells enabled rapid microscopic determination (5 min per isolate per drug) of the MICs of chloroquine, dihydroartemisinin, WR238605 (tafenoquine), and sulfadoxine. P. vivax was relatively sensitive to chloroquine (MIC = 160 ng/ml, 50% inhibitory concentration [IC50] = 49.8 ng/ml) and dihydroartemisinin (MIC = 0.5 ng/ml, IC50 = 0.47 ng/ml). The poor response of P. vivax to both tafenoquine (MIC = 14,000 ng/ml, IC50 = 9,739 ng/ml) and sulfadoxine (MIC = 500,000 ng/ml, IC50 = 249,000 ng/ml) was due to the slow action of these drugs and the innate resistance of P. vivax to sulfadoxine. The in vitro assay developed in our study should be useful both for assessing the antimalarial sensitivity of P. vivax populations and for screening new antimalarials in the absence of long-term P. vivax cultures.
Each year, Plasmodium vivax is responsible for approximately 70 to 80 million cases of malaria worldwide (7). Although P. vivax causes less mortality than P. falciparum, it still causes significant worldwide mortality. Antimalarial drug resistance in P. vivax was first reported in 1989, some 30 years after chloroquine resistance was reported in P. falciparum (11).
Simple in vitro susceptibility tests for P. falciparum have been available since 1968 (9). However, the development of similar in vitro susceptibility tests for P. vivax has been problematic because of the poor in vitro growth of P. vivax. In the absence of an in vitro test for determining the susceptibility of P. vivax to antimalarials, investigators have been limited to in vivo protocols. Although in vivo protocols are a direct measure of the clinical efficacy of antimalarials, they are often difficult to carry out and subject to individual variations in patient immune status. In vitro tests provide a valuable adjunct to in vivo drug susceptibility tests, especially when comparing temporal and spatial variations in Plasmodium sp. susceptibility to antimalarials.
Recent advances in in vitro culture techniques (i.e., specially modified and serum-enriched media) have enabled the short-term culture of P. vivax (3). The aim of this study was to develop a simple, practical, and effective in vitro method for determining the sensitivity of fresh erythrocytic P. vivax isolates to a range of antimalarials. All of the antimalarials tested in this study, except WR238605 (tafenoquine), specifically target the erythrocytic stages of Plasmodium sp. WR238605 is a long-acting 8-aminoquinoline related to primaquine and is active against all stages of Plasmodium sp.
MATERIALS AND METHODS
Predosed drug plates.
Chloroquine phosphate was purchased from Sterling-Winthrop (Sydney, Australia). The antifolate sulfadoxine (fanasil) was obtained from Hoffmann-La Roche (Basel, Switzerland). The Walter Reed Army Institute of Research (Washington, D.C.) supplied WR238605 (tafenoquine) and dihydroartemisinin. A stock solution of each drug was prepared in methanol in glass vials coated with a 0.2% (vol/vol) AquaSil water solution (Pierce) to prevent the drug from binding to the glass surface. One or two dilutions of each drug were subsequently made in methanol to obtain the drug concentration desired for testing. Twenty microliters of the final drug solution was added to each well of a 96-well microculture plate (Nunc), and twofold serial dilutions were made in methanol. The charged plates were dried in an incubator at 37°C overnight, covered with Plate Sealer (Linbro), and stored at 4°C. Randomly selected, predosed plates were checked against the K1 P. falciparum strain before and after the study to assess drug activity.
Parasites.
Plasmodium sp. isolates were collected from malaria patients reporting to the Mae Sod (Thailand) government malaria clinic and to Australian Army health service facilities in East Timor and Australia. All patients were briefed on the project and provided informed consent prior to collection of blood by venipuncture. Blood was collected in sodium or lithium heparin collection tubes. The chloroquine- and pyrimethamine-resistant K1 strain of P. falciparum used in this study is maintained in continuous culture at the Australian Army Malaria Institute (Brisbane).
Drug assay.
The drug assay method used was based on the standard World Health Organization microtest (10) for determination of P. falciparum drug sensitivity. This methodology was modified by removing leukocytes from the malaria-infected blood sample and using a growth medium supplemented with AB+ human serum. Details of this modification are given below.
Prior to the start of the assay, a 10-ml syringe tipped with a small quantity of glass wool was filled with 5 ml of CF11 cellulose powder (Whatman), covered with foil, and then autoclaved. When ready to use, the CF11 column was wetted with 4 ml of a phosphate-buffered saline (PBS) solution (Polysciences, Inc.). Two milliliters of whole blood parasitized with 1,000 to 50,000 ring stage P. vivax parasites per μl was gently mixed with 2 ml of PBS and added slowly to the CF11 column. The blood was then washed through the column by the addition of approximately 5 ml of PBS. When the blood dripping into the collection tube from the column started to turn clear, the collection tube was centrifuged at 200 × g for 5 min. The supernatant was removed, the pellet was resuspended in 5 ml of RPMI medium (Gibco), and the mixture was centrifuged at 200 × g for 5 min. The supernatant was removed, and the pellet was resuspended to a hematocrit of 40% in AB+ human serum. One milliliter of this blood-serum mixture was added to 9 ml of McCoy's 5A medium (Gibco) containing 20% AB+ human serum. Fifty microliters of this blood-medium mixture (4% hematocrit) was added to each well of the predosed drug plates. Drug plates containing the blood-medium mixture were incubated in a gas chamber containing 5% CO2, 5% O2, and 90% N2 at 37.5°C, until ≥50% of the ring stage parasites had matured to schizonts (24 to 36 h). After incubation, the plates were allowed to stand for 30 min in a semivertical position. The supernatant was then removed, erythrocytes were resuspended in the remaining fluid, and a thick blood film was made from each well. Blood films were then subjected to Romanowsky staining and examined by oil immersion microscopy (magnification, ×1,000). The number of normal schizonts per 200 asexual stage parasites was determined in each blood film. Results were calculated as follows: % control = (number of schizonts/treated well)/number of schizonts in control wells.
The MIC of drug was the minimum concentration at which more than 99% of the parasites, relative to the control, were inhibited from developing to schizonts (parasites with six or more chromatin dots). Degenerate schizonts (normal appearance except that the chromatin dot appears smudged and out of shape) were not counted. The dose-response data were analyzed by nonlinear regression analysis (Table Curve software; Jandel Scientific) to obtain the 50% inhibitory concentrations (IC50s) (geometric means). The experiment was repeated twice for each isolate.
RESULTS
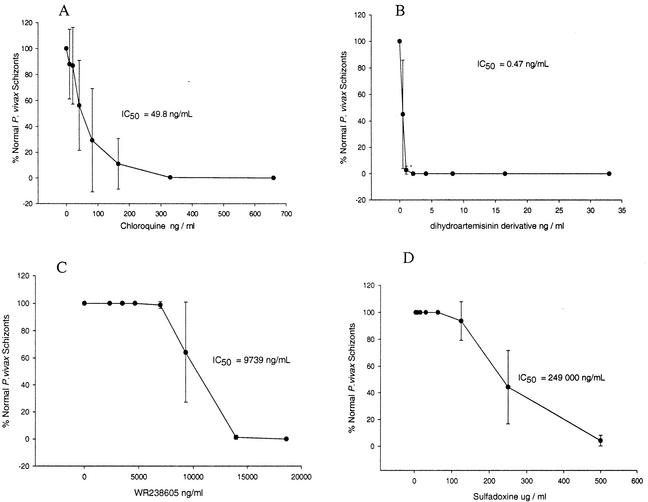
Eight-seven percent (34 of 39) of the P. vivax isolates collected at the Mae Sod malaria clinic were successfully evaluated. A mean of 66.5% (standard deviation = 5.9%) ring stage parasites in the control wells developed into schizonts. This level of development allowed rapid (5 min per isolate per drug) microscopic determination of the MICs of chloroquine, dihydroartemisinin, tafenoquine, and sulfadoxine (Table 1). The MIC of artemisinin primarily affected ring stage parasites. The small number of parasites that progressed to the pigmented trophozoite stage showed extensive vacuolation of the cytoplasm. The sensitivity of P. vivax field isolates from Mae Sod to dihydroartemisinin can be seen in Fig. 1B (IC50 = 0.47 ng/ml). MICs of chloroquine, WR238605, and sulfadoxine halted the growth of P. vivax at the pigmented trophozoite and late amoeboid stages. Abnormal schizonts were present at all concentrations of WR238605 and sulfadoxine except the highest. Abnormal schizonts had chromatin dots that were irregular in shape and size and contained large quantities of hemozoin pigment in a patchy, poorly staining cytoplasm. The relatively low activity of chloroquine (IC50 = 49.8 ng/ml) and the very low activities of WR238605 (IC50 = 9,739 ng/ml) and sulfadoxine (IC50 = 249,000 ng/ml) are presented in Fig. 1A, C, and D, respectively. Quality control checks of the predosed drug plates before and 1 month after the study revealed no significant loss of antimalarial activity against the K1 strain of P. falciparum.
TABLE 1.
Median MICs of various antimalarial compounds obtained from field isolates of P. vivax and P. falciparum from the Mae Sod malaria clinic and from other isolates of P. vivax and P. falciparum and in vivo concentrations of the antimalarials tested
| Antimalarial test agent | MIC99 (ng/ml)
|
Mean maximum in vivo concn (ng/ml) | |
|---|---|---|---|
| P. vivax | P. falciparum | ||
| Chloroquine | 160,a 330c | 660,b 660,d 40e | 141 (whole blood) (25 mg of base/kg in three doses over a 48-h period [1]) |
| Dihydroartemisinin | 0.5a | 4.0,b 1.0d | 100 (plasma) (K. Rieckmann and B. Kotecka, unpublished data) |
| WR238605 | 14,000a | 5,000,b 7,000,d 14,000f | 1,200 (whole blood) (M. D. Edstein, unpublished data) |
| Sulfadoxine | 500,000a | 500,0000,d 30,000e | 150,000 (plasma) (K. Rieckmann, unpublished data) |
Isolates from Mae Sod malaria clinic patients successfully treated with chloroquine (n = 34).
Isolates from Mae Sod malaria clinic patients (n = 5).
Isolates from Australian Army malaria patients deployed in Papua New Guinea who were not be cured by chloroquine (K. Rieckmann and B. Kotecka, unpublished data) (n = 6).
K1 strain of P. falciparum (resistant to chloroquine and sulfadoxine).
FC27 strain of P. falciparum (sensitive to chloroquine and sulfadoxine).
Isolates from East Timor (Russell et al., unpublished data).
FIG. 1.
In vitro responses of P. vivax field isolates (n = 34) to various concentrations of chloroquine (A), dihydroartemisinin (B), WR238605 (C), and sulfadoxine (D), measured as the mean percentage of parasites developing to mature schizonts ± the standard deviation, after 28 to 36 h of incubation. The IC50s shown on the graphs were calculated on a logistic dose-response curve (Jandel Scientific) that is not shown on the graphs.
DISCUSSION
This study demonstrated that it is possible to measure the sensitivity of fresh erythrocytic isolates of P. vivax to a range of antimalarials in a field setting. There have been no reported cases of chloroquine-resistant P. vivax in Thailand, and all of the P. vivax patients from the Mae Sod malaria clinic who were enrolled in this study were satisfactorily treated with chloroquine. Although the patients responded well to chloroquine treatment, our in vitro studies revealed that the MIC for P. vivax in Mae Sod was fourfold higher than that for a chloroquine-sensitive strain of P. falciparum, FC27 (Table 1). However, the MICs for isolates of P. vivax from Australian Army patients whose chloroquine treatment failed were half of those for P. falciparum with a much higher level of resistance. These observations may be due to chloroquine resistance mechanism differences between P. vivax and P. falciparum (13) or may result from specific features of the in vitro test. Unlike P. falciparum, we have no knowledge of chloroquine uptake or efflux in P. vivax and until such work is done, it will be difficult to speculate on the reason for the different chloroquine MICs for P. vivax and P. falciparum (2, 6).
Dihydroartemisinin is an active metabolite of artesunate, an antimalarial commonly used in Thailand for the treatment of P. falciparum infection. P. vivax was highly sensitive to dihydroartemisinin in comparison to the other drugs and P. falciparum (Table 1). Our results agree with those of Pukrittayamee et al. (8), who found that the artemisinin derivatives were the most potent and rapidly acting of the antimalarials tested in vivo against P. vivax infections. As with P. falciparum, we observed that dihydroartemisinin inhibited P. vivax at the early ring stage. The apparent vacuolation observed in the cytoplasm of P. vivax parasites treated with dihydroartemisinin may be due to the oxidative action common to all artemisinin-derived antimalarials (12). In combination with a longer-acting drug such as chloroquine, artesunate may prove to be an effective antimalarial treatment that can delay the onset of chloroquine resistance in P. vivax in Thailand.
The poor in vitro response of P. vivax to both WR238605 and sulfadoxine may be due to the slow action of these drugs and/or the innate resistance of P. vivax to sulfadoxine (4, 5). We have sequenced the dhps gene from four of the Mae Sod P. vivax isolates and found sequence polymorphisms consistent with the resistance phenotype shown in Table 1 (Qin Chen, personal communication). Another major factor contributing to the poor response of P. vivax to sulfadoxine is the presence of p-aminobenzoic acid and folate in the media used (Fig. 1D). The presence of these agents in the media shortcut the antifolate action of sulfadoxine. Unfortunately folate-reduced media, such as RPMI LPLF (Gibco), usually used in antifolate drug assays, do not support P. vivax growth. This emphasizes the need to establish new (and elevated) baseline MICs of antifolates for P. vivax.
Unlike those of artemisinin and chloroquine, the MICs of both WR238605 and sulfadoxine were significantly greater than the mean maximum concentrations of these drugs after their administration in vivo. Although there are problems with comparing drug concentrations from in vitro blood-medium mixtures (4% hematocrit) to in vivo concentrations (40% hematocrit), this information is included in Table 1 as a point of clinical reference for our in vitro data. Longer incubation periods may reduce the MICs so that they fall within the range of drug concentrations observed in vivo.
The absence of long-term P. vivax cultures and the difficulties associated with the use of a primate model have slowed the development of new antimalarials against P. vivax. Although this assay technique is best suited to longitudinal studies of the susceptibility of P. vivax to antimalarial drugs, it may also have utility for the screening of new drugs targeting this species. Such screening can best be done in areas where the drug susceptibility of fresh isolates of P. vivax has been well characterized.
The in vitro assay developed during this study used a wide range of drug concentrations (i.e., 5, 10, 20, 40, 80, 160, 320, and 660 ng of chloroquine/ml) to determine, very broadly, the levels of antimalarial susceptibility in previously unstudied areas. For more precise determination of drug susceptibility, the results of the initial assay should be used to target a particular drug range with smaller concentration intervals. For example, if parasite maturation is inhibited between 80 and 320 ng/ml, the new plate concentrations should be 80, 120, 160, 200, 240, 280, and 320 ng/ml, thus removing possible statistical biases inherent in the use of nonstandard intervals. The narrowing of drug concentrations will be more sensitive in detecting the emergence of R1 drug resistance and provide better information for future studies correlating the in vivo and in vitro responses of P. vivax to drugs. MICs calculated microscopically (directly from the slide rather than extrapolated from a mathematical model) by using well-defined drug concentration intervals are easier to calculate and may prove to be more clinically relevant than IC50s.
This assay technique provides field scientists with an average ability in microscopy a relatively simple method by which to determine the susceptibility of P. vivax to various drugs (drug MICs). This technique avoids the use of expensive or dangerous reagents (monoclonal antibodies or radioisotopes), expensive equipment (beta counters or robotic plate washers and dispensers), complex parasite age grading techniques, and mathematical models. Furthermore, the whole procedure can be carried out on the bench (without a laminar-flow hood) in 25 to 37 h. It is hoped that this procedure will provide a valuable adjunct to clinical studies in areas of endemicity, where the true drug susceptibility of P. vivax can be obscured by various degrees of immunity acquired by patients before their treatment with antimalarial drugs.
Acknowledgments
The U.S. Army Medical Research and Material Command, the Australian Defense Health Services Branch, and the Queens Trust for Young Australians funded this study.
We are indebted to the staff of the Mae Sod malaria clinic for cooperation and support. We thank Catherine Wong for statistical advice. The following staff from AFRIMS and the Department of Pathobiology, Mahidol University, provided valuable technical support: Nongnuch Yimamnuaychoke, Rossarin Suwanarusk, and Morakot Kaewthamasorn. We are grateful to Nick White for helpful comments and suggestions.
REFERENCES
- 1.Baird, J. K., B. Leksana, S. Masbar, D. J. Fryauff, M. A. Sutanihardja Suradi, F. S. Wignall, and S. L. Hoffman. 1997. Diagnosis of resistance to chloroquine by Plasmodium vivax: timing of recurrence and whole blood chloroquine levels. Am. J. Trop. Med. Hyg. 56:621-626. [DOI] [PubMed] [Google Scholar]
- 2.Bayoumi, R. A., H. A. Babiker, and D. E. Arnot. 1994. Uptake and efflux of chloroquine-resistant Plasmodium falciparum clones recently isolated in Africa. Acta Trop. 58:141-149. [DOI] [PubMed] [Google Scholar]
- 3.Chotivanich, K., K. Silamut, R. Udomsangpetch, K. A. Stepniewska, S. Pukrittayakamee, S. Looareesuwan, and N. J. White. 2001. Ex-vivo short-term culture and developmental assessment of Plasmodium vivax. Trans. R. Soc. Trop. Med. Hyg. 95:677-680. [DOI] [PubMed] [Google Scholar]
- 4.Findlay, G. M. 1951. Recent advances in chemotherapy, 3rd edition, vol 3. J. & A. Churchill Ltd., London, United Kingdom.
- 5.Imwong, M., S. Pukrittakayamee, S. Looareesuwan, G. Pasvol, J. Poirreiz, N. J. White, and G. Snounou. 2001. Association of genetic mutations in Plasmodium vivax dhfr with resistance to sulfadoxine-pyrimethamine: geographical and clinical correlates. Antimicrob. Agents Chemother. 45:3122-3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krogstad, D. J., I. Y. Gluzman, D. E. Kyle, A. M. J. Oduola, S. K. Martin, W. K. Milhous, and P. H. Schlesinger. 1987. Efflux of chloroquine from Plasmodium falciparum: mechanism of chloroquine resistance. Science 238:1283-1285. [DOI] [PubMed] [Google Scholar]
- 7.Mendis, K., S. J. Sina, P. Marchesini, and R. Carter. 2001. The neglected burden of Plasmodium vivax malaria. Am. J. Trop. Med. Hyg. 64:97-106. [DOI] [PubMed] [Google Scholar]
- 8.Pukrittayakamee, S., A. Chantra, J. A. Simpson, S. Vanijanonta, R. Clemens, S. Looareesuwan, and N. J. White. 2000. Therapeutic responses to different antimalarial drugs in vivax malaria. Antimicrob. Agents Chemother. 44:1680-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rieckmann, K. H., J. V. McNamara, H. Frischer, T. A. Stockert, P. E. Carson, R. D. Powell. 1968. Effects of chloroquine, quinine, and cycloguanil upon the maturation of asexual erythrocytic forms of two strains of Plasmodium falciparum in vitro. Am. J. Trop. Med. Hyg. 17:661-671. [DOI] [PubMed] [Google Scholar]
- 10.Rieckmann, K. H., L. J. Sax, G. H. Campbell, and J. E. Mrema. 1978. Drug sensitivity of Plasmodium falciparum: an in vitro microtechnique. Lancet i:22-23. [DOI] [PubMed]
- 11.Rieckmann, K. H., D. R. Davis, D. C. Hutton. 1989. Plasmodium vivax resistance to chloroquine? Lancet ii:1183-1184. [DOI] [PubMed]
- 12.Scott, M. D., S. R. Meshnick, R. A. Williams, D. T. Chiu, H. C. Pan, B. Lubin, and F. A. Kuypers. 1989. Quighaosu-mediated oxidation in normal and abnormal erythrocytes. J. Lab. Clin. Med. 114:401-406. [PubMed] [Google Scholar]
- 13.Wellems, T. E., and C. V. Plowe. 2001. Chloroquine-resistant malaria. J. Infect. Dis. 184:770-776. [DOI] [PubMed] [Google Scholar]