Abstract
OBJECTIVE
Determine the impact of fracture, coronary disease, and diabetes on changes in rates of discontinuation and initiation of estrogen therapy with (EPT) and without (ET) progestin, before (September 1, 1999 to June 30, 2002, baseline) versus 5 months after (follow-up) release of the Women's Health Initiative EPT trial results (WHI).
DESIGN, SETTING, AND PARTICIPANTS
Observational cohort; 169,586 women 40 to 80 years old from 5 U.S. HMOs.
METHODS
We used pharmacy data to identify ET and EPT users. A woman was a user any month she filled ≥1 estrogen prescription and in subsequent months based upon the number of pills/patches dispensed. We used inpatient and outpatient claims to identify fracture January 1, 1999 to June 30, 2002 and pharmacy data to identify disease-based groups of medications for diabetes and cardiovascular disease.
MEASURES
EPT/ET prevalence, initiation, and discontinuation rates.
RESULTS
Baseline to follow-up EPT and ET prevalence declined 45% and 22%, respectively, with no difference by comorbidity. Follow-up EPT initiation was half the baseline rate irrespective of comorbidity. Compared to baseline, follow-up EPT discontinuation rates increased among women with diabetes (relative risk [RR], 6.9; 95% confidence interval [CI], 5.6 to 8.4), cardiovascular disease (RR, 5.5; 95% CI, 4.9 to 6.2), fracture (RR, 3.8; 95% CI, 2.4 to 5.7), and no comorbidity (RR, 4.4; 95% CI, 3.9 to 4.9). The RRs for follow-up versus baseline EPT discontinuation were higher among women with diabetes (P <.01) and cardiovascular disease (P <.01) versus women without these comorbidities. ET discontinuation rates among these same groups were elevated 2- to 2.8-fold.
CONCLUSIONS
Diabetes and cardiovascular disease were associated with higher EPT discontinuation rates post-WHI compared to women without comorbidity; comorbidity had little impact on changes in prevalence or initiation of ET/EPT after release of the WHI.
Keywords: hormone therapy, women, menopause, estrogen, fracture
The findings from the Women's Health Initiative (WHI) estrogen plus progestin trial dramatically changed our understanding of the risks and benefits of hormone therapy (HT).1–6 This trial found that estrogen plus progestin (EPT) was associated with expected increases in risks for breast cancer, stroke, and pulmonary embolism, and decreases in risks for fracture and colon cancer.1,4,6 The findings of an increase in risk of coronary events,1 dementia, and mild cognitive impairment,2,3 however, contradicted the results of observational studies and led many to conclude that the overall risks outweighed the benefits of EPT.1 The translation of these results into prescribing practices is gradually becoming evident.7–11 Overall use of estrogen, with or without (ET) progestin, is estimated to have decreased by 30% to 66%.7,8,10 Nevertheless, millions of midlife and older women continue to use estrogen, suggesting use for conditions other than short-term treatment of menopausal symptoms.7,8,10,11 How changes in use have varied with conditions thought to be affected by HT, such as coronary disease and fracture, has received little attention. Therefore, we explored the use of EPT and ET, before and after the release of WHI EPT trial results, among women aged 40 to 80 years. We hypothesized that women with fracture might be more likely to continue EPT and ET, and that women with cardiovascular disease or diabetes (who are at high risk for cardiovascular disease) might be more likely to discontinue EPT and ET, based on the WHI findings regarding fracture prevention and cardiovascular risk.1
METHODS
The study methods have been described elsewhere.7 Briefly, this study was conducted within the Cancer Research Network, a consortium of nonprofit health maintenance organizations (HMOs), and takes advantage of the HMO Research Network's Center for Education and Research on Therapeutics (CERT) Patient Safety Study Cohort.12 The CERT cohort is a representative sample of approximately 200,000 members, of any age, enrolled at each of 10 participating HMOs. Overall, enrollees at these health plans are representative of their local populations, but those of high wealth or poverty, the unemployed, and the very old may be underrepresented at some sites. Eligibility criteria included a pharmacy prescription benefit sometime during the initial study period (January 1, 1999 to June 30, 2001). We extended the CERT cohort study period through December 31, 2002 and constructed a dynamic cohort of all women aged 40 to 80 years from 5 of the participating HMOs: Fallon Community Health Plan, Worcester, MA; Group Health Cooperative, Seattle, WA; Harvard Pilgrim Health Care, Boston, MA; HealthPartners, Minneapolis, MN; and Kaiser Permanente Colorado, Denver, CO. The human subjects committee from each participating institution reviewed and approved the study.
Hormone Use
We estimated the prevalence of EPT and ET use and the incidence of EPT and ET discontinuation and initiation using automated pharmacy data. We use National Drug Codes to identify all prescriptions for oral or transdermal estrogen or progestin filled during the study.7 We defined EPT users as women who filled combination products or estrogen and progestin preparations, and ET users as women who filled estrogen preparations with no progestin fills. A woman was considered a progestin user if she filled at least 1 progestin-containing prescription during the study period. The duration of each prescription and its run-out date were established using number of pills or patches dispensed. We considered a woman to be a continuous user if her prescription was refilled within 60 days of the run-out date. Each successive prescription set a new run-out date.
Enrollment
We tracked HMO enrollment using membership data. Membership lapses of less than 2 months were considered continuous enrollment.
Comorbidities
We used automated inpatient and outpatient International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes 800–829 to identify any fracture occurring January 1999 through June 2001 (original CERT Patient Safety Study observation period). We used the Chronic Disease Score (CDS), a validated measure of comorbidity constructed from 6 months of pharmacy data, to identify women with diabetes or cardiovascular disease (Table 1).13 The drugs in the CDS are prescribed for chronic conditions. The CDS is calculated by applying empirically derived weights for age, gender, and disease-based groups of prescriptions (e.g., drugs for diabetes), to predict total annual health care costs. The CDS increases with age, and is strongly associated with mortality, health care utilization, and hospitalization.13 We calculated the CDS for each woman, for each study month. Once a woman was identified as having a condition she was coded as having it throughout the remaining follow-up time. Conditions were not mutually exclusive; women with more than 1 condition were counted for each condition (e.g., a woman with a fracture and diabetes would be yes in the models for fracture and diabetes).
Table 1.
Drugs Used as Indicators for Diabetes and Cardiovascular Disease
| Condition | Indicator Drugs (Generic) |
|---|---|
| Diabetes | Acetohexamide, acarbose, chlorpropamide, glipizide, glyburide, insulin, insulin aspart, insulin glargine, metformin, miglitol, tolazamide, tolbutamide, pioglitazone, rosiglitazone, natelglinide, troglitazone, repaglinide |
| Cardiovascular disease | |
| Heart disease and hypertension | Acebutolol, amlodipine, atenolol, bepridil, diltiazem, esmolol, felodipine, isradipine, metoprolol, nadolol, nicardipine, nifedipine, nimodipine, pindolol, propranolol, sotalol, timolol, verapamil |
| Cardiac disease | Adenosine, amiodarone, amyl nitrite, bosentan, bretylium, bumetanide, deslanoside, digitoxin, digoxin, disopyramide, dofetilide, encainide, eptifibatide, erythrityl tetranitrate, ethacrinic acid, flecainide, furosemide, isosorbide dinitrate, isosorbide mononitrate, mexilitine, moricizine, nesiritide, nitroglycerin, ouabain, pentaerthritol tetranitrate, procainamide, propafenone, quinidine, tirofiban, tocainide, torsemide |
| Coronary and peripheral vascular disease | Bivalirudin, clopidogrel, cyclandelate, dicumarol, diprydamole, nocoumalone, cilostazol, isoxsuprine, nicoumaline, pentoxifylline, tioclopidine, tinzaparin, warfarin |
Analysis
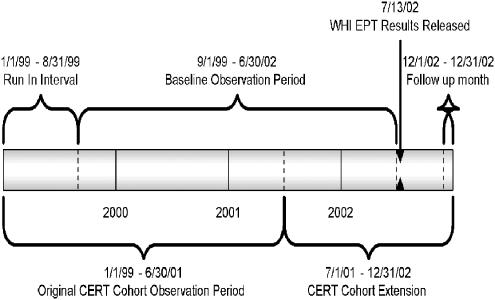
Our analyses required 7 months of pharmacy data to allow sufficient run-in time for estimating true initiation rates. The CERT request process and the timing of study funding limited us to follow up through December 2002. We estimated baseline rates combining data from September 1999 through June 2002, a period of relatively stable rates, and compared these to rates in December 2002 (follow-up to 5 months post-WHI) (Fig. 1). We examined EPT use and ET use separately. To calculate age-specific prevalence, women contributed information for each age group they belonged to during the study period. Women were allowed to age in and out of the cohort. We censored (deleted) women in the analysis from their date of HMO disenrollment. However, women who re-enrolled in the HMO re-joined the analyses 7 months after re-enrolling.
FIGURE 1.
Study time line. WHI, Women's Health Initiative estrogen plus progestin (EPT) primary results publication; CERT, HMO Research Network's Center for Education and Research on Therapeutics Patient Safety Study Cohort.
We used Poisson regression with a log link to model the incidence of initiation and discontinuation of EPT and ET at baseline and for each month following the release of the WHI results in July 2002, adjusting for age and HMO. We used a transition model,14 assuming a first-order Markov process, to account for possible serial correlation among repeated observations within each woman. We modeled EPT and ET use conditional on use/no use in the previous month, which is equivalent to modeling the incidence of initiation (use in 1 month given no use in the previous month) and discontinuation (no use in 1 month given use in the previous month). The relative risks (RR) and corresponding 95% confidence intervals (95% CI) compare the prevalence and incidence of initiation and discontinuation at follow-up (December 2002) to the baseline values within each risk group.
We used Wald's tests to compare discontinuation rates among women with a comorbidity (fracture, diabetes, cardiovascular disease) to women without that comorbidity. Because comorbidity groups were not mutually exclusive, we did not make direct statistical comparisons between them. Deciles were chosen as reasonable cut points after examining the distribution of the CDS scores. Wald's tests were used to test for trends in the relative risks for changes in prevalence, initiation, and discontinuation by CDS deciles.
Preexisting CERT data sharing agreements prohibit our reporting data by HMO. The study authors have no reported conflicts of interest.
RESULTS
The population of eligible women ranged from 22,777 to 34,958 among the 5 HMOs (data not shown), with a total of 152,876. The distribution of age (P <.0001) and CDS (P <.0001) varied among the 5 HMOS; 2 HMOs tended to have higher CDS and older women than the other 3 HMOs (data not shown). Among HMOs, the overall prevalence of chronic conditions varied from 1.3% to 2.8% for fracture, 5.9% to 7.3% for diabetes, and 24.7% to 32.1% for cardiovascular disease. The prevalence of fracture (0.9% to 4.5%), diabetes (3.1% to 12.0%), cardiovascular disease (12.4% to 55.3%), and the CDS, increased with age (Table 2).
Table 2.
Age Distribution of the Study Population, and Comorbidity by Age
| Characteristics | Age, y | ||||
|---|---|---|---|---|---|
| 40–49 | 50–59 | 60–69 | 70–80 | All | |
| Total N | 52,216 | 46,816 | 28,256 | 25,588 | 152,876 |
| % | % | % | % | % | |
| No comorbidity | 84.4 | 72.1 | 55.4 | 40.3 | 68.0 |
| Fracture prevalence* | 0.9 | 1.5 | 2.6 | 4.5 | 2.0 |
| Diabetes prevalence | 3.1 | 6.1 | 9.7 | 12.0 | 6.7 |
| Cardiovascular disease prevalence | 12.4 | 23.7 | 39.9 | 55.3 | 28.1 |
| CDS deciles (total costs in $) | |||||
| 1: 307.6–370.9 | 25.4 | 0.0 | 0.0 | 0.0 | 8.7 |
| 2: 395 | 23.4 | 21.5 | 0.0 | 0.0 | 14.6 |
| 3: 444.2–593.2 | 5.3 | 17.7 | 14.6 | 0.0 | 9.9 |
| 4: 596.9–938.4 | 10.3 | 10.2 | 4.8 | 0.0 | 7.5 |
| 5: 939.3–1136.6 | 7.4 | 5.9 | 16.2 | 12.9 | 9.5 |
| 6: 1,137.5–1,506.1 | 7.7 | 12.2 | 10.9 | 12.5 | 10.5 |
| 7: 1,506.2–1,905.8 | 6.9 | 8.6 | 12.6 | 12.0 | 9.3 |
| 8: 1,906.7–2,510.6 | 5.9 | 9.2 | 13.1 | 16.1 | 10.0 |
| 9: 2,510.8–3,497.2 | 4.4 | 8.0 | 14.3 | 20.4 | 10.0 |
| 10: 3,497.4–28,442.7 | 3.3 | 6.6 | 13.5 | 26.0 | 10.0 |
Prevalence reported as column percent, age and CDS deciles as of July 2002, diabetes and cardiovascular disease derived from drugs used for these conditions in the CDS, August 1999 through December 2002.
Fracture, any fracture between January 1999 and June 2001.
CDS, Chronic Disease Score (CDS ranges are not inclusive because only observed values are shown).
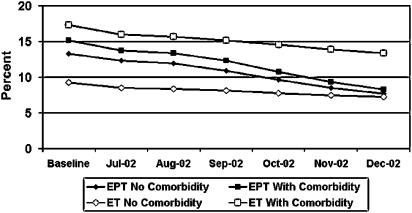
Changes in prevalence of EPT (Fig. 2), rates of EPT initiation (Fig. 3), and rates of EPT discontinuation (Fig. 4), from baseline through follow-up, were similar among women with and without comorbidities. The percent decline in EPT prevalence varied from 42.1% for women with no comorbidity to 47.7% for women with fracture (Table 3). Variation in percent decline of EPT prevalence was not statistically significant between women without comorbidity and women with fracture, diabetes, or cardiovascular disease. EPT prevalence declined 25% in the lowest CDS decile, and 41.9% in the highest CDS decile, but there was no trend in the associations between changes in EPT prevalence and CDS decile. These data are consistent with the relative risks comparing follow-up to baseline EPT prevalence, controlling for age and HMO, which show a 30 to 50% decline across all groups with virtually no difference by comorbidity.
FIGURE 2.
Prevalence of estrogen (ET) use and estrogen plus progestin (EPT), among women age 40 to 80 years, with and without comorbidity (fracture, diabetes, or cardiovascular disease) from baseline* through December 2002. *Baseline includes combined data from September 1999 to June 2002. WHI, Women's Health Initiative EPT publication.
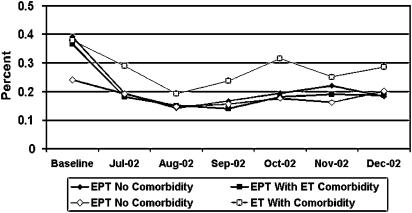
FIGURE 3.
Rate of estrogen (ET) and estrogen plus progestin (EPT) initiation among women age 40 to 80 years, with and without comorbidity (fracture, diabetes, or cardiovascular disease) from baseline* through December 2002. *Baseline includes combined data from September 1999 to June 2002. WHI, Women's Health Initiative EPT publication.
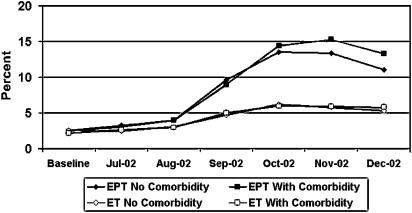
FIGURE 4.
Rate of estrogen (ET) and estrogen plus progestin (EPT) discontinuation among women age 40 to 80 years, with and without comorbidity (fracture, diabetes, or cardiovascular disease) from baseline* through December 2002. *Baseline includes combined data from September 1999 to June 2002. WHI, Women's Health Initiative EPT publication.
Table 3.
EPT Prevalence, Initiation, and Discontinuation by Comorbidity, Before (Baseline) and After (Follow-up) July 2002
| Prevalence* | |||||||
|---|---|---|---|---|---|---|---|
| N | Baseline (%) | Follow-up (%) | Decline (%) | RR (95% CI)† | Initiation RR (95% CI)† | Discontinuation RR (95% CI)† | |
| No comorbidity | 103,956 | 13.3 | 7.7 | 42.1 | 0.55 (0.49 to 0.61) | 0.44 (0.35 to 0.53) | 4.4 (3.9 to 4.9) |
| Fracture | 3,058 | 10.9 | 5.7 | 47.7 | 0.50 (0.37 to 0.66) | 0.60 (0.34 to 0.97) | 3.8 (2.4 to 5.7) |
| Diabetes | 10,243 | 11.7 | 6.3 | 46.2 | 0.53 (0.41 to 0.67) | 0.48 (0.26 to 0.78) | 6.9 (5.6 to 8.4)‡ |
| Cardiovascular disease | 42,958 | 15.5 | 8.5 | 45.2 | 0.54 (0.45 to 0.63) | 0.50 (0.37 to 0.65) | 5.5 (4.9 to 6.2)‡ |
| CDS deciles (total costs in $) | |||||||
| 1: 306.6–370.9 | 13,300 | 0.8 | 0.6 | 25.0 | 0.70 (0.51 to 0.94) | 0.15 (0.15 to 0.15) | 0.9 (0.3 to 2.3) |
| 2: 395 | 22,320 | 10.7 | 5.7 | 46.7 | 0.52 (0.48 to 0.57) | 0.34 (0.23 to 0.48) | 3.3 (2.6 to 4.2) |
| 3: 444.2–593.2 | 15,153 | 12.8 | 10.5 | 18.0 | 0.59 (0.54 to 0.64) | 0.51 (0.34 to 0.72) | 3.6 (3.0 to 4.3) |
| 4: 596.9–938.4 | 11,466 | 19.7 | 9.9 | 49.7 | 0.62 (0.53 to 0.72) | 0.56 (0.34 to 0.87) | 5.2 (4.1 to 6.4) |
| 5: 939.3–1136.6 | 14,523 | 13.6 | 6.5 | 52.2 | 0.50 (0.42 to 0.60) | 0.43 (0.28 to 0.63) | 4.8 (3.9 to 5.9) |
| 6: 1,137.5–1,506.1 | 16,052 | 19.6 | 12.1 | 38.3 | 0.57 (0.50 to 0.64) | 0.47 (0.33 to 0.66) | 5.2 (4.4 to 6.1) |
| 7: 1,506.2–1,905.8 | 14,215 | 15.3 | 8.2 | 46.4 | 0.54 (0.45 to 0.64) | 0.50 (0.32 to 0.72) | 5.0 (4.1 to 6.0) |
| 8: 1,906.7–2,510.6 | 15,288 | 16.6 | 9.5 | 42.8 | 0.55 (0.46 to 0.65) | 0.43 (0.28 to 0.63) | 5.5 (4.6 to 6.5) |
| 9: 2,510.8–3,497.2 | 15,288 | 15.7 | 8.8 | 43.9 | 0.55 (0.46 to 0.66) | 0.51 (0.33 to 0.75) | 5.1 (4.2 to 6.0) |
| 10: 3,497.4–28,442.7 | 15,288 | 13.6 | 7.9 | 41.9 | 0.57 (0.47 to 0.69) | 0.59 (0.37 to 0.89) | 5.0 (4.2 to 5.9) |
| χ2 trend | 0.58 | 0.18 | P =.0001 | ||||
Prevalence reported as column percent, represents women with diagnosis at any point during follow-up.
RR compares follow-up to baseline, within categories, adjusted for age (deciles), CDS, and HMO.
P <.01 versus no comorbidity.
CDS, Chronic Disease Score (deciles as of July 2002; CDS ranges are not inclusive because only observed values are shown). Fracture, any fracture January 1999 to June 2001; diabetes and cardiovascular disease derived from drugs used for these conditions in the CDS; fracture, diabetes, and cardiovascular disease are not mutually exclusive; baseline, September 1999 to June 2002, follow-up, December 2002.
RR, relative risk; CI, confidence interval.
EPT initiation declined 40% to 80% across comorbidity groups and CDS levels (Table 3). Though the RR for EPT initiation for women with no comorbidity, fracture, coronary heart disease, and diabetes varied (range, 0.4 to 0.6), the 95% confidence intervals for women with and without comorbidities overlapped almost completely. The largest declines in EPT initiation were at the lowest levels of the CDS, but there was no trend with increasing CDS.
EPT discontinuation rates increased from baseline to follow-up across comorbidity groups and CDS levels (Table 3). Women with fracture had the lowest relative risks comparing rates of discontinuation at follow-up to baseline rates (RR, 3.8; 95% CI, 2.4 to 5.7), followed by women without comorbidity (RR, 4.4; 95% CI, 3.9 to 4.9), women with cardiovascular disease (RR, 5.5; 95% CI, 4.9 to 6.2), and women with diabetes (RR, 6.9; 95% CI, 5.6 to 8.4). These RRs comparing follow-up to baseline EPT discontinuation rates were significantly greater for women with cardiovascular disease (P =.008) or diabetes (P =.002) compared to women without comorbidity. In addition, the RRs for EPT discontinuation increased from 0.9 to 5.0 from the lowest to highest CDS deciles (χ2 trend, P =.0001).
The prevalence of ET among women with comorbidities was higher than that of women without comorbidities, but overall declines in ET prevalence from baseline to follow-up were similar among women with and without comorbidities (Fig. 2;Table 4). Changes in ET initiation rates (Fig. 3) and ET discontinuation (Fig. 4) from baseline through follow-up were in the same direction but of smaller magnitude than those for EPT. Similar to EPT, variations in the percent decline in ET prevalence from baseline to follow-up across comorbidities and CDS deciles were not statistically significant (Table 4). The relative risks comparing follow-up to baseline prevalence of ET, controlling for age and HMO, demonstrated reductions of 13% to 29% across all groups, with virtually no difference by comorbidity. There was only a slight decrease in ET initiation, and the relative risks were much closer to 1 (range, 0.5 to 1.0) than those for EPT initiation, regardless of comorbidity or CDS.
Table 4.
ET Prevalence, Initiation, and Discontinuation by Comorbidity, Before (Baseline) and After (Follow-up) July 2002
| Prevalence* | |||||||
|---|---|---|---|---|---|---|---|
| N | Baseline (%) | Follow-up (%) | Decline (%) | RR (95% CI)† | Initiation RR (95% CI)† | Discontinuation RR (95% CI)† | |
| No comorbidity | 103,956 | 9.2 | 7.2 | 21.7 | 0.73 (0.69 to 0.78) | 0.78 (0.67 to 0.91) | 2.1 (1.9 to 2.3) |
| Fracture | 3,058 | 10.9 | 8.6 | 21.1 | 0.77 (0.59 to 0.98) | 0.89 (0.47 to 1.53) | 2.5 (1.6 to 3.8) |
| Diabetes | 10,243 | 14.0 | 10.7 | 23.6 | 0.74 (0.68 to 0.81) | 0.54 (0.32 to 0.84) | 2.5 (1.9 to 3.3) |
| Cardiovascular disease | 42,958 | 18.2 | 14.0 | 23.1 | 0.74 (0.64 to 0.85) | 0.74 (0.59 to 0.91) | 2.8 (2.4 to 3.2)‡ |
| CDS deciles (total costs in $) | |||||||
| 1: 306.6–370.9 | 13,300 | 1.1 | 0.8 | 27.3 | 0.73 (0.58 to 0.90) | 0.87 (0.40 to 1.63) | 1.9 (1.0 to 3.1) |
| 2: 395 | 22,320 | 4.9 | 3.4 | 30.6 | 0.69 (0.61 to 0.78) | 0.48 (0.25 to 0.82) | 1.8 (1.3 to 2.4) |
| 3: 444.2–593.2 | 15,153 | 7.0 | 6.5 | 7.1 | 0.76 (0.68 to 0.84) | 0.95 (0.64 to 1.35) | 1.7 (1.2 to 2.2) |
| 4: 596.9–938.4 | 11,466 | 10.8 | 7.9 | 26.9 | 0.87 (0.75 to 1.01) | 0.84 (0.47 to 1.37) | 2.7 (1.9 to 3.7) |
| 5: 939.3–1136.6 | 14,523 | 9.6 | 6.6 | 31.3 | 0.71 (0.63 to 0.79) | 0.58 (0.39 to 0.82) | 1.8 (1.4 to 2.3) |
| 6: 1,137.5–1,506.1 | 16,052 | 14.6 | 11.2 | 23.3 | 0.71 (0.66 to 0.78) | 0.82 (0.60 to 1.07) | 2.3 (1.8 to 2.9) |
| 7: 1,506.2–1,905.8 | 14,215 | 13.7 | 10.9 | 20.4 | 0.76 (0.69 to 0.84) | 0.94 (0.67 to 1.26) | 2.3 (1.8 to 2.8) |
| 8: 1,906.7–2,510.6 | 15,288 | 17.2 | 13.8 | 19.8 | 0.77 (0.70 to 0.84) | 0.79 (0.55 to 1.09) | 2.5 (2.0 to 3.1) |
| 9: 2,510.8–3,497.2 | 15,288 | 19.1 | 14.8 | 22.5 | 0.75 (0.67 to 0.83) | 0.74 (0.53 to 1.00) | 2.7 (2.2 to 3.3) |
| 10: 3,497.4–28,442.7 | 15,288 | 20.9 | 16.6 | 20.6 | 0.77 (0.70 to 0.85) | 0.76 (0.55 to 1.01) | 2.7 (2.2 to 3.2) |
| χ2 trend | 0.22 | 0.48 | P =.008 | ||||
Prevalence reported as column percent, represents women with diagnosis at any point during follow-up.
RR compares follow-up to baseline, within categories, adjusted for age (deciles), CDS, and HMO.
P =.001 versus no comorbidity.
CDS, Chronic Disease Score (deciles as of July 2002; CDS ranges are not inclusive because only observed values are shown). Fracture, any fracture January 1999 to June 2001; diabetes and cardiovascular disease derived from drugs used for these conditions in the CDS; fracture, diabetes, and cardiovascular disease are not mutually exclusive; baseline, September 1999 to June 2002; follow-up, December 2002.
RR, relative risk; CI, confidence interval.
There was a doubling to tripling of ET discontinuation rates depending on comorbidity. Women with no comorbidity had the lowest RRs comparing rates of ET discontinuation at follow-up to baseline rates (RR, 2.1; 95% CI, 1.9 to 2.3), and women with cardiovascular disease had the highest RR (RR, 2.2; 95% CI, 2.4 to 2.3). The relative risks comparing follow-up to baseline rates of ET discontinuation were significantly greater for women with cardiovascular disease compared to women without comorbidity (P =.001). The 95% confidence limits for the relative risk for ET discontinuation comparing follow-up to baseline for women with fracture (RR, 2.5; 95% CI, 1.6 to 3.8) overlapped completely with those for women with no comorbidity, cardiovascular disease, or diabetes. The RRs for ET discontinuation increased from 1.9 to 2.7 from the lowest to highest CDS deciles (χ2 trend, P =.008).
DISCUSSION
We found that changes in EPT and ET discontinuation rates after the release of the WHI EPT findings were associated with specific comorbidities. Women with diabetes or cardiovascular disease were most likely to discontinue EPT, while changes in EPT discontinuation rates were somewhat lower among women with fracture or no comorbidity. Results regarding ET discontinuation were in a similar direction but had a lower magnitude, and only women with cardiovascular disease had significantly increased rates of ET discontinuation compared to women with no comorbidity. There was a trend of increasing EPT and ET discontinuation rates from the lowest to highest CDS deciles. Prevalence of use and initiation rates of EPT and ET decreased in the early months after the release of the WHI EPT results, though changes were greater for EPT than ET, and comorbidities had no effect on these changes.
Our findings of the largest increases in discontinuation among women with cardiovascular disease or diabetes, and the smallest increase in discontinuation among those with fracture, are consistent with the EPT trial results and with our initial hypotheses. The findings on the WHI EPT trial were widely and rapidly disseminated in both the professional and the lay literature, and heavily covered in the media.1–6 Whether there will be a rebound in EPT use, and whether women will begin using lower-dose preparations or local methods such as the vaginal estrogen ring or vaginal creams, are questions for future analyses.
The meaning of increasing rates of discontinuation with increasing deciles of the CDS is less clear. Medications for diabetes and cardiovascular diseases, including hypertension, contribute to the CDS, and it may be that changes reflect the impact of these highly prevalent conditions on decisions about HT use. The CDS also increases with age, and younger women may have been less likely to relinquish HT than older women.
The findings of significantly higher relative risks for change in discontinuation rate for cardiovascular disease (ET and EPT) and diabetes (EPT only) compared to women without comorbidities, without significant differences in prevalence by comorbidity, appear paradoxical. Because the magnitude of change in discontinuation was 10-fold that of initiation (Fig. 4 vs Fig. 3), changes in discontinuation were the primary contributor to decreased prevalence. Though the absolute cumulative decrease in prevalence varied among groups depending upon the baseline prevalence, percent declines were quite similar. Changes in rates of discontinuation were also similar among groups until 4 months post-WHI. Despite relatively large discontinuation rates in months 4 through 6 (13% to 17%), when applied to low absolute prevalences (6% to 18%), the absolute differences in change in prevalence per month by comorbidity were small. These differences were insufficient to lead to significant differences by comorbidity in the relative change in prevalence during our short period of follow-up.
In an effort to investigate the potential impact of the WHI results on clinical recommendations and dissemination to medical staff, we collected HT guidelines and relevant communications from the 5 participating HMOs. All 5 HMOs had released either a newsletter or updated guidelines by November 2002. Recommended changes were similar across sites, including suggestions to use the lowest effective dose for the shortest amount of time for menopausal symptoms. Not surprisingly, all the HMOs acknowledged the unacceptable risk of using EPT in women with coronary heart disease. New recommendations included stopping current therapy if the user was on EPT for cardiovascular indications and initiating alternate treatment for all women (current and new users) with cardiovascular disease. Changes related to the use of EPT for the prevention and treatment of osteoporosis were less consistent among the HMOs, with recommendations ranging from discontinuation, discussion of alternative treatment options with patients, to continuation of EPT use for osteoporosis in women without known coronary disease. These recommendations are consistent with our observed changes in EPT and ET use at the participating HMOs.
Several other studies have examined changes in estrogen use related to the release of the WHI.7–11 Ettinger et al. found that women who attempted to stop HRT had better self-perceived health, lower self-perceived colon cancer risk, and were less likely to have had a hysterectomy, but found no relationship with self-perceived risk of breast cancer, venous thromboembolism, or osteoporosis, body mass index, or smoking status.8 Using the same survey, Grady et al. reported that women who successfully stopped hormone therapy (stopped or decreased dose with intention of stopping) had lower self-perceived risk of hip or spine fracture and were less likely to have troublesome symptoms when stopping.9 EPT users were more likely to attempt to stop,8 and to successfully stop,9 than ET users. These self-report data from Kaiser are consistent with our findings of lower rates of EPT discontinuation among women with fracture, and among women taking ET (women with hysterectomy) versus EPT.
Findings from the WHI1–6 and the Heart and Estrogen/Progestin Replacement Study trial15 provided information on the risks and benefits of EPT, including an increase in risk of cardiovascular events and a decrease in fractures. The more recently released results from the WHI ET trial indicated no significant effect of ET on risks for coronary heart disease, breast cancer, colorectal cancer, pulmonary embolism, or total mortality, a 39% decrease in hip fracture risk, and a 40% increase in stroke risk.16 How these findings may influence subsequent rates of EPT and ET use remains to be determined.
Our study has several limitations. We only captured fractures occurring during the Patient Safety Cohort observation period of January 1999 through June 2001, and thus our prevalence ratios underestimate osteoporotic fractures. However, using incident fracture data makes us confident that we captured women with prevalent osteoporosis. Had we been able to capture osteoporosis diagnoses and previous fractures our findings might have differed, but our finding that EPT users with recent fracture had lower increases in discontinuation rates than women with diabetes or cardiovascular disease is consistent with the WHI findings of a reduction of fracture risk with EPT.1
Diabetes and cardiovascular disease were inferred from drugs used for these conditions captured in the Chronic Disease Score throughout the study. We were unable to capture women with diabetes who were not pharmacologically managed, estimated to be approximately 26% of women with diabetes.17 Our definition of cardiovascular disease was broad. Despite these limitations, we saw that women with these conditions had the highest rates of estrogen discontinuation.
Finally, we accepted the Patient Safety Cohort entry criteria of a drug benefit at any time during the observation period. Pharmacy benefits at the study health plans are quite stable; at any given time only 2.2% of cohort members are without a drug benefit (unpublished data), making the complexity of tracking the presence or absence of a benefit over time, and thus dropping individuals into and out of the cohort, unnecessary. Unless gaining or losing pharmacy benefits were related to the timing of the WHI release our approach would introduce a small amount of random misclassification, but should not have biased our findings.
The strengths of this study include the large random sample from 5 HMOs across the United States. Our findings should be generalizable to the approximately 1 in 4 U.S. residents (26.4%) enrolled in HMOs,5 if not to the larger U.S. population. Our estimates were based on prescriptions filled rather than from self-reported data or from written prescriptions that may not have been filled. Although we could not capture hormone use if women filled at non-HMO pharmacies, one of the participating HMOs (GHC) has previously reported that 95% of women aged 50 to 80 filled all or almost all of their prescriptions at their HMO pharmacies, providing reassurance that this is not a major limitation.18
In summary, we found that comorbidities were not associated with changes in EPT prevalence or initiation rates after the release of the WHI EPT trial. This should be of some concern to clinicians and those interested in public health. Although the WHI found similar relative risks in all subgroups of women, women with diabetes and a history of cardiovascular disease have higher baseline risks, and thus the greatest absolute risk of adverse outcomes with EPT.1 Our finding that there was no difference by comorbidity may suggest that the message from WHI has not been “universally” received by clinicians and patients. Clinicians should use the WHI as an opportunity to revisit the HT decision, including appraisal of the reasons for its use, and discussion of other options. We also found that women who were taking medications prescribed for diabetes and cardiovascular disease, and women with higher CDS, had higher rates of estrogen discontinuation. Whether women discontinued HT on their own or after discussion with their physician is unknown. These associations were stronger for EPT than for ET. How these associations may change after the release of the WHI ET data,16 and as further information about the risks and benefits of hormone therapy is published, remains to be determined.
Acknowledgments
This study was funded by a cooperative agreement from the National Cancer Institute (U19-CA-79689-05). The Cancer Research Network (CRN) consists of the research programs, enrollee populations, and databases of 10 HMOs that are members of the HMO Research Network. The health care delivery systems participating in the CRN are: GHC, Harvard Pilgrim Health Care, Henry Ford Health System, HealthPartners, the Meyers Primary Care Institute of the Fallon Healthcare System/University of Massachusetts, and Kaiser Permanente in five regions: Colorado, Hawaii, Northwest (Oregon and Washington), Northern California, and Southern California. The overall goal of the CRN is to increase the effectiveness of preventive, curative, and supportive interventions that span the natural history of major cancers among diverse populations and health systems, through a program of collaborative research.
Dr. Buist's time was supported in part by a grant from the American Cancer Society (CRTG-03-024-01-CCE). We would like to thank Drs. Robert Davis, Martin Brown, Marsha Raebel, Andrea Z. LaCroix, Richard Platt, and Edward Wagner, and Linda Shultz, MPH and Sarah Greene, MPH for their assistance with this project. We would also like to acknowledge all of the investigators who worked on the HMO Research Network Center for Education and Research on Therapeutics Patient Safety Project.
Data collection for the Patient Safety Project was funded by a grant from the Agency for Healthcare Research and Quality (AHRQ U18HS11843-01). We would also like to acknowledge all of the programmers from participating sites who worked on the HMO Research Network Center for Education and Research on Therapeutics Patient Safety Project (Julia Hecht, PhD, GHC; Doris Milan, Harvard Pilgrim; Jackie C. Fuller, Meyers Primary Care Institute; David L. McClure, MS, KP Colorado; and Gerald Amundson, HealthPartners).
REFERENCES
- 1.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 2.Shumaker SA, Legault C, Rapp SR, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651–62. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 3.Rapp SR, Espeland MA, Shumaker SA, et al. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2663–72. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- 4.Chlebowski RT, Hendrix SL, Langer RD, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women's Health Initiative Randomized Trial. JAMA. 2003;289:3243–53. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 5.Hays J, Ockene JK, Brunner RL, et al. Effects of estrogen plus progestin on health-related quality of life. N Engl J Med. 2003;348:1839–54. doi: 10.1056/NEJMoa030311. [DOI] [PubMed] [Google Scholar]
- 6.Wassertheil-Smoller S, Hendrix SL, Limacher M, et al. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women's Health Initiative: a randomized trial. JAMA. 2003;289:2673–84. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 7.Buist D, Newton KM, Miglioretti DL, et al. Hormone therapy prescribing patterns in the United States. Obstet Gynecol. 2004;104(5pt 1):1042–50. doi: 10.1097/01.AOG.0000143826.38439.af. [DOI] [PubMed] [Google Scholar]
- 8.Ettinger B, Grady D, Tosteson AN, Pressman A, Macer JL. Effect of the Women's Health Initiative on women's decisions to discontinue postmenopausal hormone therapy. Obstet Gynecol. 2003;102:1225–32. doi: 10.1016/j.obstetgynecol.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Grady D, Ettinger B, Tosteson AN, Pressman A, Macer JL. Predictors of difficulty when discontinuing postmenopausal hormone therapy. Obstet Gynecol. 2003;102:1233–9. doi: 10.1016/j.obstetgynecol.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 10.Lawton B, Rose S, McLeod D, Dowell A. Changes in use of hormone replacement therapy after the report from the Women's Health Initiative: cross sectional survey of users. BMJ. 2003;327:845–6. doi: 10.1136/bmj.327.7419.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291:47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- 12.Platt R, Davis R, Finkelstein J, et al. Multicenter epidemiologic and health services research on therapeutics in the HMO Research Network Center for Education and Research on Therapeutics. Pharmacoepidemiol Drug Saf. 2001;10:373–7. doi: 10.1002/pds.607. [DOI] [PubMed] [Google Scholar]
- 13.Clark D, Von Korff M, Saunders K, Baluch W, Simon G. A chronic disease score with empirically derived weights. Med Care. 1995;33:783–95. doi: 10.1097/00005650-199508000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Diggle PJ, Heagerty P, Liang KY, Zeger SL. Analysis of Longitudinal Data. New York, NY: Oxford University Press; 2002. [Google Scholar]
- 15.Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/Progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–13. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 16.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004;291:1701–12. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 17.Katon W, von Korff M, Ciechanowski P, et al. Behavioral and clinical factors associated with depression among individuals with diabetes. Diabetes Care. 2004;27:914–20. doi: 10.2337/diacare.27.4.914. [DOI] [PubMed] [Google Scholar]
- 18.Newton KM, LaCroix AZ. Association of body mass index with reinfarction and survival after first myocardial infarction in women. J Womens Health. 1996;5:433–44. [Google Scholar]