Abstract
The MAL proteolipid has been recently demonstrated as being necessary for correct apical sorting of the transmembrane influenza virus hemagglutinin (HA) in Madin–Darby canine kidney (MDCK) cells. The fact that, in contrast to MDCK cells, Fischer rat thyroid (FRT) cells target the majority of glycosylphosphatidylinositol (GPI)-anchored proteins to the basolateral membrane provides us with the opportunity to determine the role of MAL in apical transport of membrane proteins under conditions in which the majority of GPI-anchored proteins are (MDCK cells) or are not (FRT cells) targeted to the apical surface. Using an antisense oligonucleotide-based strategy to deplete endogenous MAL, we have observed that correct transport of apical transmembrane proteins associated (HA) or not (exogenous neurotrophin receptor and endogenous dipeptidyl peptidase IV) with lipid rafts, as well as that of the bulk of endogenous apical membrane, takes place in FRT cells by a pathway that requires normal MAL levels. Even transport of placental alkaline phosphatase, a GPI-anchored protein that is targeted apically in FRT cells, was dependent on normal MAL levels. Similarly, in addition to the reported effect of MAL on HA transport, depletion of MAL in MDCK cells caused a dramatic reduction in the apical delivery of the GPI-anchored gD1-DAF protein, neurotrophin receptor, and the bulk of membrane proteins. These results suggest that MAL is necessary for the overall apical transport of membrane proteins in polarized MDCK and FRT cells.
INTRODUCTION
In polarized epithelia, apical and basolateral proteins are packed into different vesicular carriers for delivery to their specific final destination (Wandinger-Ness et al., 1990). Although apical and basolateral vesicles may share elements of the protein-sorting machinery, they must certainly differ in some components to carry out specifically the processes of cargo recruitment and targeting to the appropriate surface domain. Recruitment of integral proteins for basolateral transport appears to be mediated by the recognition of sorting signals in their cytoplasmic domain by sorting machinery, probably including some elements related to proteins capable of recognizing tyrosine and dileucine determinants (Matter and Mellman, 1994). A novel mechanism for apical transport was proposed based on the selective partition of proteins into specialized glycolipid- and cholesterol-enriched membrane (GEM) microdomains or rafts (Simons and Wandinger-Ness, 1990). This model is supported by evidence obtained from the prototypical Madin–Darby canine kidney (MDCK) cell system showing that the influenza virus hemagglutinin (HA) and glycosylphosphatidylinositol (GPI)-anchored proteins are included into GEMs during biosynthetic transport to the apical surface, whereas basolateral proteins are excluded (Skibbens et al., 1989; Brown and Rose, 1992). Moreover, cholesterol removal enhances the solubility of HA into nonionic detergents and slows down apical transport of HA probably by altering GEM integrity (Keller and Simons, 1998). The GPI anchor is essential for incorporation of GPI-anchored proteins into GEMs and apical sorting (Brown et al., 1989; Lisanti et al., 1989). In the case of HA, specific residues in its membrane-spanning domain are required for these processes (Lin et al., 1998; Scheiffele et al., 1998).
Despite the parallelism between HA and GPI-anchored proteins in their route to the apical surface in MDCK cells, some differences have been reported in GPI-anchored protein transport in other cell lines (Zurzolo et al., 1993). Fischer rat thyroid (FRT) cells are a polarized cell line that sorts the majority of GPI-anchored proteins to the basolateral surface (Zurzolo et al., 1993). Biochemical analysis showed that, although FRT cells are able to assemble GEMs (Sargiacomo et al., 1993; Zurzolo et al., 1994), gD1-DAF, a chimeric GPI-anchored protein consisting of the ectodomain of the herpes simplex virus coat gD1 protein (a type I transmembrane protein) fused to the GPI attachment signal of human decay accelerating factor (Brown et al., 1989), is excluded from these specialized membranes (Zurzolo et al., 1994). Placental alkaline phosphatase (PLAP), a GPI-anchored protein that has been recently shown to be included in GEMs in FRT cells (Benting et al., 1999), is delivered apically in this cell line by a pathway that is resistant to cholesterol depletion but sensitive to treatment with fumonisin B1, an inhibitor of glycosphingolipid biosynthesis (Lipardi et al., 2000). It is not established whether the GEM-mediated pathway of transport is operative for HA and/or other apical transmembrane proteins in FRT cells.
The MAL gene was initially identified during a search for genes that are differentially expressed during human T-cell development (Alonso and Weissman, 1987). More recently, the MAL protein has been identified in rat myelin-forming cells (Kim et al., 1995) and in polarized epithelial cells including the renal MDCK cell line (Zacchetti et al., 1995; Millán et al., 1997) and thyroid FRT cells (Martín-Belmonte et al., 1998). The MAL gene encodes a nonglycosylated integral membrane protein of 17 kDa containing multiple hydrophobic segments that, in contrast to most integral membrane proteins, is highly soluble in organic solvents used to extract cell lipids (Martín-Belmonte et al., 1998). Using MDCK cells whose endogenous MAL was depleted, it has been recently demonstrated a role for MAL as an element of the apical sorting apparatus necessary for apical transport of HA and a limited set of proteins, which includes the secretory protein gp80, the transmembrane protein gp114, and a GPI-anchored protein (Cheong et al., 1999; Puertollano et al., 1999).
The fact that MAL is expressed in both polarized epithelial MDCK and FRT cells (Martín-Belmonte et al., 1998), and the reported surface targeting of GPI-anchored proteins in these cell systems, allowed us to examine whether the MAL-mediated apical pathway functions for transmembrane HA, neurotrophin receptor (p75NTR), GPI-anchored proteins, and other membrane proteins under conditions in which the majority of GPI-anchored proteins are or are not targeted to the apical surface. The results presented herein show that MAL is necessary for the overall apical transport of membrane proteins, regardless of whether they are detected in the GEM fraction, in both MDCK and FRT cells.
MATERIALS AND METHODS
Materials
The mouse hybridoma producing mAb 9E10 against the c-Myc epitope EQKLISEEDL was obtained from the American Type Culture Collection (Manassas, VA). Control anti-CD3ε antibodies were a generous gift from Dr. B. Alarcón (Centro de Biología Molecular “Severo Ochoa”). Mouse monoclonal antibodies to E-cadherin were obtained from Transduction Laboratories (Lexington, KY). Anti-herpes simplex virus gD1 protein antibodies were purchased from Serotec (Kidlington, Oxford, United Kingdom). The anti-HA mAb 12CA5 and anti-PLAP mAb were obtained from Boehringer Mannheim (Mannheim, Germany) and Dako A/S (Glostrup, Denmark), respectively. The rabbit polyclonal antibody to p75NTR and the anti-dipeptidyl peptidase IV (DPPIV) mAb were generous gifts from Dr. M. Chao (Cornell University, Ithaca, NY) and Dr. A. Quaroni (Cornell University), respectively. The mouse mAb 6D9 that recognizes the human (h) and rat (r) MAL species and the mAb 2E5 specific to dog (d) MAL have been described previously (Martín-Belmonte et al., 1998; Puertollano et al., 1999). Peroxidase-conjugated secondary anti-immunoglobulin antibodies, sulfo-N-hydroxyl-succinimido-phenyl-propionate (sulfo-SHPP), sulfo-N-hydroxyl-succinimido-biotin (sulfo-NHS-biotin), streptavidin-coupled agarose, and peroxidase-coupled streptavidin were supplied by Pierce (Rockford, IL). The DNA constructs expressing gD1-DAF and PLAP were kindly provided by Genentech (South San Francisco, CA) and Dr. L. Gerber (Roche Institute of Molecular Biology, Nutley, NJ), respectively. Compactin was obtained from Fluka Chemie (Buchs, Switzerland). Triton X-100, methyl-β-cyclodextrin, mevalonate, and octyl-glucoside were purchased from Sigma (St. Louis, MO).
Cell Culture and Infection Conditions
Epithelial MDCK II cells from canine kidney (a kind gift from Dr. M.P. Lisanti, Albert Einstein College of Medicine, Bronx, NY) were grown on Petri dishes in Dulbecco's modified Eagle's medium supplemented with 10% of fetal bovine serum (Life Technologies, Gaithersburg, MD), penicillin (50 U/ml), and streptomycin (50 μg/ml), at 37°C in an atmosphere of 5% CO2. Epithelial thyroid FRT cells (kindly provided by Dr. P. Santiesteban, Instituto de Investigaciones Biomédicas, Madrid, Spain) were grown in F-12 Coon's medium (Sigma) under the same conditions used for MDCK cells. Influenza virus A/Victoria/3/75 strain (a generous gift from Dr. J. Ortín, Centro Nacional de Biotecnología, Madrid, Spain) was grown and titered on MDCK cells. Confluent cell monolayers were incubated with influenza virus (10–15 plaque-forming units per cell) for 1 h at 37°C to allow adsorption and entry of the virus. After that (taken as time zero of infection), the inoculum was removed, and the cell cultures were incubated at 37°C for the indicated times in normal medium. The replication-defective recombinant adenovirus vector expressing human p75NTR, a kind gift from Dr. M. Chao, was used following reported procedures (Yeaman et al., 1997).
DNA Constructions, Oligonucleotides, and Transfections
The construct expressing an hMAL protein lacking the four amino acids contiguous with the initial methionine residue and bearing the 9E10 c-Myc epitope at the N terminus (hMAL-ΔN) was generated with a PCR using MAL cDNA as a template and oligonucleotide primers with the appropriate modifications. Phosphorothioate oligonucleotides were synthesized with sulfur throughout the phosphate backbone (Isogen Bioscience, Maarssen, Belgium). The 19-mer phosphorothioate oligonucleotide AS (5′-CGCCGCTGCTGGGGCCATG-3′), complementary to dMAL mRNA, and the oligonucleotide AM (5′-CGCGGCCACTCGCGTCGTG-3′), similar in composition to AS but with some replacements to prevent pairing with endogenous MAL mRNA, have been described previously (Puertollano et al., 1999). Oligonucleotides (20 μM) were introduced into FRT cells by transfection using the calcium phosphate precipitation procedure (Sambrook et al., 1989). For MDCK cells we used the electroporation protocol of Puertollano et al. (1999). Parallel experiments to measure the uptake of oligonucleotides using phosphorothioate oligonucleotides labeled at the 5′ end with Texas Red indicated that the efficiency of transfection varied between 80 and 99% of the cells as assayed by immunofluorescence analysis (our unpublished results). Transfection of MDCK or FRT cells with plasmid DNA was carried out by electroporation with the Electro Cell Manipulator 600 equipment (BTX, San Diego, CA). Stable transfectants were selected by treatment with 0.5 mg/ml G-418 sulfate (Life Technologies) or 0.75 μg/ml puromycin (Sigma) for at least 4 wk after transfection. Drug-resistant cells were selected and screened by immunofluorescence analysis, and the clones resulting positive were maintained in drug-free medium. After several passages in this medium, >95% of cells within the selected positive clones retained expression of the ectopic protein.
Detergent Extraction Procedures
GEMs were isolated by standard procedures (Brown and Rose, 1992). Cells grown to confluency in 100-mm dishes were rinsed with PBS and lysed for 20 min in 1 ml of 25 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA, and 1% Triton X-100 at 4°C. The lysate was scraped from the dishes with a cell lifter, the dishes were rinsed with 1 ml of the same buffer at 4°C, and the lysate was homogenized by passing the sample through a 22-gauge needle. The extract was finally brought to 40% sucrose in a final volume of 4 ml and placed at the bottom of an 8-ml 5–30% linear sucrose gradient. Gradients were centrifuged for 18 h at 39,000 rpm at 4°C in a Beckman Instruments (Palo Alto, CA) SW41 rotor. Fractions of 1 ml were harvested from the bottom of the tube, and aliquots were subjected to immunoblot analysis. The method of Skibbens et al. (1989) was adopted to analyze the partition of HA or gD1-DAF into insoluble membranes during biosynthetic transport. Briefly, cell monolayers were extracted for 20 min on ice with 25 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA, and 1% Triton X-100 supplemented with a mixture of protease inhibitors. The extracts were then centrifuged in a refrigerated Hettich (Tuttlingen, Germany) microfuge at 14,000 rpm for 3 min. The supernatant (soluble fraction) was removed, and a small amount of the remaining soluble material was recovered from the pellet (insoluble fraction) after a second centrifugation. The soluble material was pooled, and the pellet was resuspended in buffer for SDS-PAGE. Finally, equivalent aliquots from the soluble and insoluble fractions were either directly subjected to SDS-PAGE (HA) or immunoprecipitated with anti-gD1 antibodies and fractionated by SDS-PAGE (gD1-DAF). The proteins were finally monitored by autoradiography.
Immunoblot and Immunoprecipitation Analyses
For immunoblot analysis, samples were subjected to SDS-PAGE in 15% acrylamide gels under reducing conditions and transferred to Immobilon-P membranes (Millipore, Bedford, MA). After blocking with 5% (wt/vol) nonfat dry milk and 0.05% (vol/vol) Tween 20 in PBS, blots were incubated with the indicated primary antibody. After several washings, blots were incubated for 1 h with goat anti-mouse or anti-rat immunoglobulin G antibodies coupled to horseradish peroxidase, washed extensively, and developed using an enhanced chemiluminescence Western blotting kit (Amersham Pharmacia Biotech, Uppsala, Sweden). Quantitative analyses were done with a computing densitometer.
For metabolic labeling, cells were starved in culture medium lacking methionine and cysteine for 30 min and incubated with 100–500 μCi of a [35S]methionine/cysteine mixture (ICN, Costa Mesa, CA) for 5 min at 37°C. After this period, the medium was removed and replaced with standard culture medium. For immunoprecipitation of gD1-DAF during biosynthetic transport, antibodies were prebound overnight at 4°C to protein G-Sepharose in 10 mM Tris-HCl, pH 8.0, 0.15 M NaCl, and 1% Triton X-100. Cell extracts were incubated for 4 h at 4°C with an control anti-CD3ε antibody bound to protein G-Sepharose, and the supernatant was immunoprecipitated by incubation for 4 h at 4°C with the appropriate antibodies bound to protein G-Sepharose. After collection, the immunoprecipitates were washed six times with 1 ml of 10 mM Tris-HCl, pH 8.0, 0.15 M NaCl, and 1% Triton X-100 and analyzed by SDS-PAGE under reducing conditions. Immunoprecipitation of surface-biotinylated proteins was carried out with streptavidin-agarose using a protocol similar to that described for immunoprecipitation with antibodies bound to protein G-Sepharose. To detect 35S labeling, dried gels were finally exposed to Fujifilm imaging plates (Fuji Photo Film, Tokyo, Japan).
Domain-selective Biotinylation
For separate access to apical or basolateral domains, FRT or MDCK cells were seeded at confluent levels on 24-mm polyester tissue culture inserts of 0.4 μm pore size (Transwell; Costar, Cambridge, MA). The integrity of the cell monolayer was monitored by measuring the transepithelial electrical resistance using the Millicell ERS apparatus (Millipore). For metabolic labeling of cells in filters, cells infected with influenza virus for 2.5 h were starved in media lacking methionine and cysteine. After 30 min, 250 μCi of [35S]methionine/cysteine were added to the basolateral compartment, and filters were incubated for 1 h at 37°C. After repeated washings with ice-cold PBS containing 0.1 mM CaCl2 and 1 mM MgCl2, 0.5 mg/ml sulfo-NHS-biotin were added either to the apical or basolateral compartment of the filter chamber. After 30 min at 4°C, the solution was removed, and remaining unreacted biotin was quenched by incubation with ice-cold serum-free culture medium. Cell monolayers were finally washed with PBS and extracted with 0.5 ml of 25 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, and 60 mM octyl-glucoside for 30 min on ice. Extracts were immunoprecipitated with streptavidin-agarose, and the immunoprecipitates were fractionated by SDS-PAGE. To detect the presence of 35S-labeled HA on the cell surface, blots were exposed to imaging plates. To detect surface E-cadherin, the streptavidin-agarose immunoprecipitates were analyzed by immunoblot with anti-E-cadherin antibodies.
To determine cell surface delivery of exogenously expressed gD1-DAF and PLAP or endogenous DPPIV, cells were pretreated at both the apical and basolateral compartments with 0.5 mg/ml sulfo-SHPP (which lacks a biotin moiety) in PBS containing 0.1 mM CaCl2 and 1 mM MgCl2 for 10 min to quench free amino groups. The solution was then removed, and the treatment was repeated five times to quench residual free amino groups (Lisanti et al., 1990). After incubation for 7 h at 37°C, the appearance of newly delivered molecules on the cell surface was monitored by domain-selective labeling with sulfo-NHS-biotin, followed by immunoprecipitation with the appropriate antibodies coupled to protein G-Sepharose, and immunoblotting with streptavidin-peroxidase.
RESULTS
Depletion of Endogenous MAL in MDCK and FRT Cells by Using an Antisense Oligonucleotide-based Strategy
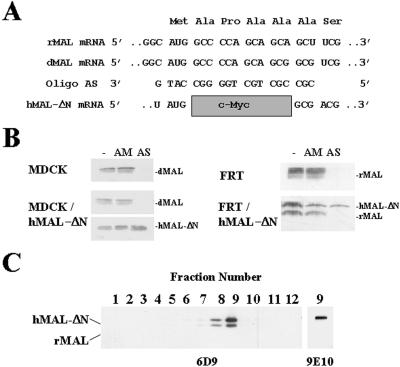
We previously reported the expression of the MAL proteolipid in MDCK and FRT cells (Martín-Belmonte et al., 1998). Figure 1A shows the sequence of the antisense 19-mer phosphorothioate oligonucleotide AS, which pairs the sequence surrounding the AUG translation initiation site of dMAL and rMAL mRNA species. The oligonucleotide AM (Puertollano et al., 1999), which has a composition similar to that of AS but differs in seven nucleotides scattered along its sequence, was used as a control. These oligonucleotides were transfected by electroporation into MDCK cells or by the calcium phosphate technique into FRT cells. Forty-eight hours after transfection, cell extracts were analyzed by immunoblotting with either mAb 2E5 (dMAL) or mAb 6D9 (rMAL). Figure 1B shows that whereas the control oligonucleotide AM did not affect the levels of MAL, transfection of oligonucleotide AS greatly diminished the amount of endogenous MAL in both MDCK and FRT cells. Parallel experiments using Texas Red-labeled phosphorothioate oligonucleotides indicated that the efficiency of transfection varied between 90–99% of the cells as assayed by immunofluorescence analysis (our unpublished results). The MAL levels obtained in cells transfected with oligonucleotide AS were usually 10–20% of the amount found in cells transfected with oligonucleotide AM.
Figure 1.
Depletion of endogenous MAL by transfection with an antisense phosphorothioate oligonucleotide. (A) Nucleotide sequence of the oligonucleotides used. The sequence of the antisense oligonucleotide AS, used in MAL depletion experiments, and its alignment with the rMAL, dMAL, and hMAL-ΔN mRNA species are shown. Note that the deleted sequence in hMAL-ΔN mRNA prevents pairing with oligonucleotide AS. (B) Top panels, transfection of oligonucleotide AS in MDCK and FRT cells causes a drop in endogenous MAL protein levels. Cells were transfected with AM or AS oligonucleotide or not and incubated at 37°C. After 48 h, cell extracts were subjected to immunoblot analysis with anti-MAL mAb 2E5 (MDCK cells) or 6D9 (FRT cells). Bottom panels, hMAL-ΔN is resistant to depletion by oligonucleotide AS. Cells stably expressing hMAL-ΔN were transfected with AM or AS oligonucleotide and incubated at 37°C. After 48 h, cell extracts were subjected to immunoblot analysis with anti-MAL mAb 2E5 and anti-tag mAb 9E10 (MDCK cells) or anti-MAL 6D9 mAb (FRT cells), which recognizes both endogenous (rMAL) and exogenous (hMAL-ΔN) MAL species. (C) An intact amino terminus is not required for incorporation of hMAL into GEMs. FRT cells stably expressing a c-Myc-tagged hMAL protein bearing a deletion of the four amino acids adjacent to the initial methionine residue (hMAL-ΔN) were lysed with 1% Triton X-100 at 4°C, and the extract was centrifuged to equilibrium. After fractionation from the bottom of the tube, aliquots from each fraction were analyzed by immunoblotting with mAb 6D9. An aliquot of fraction 9 from the gradient was analyzed by immunoblotting with anti-tag mAb 9E10 to identify unambiguously tagged hMAL-ΔN. Note that because of the 6-amino-acid difference (10 amino acids from the c-Myc tag minus 4 amino acids from the deleted region) hMAL-ΔN migrates slower than rMAL.
As a control for the specificity of the effect of oligonucleotide AS, we used MDCK cells (MDCK/hMAL-ΔN cells) and FRT cells (FRT/hMAL-ΔN cells) that stably express a truncated form of hMAL, named hMAL-ΔN, lacking the four-amino-acid sequence contiguous with the initial methionine residue of the human MAL protein (Figure 1A). The deleted sequence was demonstrated to be dispensable for targeting of MAL to GEMs, as demonstrated by immunoblot analysis of GEMs isolated from FRT/hMAL-ΔN cells with anti-MAL 6D9, which recognizes both the endogenous (rMAL) and the exogenous (hMAL-ΔN) MAL protein, or anti-tag 9E10 mAb (Figure 1C). The levels of exogenous MAL in these cells were estimated to be ∼1.5-fold higher than those of the endogenous protein. When the effect of oligonucleotide AS was assayed on FRT/hMAL-ΔN cells, we found that whereas the endogenous MAL protein was depleted, the level of the exogenously expressed protein was completely unaffected (Figure 1B, right panel), because the deletion made in the hMAL-ΔN cDNA covers most of the sequence that pairs with the antisense AS oligonucleotide (Figure 1A). An identical result was obtained in MDCK/hMAL-ΔN cells (Figure 1B, left panel). Our previous method to restore MAL expression consisted of the ectopic expression of tagged hMAL in cells whose endogenous protein was depleted, because tagged hMAL expression was minimally affected by the antisense oligonucleotide AS (Puertollano et al., 1999). The use of hMAL-ΔN cDNA has the advantage over our previous method in that its expression is not affected at all by transfection with the AS oligonucleotide.
MAL Is Necessary for Apical Transport and Accurate Sorting of gD1-DAF and p75NTR in MDCK Cells
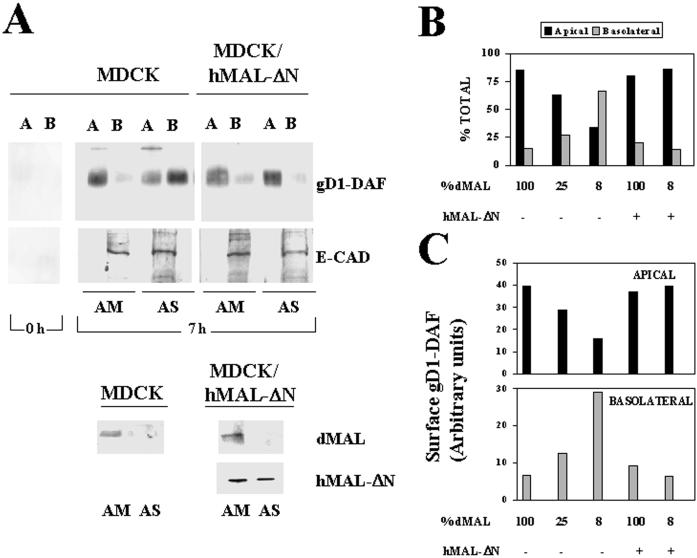
The chimeric gD1-DAF protein has been widely used as a prototype of GPI-anchored proteins in both MDCK and FRT cells (Brown et al., 1989; Lisanti et al., 1990; Zurzolo et al., 1993, 1994). To use gD1-DAF as a GPI-anchored marker in subsequent studies, we first analyzed the requirement of MAL for apical transport of this protein in MDCK cells. Cells stably expressing gD1-DAF were transfected with either AM or AS oligonucleotide and seeded at high density on filter culture inserts. After 48 h at 37°C, the integrity of the cell monolayers was checked. To detect new surface delivery of gD1-DAF, intact cell monolayers were treated with sulfo-SHPP (which lacks the biotin moiety) to quench the free amino groups already preexisting in the cell surface that would otherwise react with sulfo-NHS-biotin (Lisanti et al., 1990). An example of the efficiency of the sulfo-HSPP treatment is shown in Figure 2A, left lane. The new arrival of gD1-DAF either at the apical or basolateral membrane was traced by domain-selective labeling with sulfo-NHS-biotin 7 h after treatment with sulfo-SHPP. Using this procedure, it was previously determined that of the newly arrived population of gD1-DAF, 95% is due to biosynthetic delivery, whereas the remaining 5% may represent a recycling pool (Lisanti et al., 1990). After immunoprecipitation with anti-gD1 antibodies, surface gD1-DAF was visualized by immunoblotting with streptavidin-peroxidase. As an internal control, the sorting of E-cadherin, a basolateral protein of MDCK cells (Puertollano et al., 1999), was determined in parallel using a similar procedure. The extent of MAL depletion obtained in each experiment was quantified by densitometric scanning of immunoblots of the initial lysates with anti-dMAL 2E5 mAb. A representative experiment in which MAL levels dropped to ∼8% of those in control cells is shown in Figure 2A. Whereas only 15% of surface gD1-DAF was present on the basolateral membrane in control MDCK cells, missorting to this domain increased to ∼65% in the cells with reduced MAL levels. To confirm that the observed effects were due to MAL depletion, we used MDCK/hMAL-ΔN cells. Figure 2A also shows that the exogenous expression of hMAL-ΔN restored apical transport of gD1-DAF and prevented gD1-DAF missorting to the basolateral membrane, despite the drop of endogenous MAL to ∼8% of that in normal cells. A compilation of the results obtained on gD1-DAF transport to the apical or basolateral membranes from experiments in which MAL was depleted to different extents is shown in Figure 2B. Quantitative analysis of the amount of gD1-DAF on the apical or the basolateral domains indicates that the altered ratio of apical to basolateral gD1-DAF targeting observed upon MAL depletion was due to both decreased gD1-DAF transport to the apical surface and increased transport of this molecule to the basolateral surface (Figure 2C). In summary, Figure 2 indicates clear correlations among MAL depletion, progressive reduction of apical sorting of gD1-DAF, and concomitant increased transport of gD1-DAF to the basolateral membrane.
Figure 2.
MAL depletion in MDCK cells causes reduced transport of gD1-DAF to the apical surface and missorting to the basolateral membrane. (A) Normal MDCK cells or MDCK/hMAL-ΔN cells stably expressing gD1-DAF were transfected with AM or AS oligonucleotide, plated on 24-mm-diameter tissue culture inserts, and incubated at 37°C for 48 h. To study surface delivery of gD1-DAF, cells were repeatedly treated with sulfo-SHPP to quench the free amino groups on the cell surface from both the apical and basolateral faces of the insert. After incubation at 37°C for 0 or 7 h, the appearance of new free surface amino groups was monitored by domain-selective labeling with sulfo-NHS-biotin. After cell lysis, the extracts were immunoprecipitated with anti-gD1 antibodies, and newly surface delivered gD1-DAF was detected by immunoblotting with streptavidin-peroxidase. As a control, a similar analysis was done to detect surface delivery of E-cadherin, a basolateral protein of MDCK cells. The endogenous (dMAL) or exogenous (hMAL-ΔN) MAL levels in these cells were examined by immunoblot analysis with mAb 2E5 or 9E10, respectively. Note the efficiency of the treatment with sulfo-SHPP (0 h). (B and C) Quantitative analysis of the effect of MAL depletion on the polarized delivery of gD1-DAF. The intensity of the apical and basolateral signal corresponding to newly delivered surface gD1-DAF from experiments in which MAL was depleted at different extensions were quantified. The values obtained are represented as percentages of biotinylated gD1-DAF on the apical (black bars) or basolateral (gray bars) surface (B) or expressed in arbitrary units as apical (black bars) or basolateral (gray bars) absolute surface content of biotinylated gD1-DAF (C). The results of some representative experiments are shown. The effects observed in other experiments in which MAL was depleted to different extents (our unpublished results) were consistent with those shown.
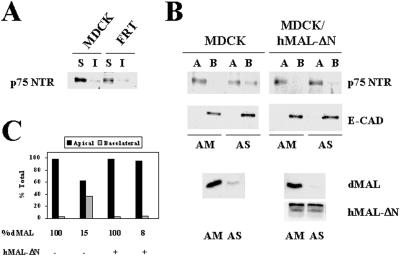
We have previously shown that normal apical transport and accurate sorting of influenza virus HA, a transmembrane protein detected in the GEM fraction, is dependent on normal MAL levels (Puertollano et al., 1999). To investigate whether transmembrane proteins that are found excluded from GEMs depend also on MAL levels to be delivered apically, we expressed p75NTR using a recombinant adenovirus vector. p75NTR was indeed excluded from the GEM fraction in both MDCK and FRT cells as assayed by immunoblot analysis (Figure 3A) and pulse–chase experiments (our unpublished results), in agreement with previous reports (Lipardi et al., 2000). Figure 3B shows that, similar to HA (Puertollano et al., 1999) and gD1-DAF (Figure 2), lowering MAL levels to ∼15% of those in control cells diminished approximately to the half the absolute apical delivery of p75NTR (Figure 2A) and increased missorting of surface p75NTR to the basolateral subdomain of MDCK cells from 2 to 37% (Figure 2, A and B). As a control, we observed that the basolateral expression of E-cadherin was unaffected by the treatment (Figure 2A). Thus, normal MAL levels are required for correct transport of HA and p75NTR, representatives of transmembrane proteins included and excluded from GEMs, respectively, and the GPI-anchored protein gD1-DAF in MDCK cells.
Figure 3.
MAL depletion in MDCK cells causes reduced transport of p75NTR to the apical surface and missorting to the basolateral membrane. (A) MDCK and FRT cells were infected with a recombinant adenovirus expressing p75NTR, and 8 h later the cell surface was labeled with sulfo-NHS-biotin. After extraction with 1% Triton X-100 at 4°C, the soluble and insoluble fractions were separated by centrifugation and were subjected to immunoprecipitation with anti-p75NTR antibodies. Surface p75NTR was detected by immunoblotting with streptavidin-peroxidase. Quantification of the signals obtained indicates that >90% of p75NTR was excluded from the GEM fraction in both MDCK and FRT cells. (B) MDCK cells or MDCK/hMAL-ΔN cells were transfected with AM or AS oligonucleotide, plated on tissue culture inserts, and incubated at 37°C for 48 h. Cells were then infected with a recombinant adenovirus expressing p75NTR, and 8 h later the cell surface was labeled with sulfo-NHS-biotin from either the apical or the basolateral faces of the insert. After cell lysis, the extracts were immunoprecipitated with anti-p75NTR antibodies, and surface p75NTR was detected by immunoblotting with streptavidin-peroxidase. As a control, surface expression of E-cadherin was analyzed. The endogenous (dMAL) or the exogenous tagged hMAL-ΔN levels in these cells were examined by immunoblot analysis with mAb 2E5 or 9E10, respectively. (C) Quantitative analysis of the effect of MAL depletion on the polarized delivery p75NTR. The intensity of the apical and basolateral signal corresponding to newly delivered surface p75NTR was quantified. The values obtained are represented as percentages of biotinylated p75NTR on the apical (black bars) or basolateral (gray bars) surface.
Influenza Virus HA Access to GEMs during Biosynthetic Transport in FRT Cells
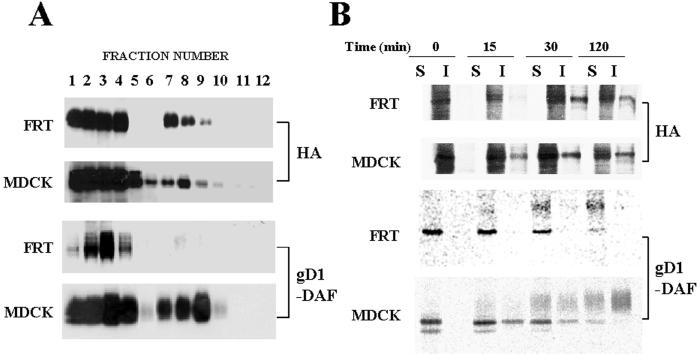
Although FRT cells are able to assemble glycolipids and cholesterol to form GEM rafts, gD1-DAF as well as other GPI-anchored proteins and endogenous transmembrane proteins examined are excluded from these specialized membranes (Zurzolo et al., 1994). To investigate whether HA is able to access GEMs in FRT cells, cells were infected with influenza virus and extracted with 1% Triton X-100 at 4°C after 4 h of infection. The extracts were then centrifuged to equilibrium in sucrose density gradients, and the presence of HA in GEMs was analyzed by immunoblotting of the different fractions with anti-HA 12CA5 mAb. A similar experiment was carried out in parallel using infected MDCK cells as a control of the fractionation procedure. Figure 4A shows that HA was found in GEMs at similar levels in both FRT and MDCK cells. As a further control we analyzed the presence of the GPI-anchored gD1-DAF protein in GEMs by immunoblotting using FRT and MDCK cells stably expressing this chimera. Consistent with previous reports (Zurzolo et al., 1994), gD1-DAF protein was found exclusively in the soluble fractions in FRT cells, whereas a significant fraction of gD1-DAF was found in GEMs in MDCK cells.
Figure 4.
HA becomes incorporated into GEMs during biosynthetic transport in FRT cells. (A) The levels of influenza virus HA in GEMs are similar in infected FRT and MDCK cells. FRT and MDCK cells were infected with influenza virus and 4 h afterward were extracted with 1% Triton X-100 at 4°C. Lysates were centrifuged to equilibrium in a sucrose density gradient and fractionated from the bottom of the tube. Aliquots from the different fractions were analyzed with anti-HA 12CA5 mAb. In parallel, FRT and MDCK cells stably expressing gD1-DAF were subjected to a similar analysis with anti-gD1 antibodies. Fractions 1–4 are the 40% sucrose layer and contain the bulk of cellular membranes and cytosolic proteins, whereas fractions 5–12 are the 5–30% sucrose layer and contain GEMs. Occasionally, as was the case shown for gD1-DAF in FRT cells, fractions 1 and 4 of the bottom sucrose layer are distorted during the procedure, and soluble proteins concentrate in fractions 2 and 3. Quantification of the distribution of HA in the different fractions indicates that ∼20% of HA was insoluble in MDCK and FRT cells, whereas ∼40 and 3% of gD1-DAF partitioned in the insoluble fraction in MDCK and FRT cells, respectively. (B) HA is incorporated into GEMs during biosynthetic transport in FRT cells. FRT and MDCK cells were infected with influenza virus and after 2.5 h of infection were metabolically labeled with a 5-min pulse of [35S]methionine/cysteine. Cells were then incubated for the indicated times in normal medium lacking radioactive precursors and extracted with 1% Triton X-100 at 4°C, and the soluble (S) and insoluble (I) fractions were separated by centrifugation. Equivalent aliquots from these fractions were subjected to SDS-PAGE, and HA was monitored by autoradiography. In parallel, FRT and MDCK cells stably expressing gD1-DAF were subjected to a similar analysis. Newly synthesized gD1-DAF was monitored by autoradiography of the immunoprecipitates obtained with anti-gD1 antibodies.
Internal GEM rafts have been proposed as being platforms for polarized delivery of apical proteins in epithelial MDCK cells (Simons and Wandinger-Ness, 1990). Proteins using this pathway of transport become insoluble after biosynthesis (Skibbens et al., 1989; Brown and Rose, 1992). To compare the kinetics of acquisition of insolubility of HA in FRT and MDCK cells, we incubated the cells in the presence of [35S]methionine/cysteine for 5 min after 2.5 h of infection and analyzed the presence of radiolabeled HA in the soluble and insoluble (GEM) fractions at different times of chase using the procedure of Skibbens et al. (1989). Figure 4B shows that, after biosynthesis, HA became progressively incorporated into GEMs in both FRT and MDCK cells. As described previously (Zurzolo et al., 1994), we observed that gD1-DAF incorporated into GEMs in MDCK cells but not in FRT cells.
HA Transport to the Apical Surface Requires Intact GEMs in FRT Cells
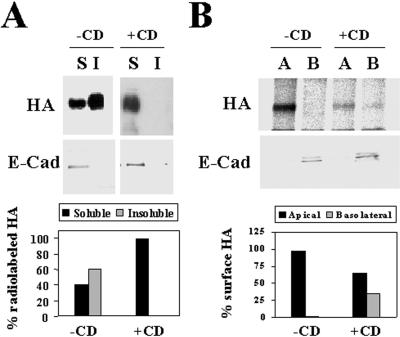
The observation that the majority of GPI-anchored proteins do not incorporate into GEMs in FRT cells and become basolaterally transported in this cell line indicates that the GEM-mediated pathway is not operative for the majority of GPI-anchored proteins in FRT cells (Zurzolo et al., 1994). To investigate whether HA depends on intact GEMs to be correctly targeted to the apical surface in FRT cells, we carried out experiments using cells whose endogenous cholesterol levels were depleted. FRT cells were treated with 25 μM compactin, an inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase, to inhibit cholesterol biosynthesis, in medium supplemented with 200 μM mevalonate to provide substrate for nonsterol biosynthetic processes. After 48 h, cells were infected with influenza virus and 2 h later were treated with 10 mM methyl-β-cyclodextrin to extract cholesterol from cellular membranes (Keller and Simons, 1998). Cells were then labeled with [35S]methionine/cysteine for 5 min and incubated for 30 min in normal medium. The soluble and insoluble fractions were separated by centrifugation, and the partition of HA was analyzed by autoradiography. Figure 5A shows that HA insolubility was sensitive to methyl-β-cyclodextrin treatment, suggesting that, consistent with previous findings in MDCK cells (Keller and Simons, 1998; Lin et al., 1998), HA requires intact cholesterol levels to become insoluble in FRT cells.
Figure 5.
Both insolubility and apical transport of HA in FRT cells are sensitive to methyl-β-cyclodextrin treatment. (A) FRT cells were pretreated or not in medium containing 25 μM compactin and 200 μM mevalonate for 48 h and infected with influenza virus. After 2 h of infection, the cultures pretreated with compactin and mevalonate were treated with 10 mM methyl-β-cyclodextrin (+CD) for 1 h, and then all cell cultures were labeled for 5 min with [35S]methionine/cysteine. After incubation in normal medium for 30 min at 20°C, cells were lysed with 1% Triton X-100 at 4°C, and the soluble (S) and insoluble (I) fractions were separated by centrifugation. Aliquots from these fractions were subjected to SDS-PAGE. The partition of newly synthesized HA was analyzed by autoradiography. As an internal control, the same samples were subjected to immunoblot analysis with anti-E-cadherin antibodies to show that E-cadherin was present exclusively in the soluble fractions. The bottom panel shows a quantitative analysis of the effect of the treatment with methyl-β-cyclodextrin on HA solubility. (B) FRT cells grown in filter culture inserts were pretreated or not with compactin plus mevalonate as in A and then infected with influenza virus. After 2 h, 10 mM methyl-β-cyclodextrin was added to both the apical and basolateral compartments of the pretreated cell cultures. After 1 h, cells were labeled for 5 min with [35S]methionine/cysteine and incubated in normal medium for 1 h at 37°C. Cells were surface biotinylated from either the apical or the basolateral compartment and lysed with 1% Triton X-100 plus 60 mM octyl-glucoside, and the lysates were immunoprecipitated with streptavidin-agarose. As a control, we observed that the total levels of newly synthesized radiolabeled HA were similar in control cells and in cells treated with methyl-β-cyclodextrin (our unpublished results). The apical or basolateral delivery of HA was monitored by autoradiography of the corresponding immunoprecipitates. As an internal control, the surface distribution of E-cadherin, a basolateral protein, was determined by immunoblot analysis of the streptavidin-agarose immunoprecipitates. The bottom panel shows a quantitative analysis of the effect of the treatment with methyl-β-cyclodextrin on the polarized delivery of HA to the cell surface.
To demonstrate that HA requires GEM to be correctly transported to the apical membrane in FRT cells, cells grown on filter culture inserts were preincubated with compactin and mevalonate for 48 h and were then infected with influenza virus. After 2 h of infection, cells were treated with methyl-β-cyclodextrin for 1 h, and newly synthesized HA was labeled with a 5-min pulse of [35S]methionine/cysteine. After 1 h, cells were biotinylated from either the apical or basolateral compartments and lysed in the presence of 1% Triton X-100 and 60 mM octyl-glucoside. The arrival of radiolabeled HA at the apical or basolateral domains was monitored by autoradiography of the streptavidin-agarose immunoprecipitates obtained using the corresponding biotinylated extracts. Figure 5B shows that, similar to the case in MDCK cells (Keller and Simons, 1998), intact GEMs are also required in FRT cells for normal delivery of HA to plasma membrane and for its accurate sorting to the apical surface. As an internal control, the sorting of E-cadherin, a basolateral protein of FRT cells, was determined by immunoblot analysis with anti-E-cadherin antibodies of the streptavidin-agarose immunoprecipitates. Finally, we addressed whether intact GEMs are necessary for the basolateral sorting of gD1-DAF in FRT cells. Treatment with compactin/methyl-β-cyclodextrin did not show any effect on the surface delivery of gD1-DAF (our unpublished results), indicating that, in FRT cells, this molecule is not targeted to the apical surface even under conditions in which HA is partially missorted to the basolateral surface because of the absence of fully functional GEMs.
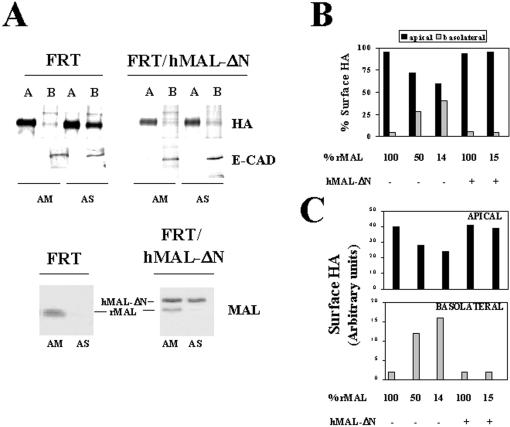
Correct Apical Transport of HA, p75NTR, and DPPIV in FRT Cells Requires Expression of the MAL Proteolipid
Depletion of endogenous MAL in MDCK cells causes reduction of apical transport and partial missorting of HA to the basolateral membrane (Puertollano et al., 1999). Although the MAL-mediated pathway of apical transport is not functional for the majority of GPI-anchored proteins in FRT cells, because this type of protein is basolateral in this cell line (Zurzolo et al., 1993), we examined whether the MAL-dependent apical transport pathway is operative for HA in FRT cells. To this end, we compared the apical and basolateral delivery of HA in FRT cells with either normal or depleted levels of MAL. Cells were transfected with either AM or AS oligonucleotide and seeded at high density (3.5–5.0 × 105 cells/cm2) on filter culture inserts. After 48 h at 37°C, the integrity of the cell monolayers was checked, and intact cell monolayers were infected with influenza virus. Two and one-half hours after removal of the inoculum, newly synthesized proteins were labeled with [35S]methionine/cysteine for 2 h. Surface proteins were then separately biotinylated from the apical or basolateral surface and immunoprecipitated with streptavidin-agarose. The apical or basolateral surface expression of HA was determined by autoradiography of the corresponding streptavidin-agarose immunoprecitate. As an internal control, the sorting of E-cadherin was determined by immunoblot analysis with anti-E-cadherin antibodies of the streptavidin-agarose immunoprecipitates. The extent of MAL depletion obtained in each experiment was quantified by densitometric scanning of immunoblots of the initial lysates with anti-MAL 6D9 mAb. A representative experiment in which MAL levels dropped to ∼14% of those in control cells is shown in Figure 6A, left panel. Whereas only 5% of surface HA was on the basolateral membrane in control FRT cells, missorting to this domain increased to ∼40% in the cells with reduced MAL levels. To confirm that the observed effects were due to MAL depletion and not to spurious effects of the AS oligonucleotide, we took advantage of the selectivity of oligonucleotide AS in blocking expression of endogenous rMAL but not of hMAL-ΔN in FRT/hMAL-ΔN cells to demonstrate whether ectopic expression of MAL rescues the effects observed in MAL-depleted FRT cells. Figure 6A, right panel, shows that the expression of exogenous MAL restored apical transport of HA and prevented HA missorting to the basolateral membrane, despite the drop of endogenous MAL to ∼15% of that in normal cells. A compilation of the results obtained on HA transport to the apical or basolateral membranes from experiments in which MAL was depleted to different extents is shown in Figure 6B. Quantitative analysis of the amount of HA on the apical or basolateral domains indicates that, as occurs in MDCK cells (Puertollano et al., 1999), the altered ratio of apical to basolateral HA targeting observed upon MAL depletion was due not only to decreased HA transport to the apical surface but also to a concomitant increased transport of HA to the basolateral surface (Figure 6C).
Figure 6.
MAL is necessary for correct transport of influenza virus HA in FRT cells. (A) Wild-type FRT cells or FRT/hMAL-ΔN cells were transfected with AM or AS oligonucleotide and plated on 24-mm-diameter tissue culture inserts. After 48 h, cells were infected with influenza virus and 2.5 h later were labeled with [35S]methionine/cysteine for 2 h. Apical or basolateral surface proteins were then labeled in separate culture inserts with sulfo-NHS-biotin. After cell lysis, biotinylated proteins were immunoprecipitated with streptavidin-agarose. To detect apical or basolateral surface expression of HA and E-cadherin, the immunopre-cipitates were subjected to autoradiography to detect radiolabeled HA or to immunoblot analysis, respectively. The endogenous (rMAL) or the exogenous hMAL-ΔN levels in these cells were examined by immunoblot analysis with mAb 6D9. (B and C) Quantitative analysis of the effect of MAL depletion on the polarized delivery of HA in FRT cells. The intensity of the apical and basolateral signal corresponding to radiolabeled HA from experiments in which MAL was depleted at different extensions was quantified. The values obtained are represented as percentages of radiolabeled HA on the apical or basolateral surface (B) or expressed in arbitrary units as apical and basolateral absolute surface content of radiolabeled HA (C). The results of some representative experiments are shown. The effects observed in other experiments in which rMAL was depleted to different extents (our unpublished results) were consistent with those shown. Black bars, apical; gray bars, basolateral.
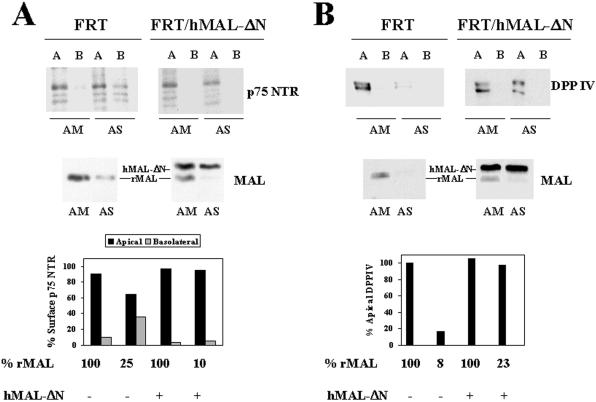
To investigate whether the dependence on MAL expression observed for normal surface transport of HA in FRT cells is extended to apical transmembrane proteins that are excluded from GEMs, we analyzed the effect of MAL depletion on the targeting of exogenous p75NTR and endogenous DPPIV protein (Zurzolo et al., 1994). Figure 7A shows that, similar to HA (Figure 6), a drop in MAL content to ∼25% of that in control cells reduced by approximately one-fourth the absolute delivery of p75NTR to the apical subdomain and increased missorting of surface p75NTR to the basolateral subdomain (bottom panel). In the case of endogenous DPPIV, the absolute apical delivery was reduced to ∼17% of that in control cells when MAL levels were lowered to 8% (Figure 7B), but missorting to the basolateral subdomain was not detected. Thus, MAL is also required for normal transport of the transmembrane p75NTR and DPPIV molecules in FRT cells.
Figure 7.
MAL depletion affects the polarized transport of p75NTR in FRT cells. (A) FRT cells or FRT/hMAL-ΔN cells were transfected with AM or AS oligonucleotide, plated on tissue culture inserts, and incubated at 37°C for 48 h. Cells were then infected with recombinant adenovirus expressing p75NTR, and 8 h later the cell surface was labeled with sulfo-NHS-biotin from either the apical or the basolateral faces of the insert. After cell lysis, the extracts were immunoprecipitated with anti-p75NTR antibodies, and p75NTR was detected by immunoblotting with streptavidin-peroxidase. The endogenous (rMAL) or exogenous (hMAL-ΔN) MAL levels in these cells were examined by immunoblot analysis with mAb 6D9. The intensity of the apical and basolateral signal corresponding to surface p75NTR was quantified. The values obtained are represented as percentages of surface p75NTR on the apical (black bars) or basolateral (gray bars) subdomain. (B) FRT and FRT/hMAL-ΔN cells were transfected with AM or AS oligonucleotide, plated on tissue culture inserts, and incubated at 37°C for 48 h. To study surface delivery of endogenous DPPIV, cells were repeatedly treated with sulfo-SHPP from both the apical and basolateral faces of the insert. After incubation at 37°C for 7 h, the appearance of new DPPIV molecules was monitored by domain-selective labeling with sulfo-NHS-biotin. After cell lysis, the extracts were immunoprecipitated with anti-DPPIV antibodies and were subjected to SDS-PAGE and analyzed with streptavidin-peroxidase. Endogenous (rMAL) and exogenous (hMAL-ΔN) MAL levels were analyzed by immunoblotting with mAb 6D9. The intensity of the apical and basolateral signal corresponding to newly delivered surface DPPIV was quantified. The values obtained are represented as percentages of the DPPIV delivered to the apical surface in MAL-depleted cells relative to that in control cells during the 7-h period of chase. No basolateral signal was detectable for DPPIV under any of the conditions used.
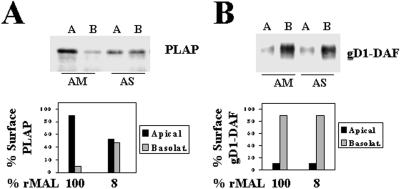
MAL Depletion Affects the Normal Transport of PLAP to the Apical Surface but Not the Basolateral Delivery of gD1-DAF in FRT Cells
To investigate the possible role of MAL in GPI-anchored protein transport in FRT cells, we used PLAP and gD1-DAF as representatives of GPI-anchored proteins targeted to the apical and the basolateral surface subdomain, respectively (Zurzolo et al., 1993; Lipardi et al., 2000). Figure 8A shows that depletion of MAL to 8% of that in control cells reduced to 50% the absolute delivery of PLAP to the apical surface and, concomitantly, caused missorting of the protein to the basolateral subdomain (bottom panel). Normal transport of PLAP was observed in FRT/hMAL-ΔN cells whose endogenous MAL protein was depleted (our unpublished results). Figure 8B shows in a similar experiment that the effect observed for PLAP is specific for this apical GPI-anchored protein, because MAL depletion did not affect the basolateral delivery of gD1-DAF in FRT cells.
Figure 8.
Effect of MAL depletion on the polarized transport of PLAP and gD1-DAF in FRT cells. FRT cells stably expressing PLAP (A) or gD1-DAF (B) were transfected with AM or AS oligonucleotide, plated on tissue culture inserts, and incubated at 37°C for 48 h. To study surface delivery of newly synthesized molecules, cells were repeatedly treated with sulfo-SHPP from both the apical and basolateral faces of the insert. After incubation at 37°C for 7 h, the appearance of new free surface amino groups was monitored by domain-selective labeling with sulfo-NHS-biotin. After cell lysis, the extracts were immunoprecipitated with the appropriate antibodies, and new surface-delivered PLAP and gD1-DAF were detected by immunoblotting with streptavidin-peroxidase. The levels of endogenous rMAL were analyzed by immunoblotting with mAb 6D9. The bottom panels show a quantitative analysis of the effect of MAL depletion on the polarized delivery of these two GPI-anchored molecules. The intensity of the apical and basolateral signal corresponding to newly delivered surface molecules was quantified, and the values obtained are represented as percentages of surface PLAP or gD1-DAF on the apical (black bars) or basolateral (gray bars) subdomain.
MAL Is Required for the Overall Apical Transport of Membrane Proteins in MDCK and FRT Cells
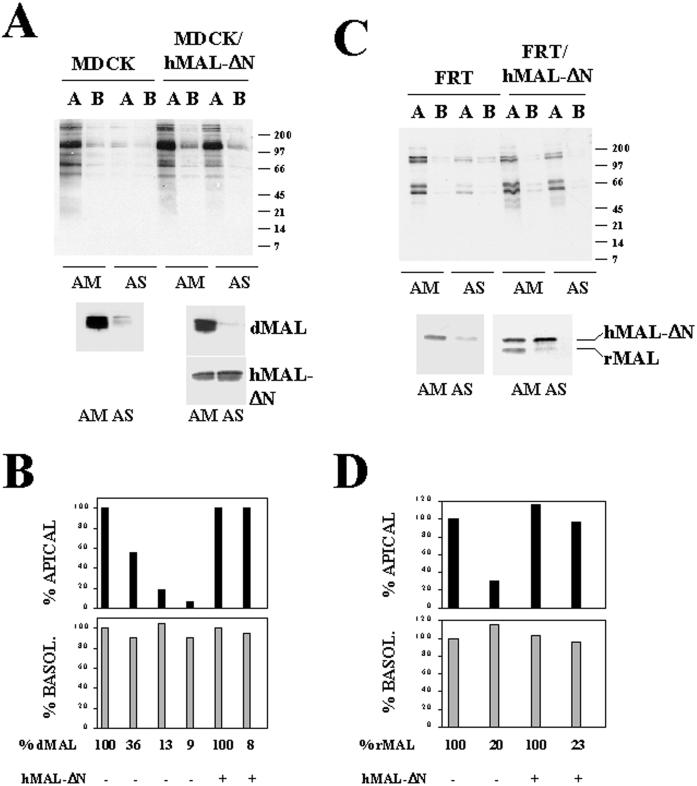
The observations that HA, p75NTR, and gD1-DAF require normal MAL levels to be apically transported in MDCK cells prompted us to investigate whether the overall apical transport in these cells is dependent on MAL levels. MDCK cells were transfected with AM or AS oligonucleotide and seeded at high density on filter culture inserts. After 48 h at 37°C, intact cell monolayers were treated with sulfo-SHPP and 7 h later were subjected to domain-selective labeling with sulfo-biotin. After cell lysis, aliquots from the extracts were analyzed by immunoblotting with streptavidin-peroxidase to detect newly surface-delivered proteins. No effect on the overall protein synthesis was observed by the transfection with the oligonucleotides as assayed by incorporation of [35S]methionine/cysteine into 10% trichloroacetic acid-insoluble material (our unpublished results). The extent of MAL depletion obtained in each experiment was quantified by densitometric scanning of immunoblots of the initial lysates with anti-dMAL 2E5 mAb. Although at steady state the basolateral surface has a higher content in membrane proteins than does the apical subdomain (Sargiacomo et al., 1989; our unpublished results), Figure 9A, left panel, shows that in control cells the delivery of membrane proteins after 7 h of chase was higher in the apical than on the basolateral surface (AM). Consistent with the results obtained for HA and gD1-DAF, when MAL levels dropped to ∼9% (AS) the overall apical transport was reduced to 7% of that in control cells. In contrast with the results obtained for HA and gD1-DAF, no detectable missorting of proteins to the basolateral surface was observed. Furthermore, apical transport was restored in MDCK cells by the exogenous expression of hMAL-ΔN despite the drop in the endogenous dMAL levels (Figure 9A, right panel). Results from experiments in which MAL was depleted to different extents are compiled in Figure 9B. Finally, to examine whether the results obtained for transport of HA, p75NTR, DPPIV, and PLAP in FRT cells can be extrapolated to the rest of apical membrane proteins, we carried out experiments similar to that illustrated in Figure 9A using this cell line. Figure 9, C and D, show that, even in the absence of apical targeting of GPI-anchored proteins, the overall apical transport of membrane proteins was dependent on the level of MAL expression. In summary, Figure 9 clearly indicates that MAL is necessary for the overall apical transport of membrane proteins in both MDCK and FRT cells.
Figure 9.
Effect of MAL depletion on the overall apical delivery of membrane proteins in MDCK and FRT cells. MDCK and MDCK/hMAL-ΔN cells (A and B) or FRT and FRT/hMAL-ΔN cells (C and D) were transfected with AM or AS oligonucleotide, plated on tissue culture inserts, and incubated at 37°C for 48 h. To study surface delivery of membrane proteins, cells were repeatedly treated with sulfo-SHPP from both the apical and basolateral faces of the insert. After incubation at 37°C for 7 h, the appearance of new surface proteins was monitored by domain-selective labeling with sulfo-NHS-biotin. After cell lysis, the extracts were subjected to SDS-PAGE and analyzed with streptavidin-peroxidase. Endogenous MAL and exogenous hMAL-ΔN were separately analyzed by immunoblotting with mAb 2E5 and 9E10 (MDCK cells), respectively, or simultaneously with mAb 6D9 (FRT cells). (A and C) Representative experiment. (B and D) Quantitative analysis of the effect of MAL depletion on the polarized delivery of membrane proteins. The intensity of the apical and basolateral signal corresponding to newly delivered surface proteins from experiments in which MAL was depleted at different extensions was quantified by densitometric analysis of the entire lanes. The values obtained are expressed as percentages of the apical (black bars) or (gray bars) basolateral delivery obtained in normal cells transfected with the control AM oligonucleotide. The results of some representative experiments are shown. The effects observed in other experiments in which MAL was depleted to different extents (our unpublished results) were consistent with those shown.
DISCUSSION
The MAL-mediated Route of Apical Transport Is Operative Even Under Conditions in Which It Is Not Functional for the Majority of GPI-anchored Proteins
FRT cells are able to assemble GEM clusters, but the majority of GPI-anchored proteins are excluded from them and become transported basolaterally (Zurzolo et al., 1993, 1994). Our results show that, contrary to the case of the GPI-anchored gD1-DAF protein, HA incorporates into GEMs in FRT cells during biosynthetic transport with an efficiency similar to that in MDCK cells. Both HA insolubility and targeting to the apical membrane in FRT cells were dependent on GEM integrity, because treatments that lower cellular cholesterol levels diminished the presence of HA in GEMs and reduced its targeting to the apical surface with a concomitant partial missorting to the basolateral domain. Moreover, MAL depletion produced an effect on the polarized transport of HA similar to that obtained by decreasing the cellular cholesterol levels, indicating that MAL is necessary for GEM-mediated apical targeting of HA in FRT cells. The finding that gD1-DAF is unable to reach the apical surface in FRT cells even under conditions in which GEMs are disrupted or MAL is depleted suggests that the existence of a functional GEM-mediated apical pathway is not necessary for the basolateral sorting of GPI-anchored proteins in this cell line. The demonstration that the MAL-mediated apical pathway of transport is functional for HA and PLAP but not for gD1-DAF in FRT cells together with the observation that MAL is necessary for apical transport of GPI-anchored proteins in MDCK cells indicates that gD1-DAF and the majority of endogenous GPI-anchored proteins of FRT cells have additional requirements for apical sorting.
For secretory proteins, N-glycans appear to act as apical targeting signals (Scheiffele and Simons, 1995). It has been hypothesized that a transmembrane lectin might mediate the recruitment of secretory proteins into GEM rafts (Fiedler and Simons, 1995). Similarly, clustering of GPI-anchored proteins might be dependent on a transmembrane receptor recognizing sorting features in their GPI-anchor and/or ectodomain. The observations that apical sorting of gD1-DAF occurs in the absence of N-glycosylation (Lisanti et al., 1990), and that gp80, a secretory glycoprotein that requires intact GEMs to be correctly transported (Keller and Simons, 1998), is apically secreted in both MDCK and FRT cells (Graichen et al., 1996), argue against the secretory and GPI-anchored proteins depending on the same clustering factor for apical sorting. Although MAL is required for apical transport of gD1-DAF, obviously our results in FRT cells indicate that it does not function as a clustering factor for the majority of GPI-anchored proteins. Rather, our previous results suggest that MAL functions in other steps of apical transport such as vesiculation of GEM rafts (Puertollano et al., 1997) and in the regulation of the traffic of apically destined vesicles (Puertollano and Alonso, 1998, 1999).
MAL Is an Essential Element for the Overall Transport of Apical Membrane Proteins in MDCK and FRT Cells
The influenza virus HA molecule has been considered to be a paradigm of transmembrane apical proteins. However, whereas HA becomes insoluble soon after biosynthesis, most of the apical integral proteins analyzed do not. This has led to the idea that there are both GEM-mediated and -independent pathways for apical transport (Zurzolo et al., 1994). However, it is still possible that the GEM-mediated pathway is the only direct route to the apical surface and that insolubility in nonionic detergent at low temperatures, despite being a useful operational assay for investigating the incorporation of certain proteins into rafts, might not be a valid general test to determine the presence of proteins in rafts in vivo. The fact that MAL depletion in MDCK cells affects apical transport of transmembrane proteins included (HA) or excluded (p75NTR) from GEMs might be interpreted either as that 1) two types of apical vesicles exist for transmembrane proteins, one enriched in GEM-associated proteins and the other enriched in proteins excluded from GEMs; and 2) only one type of apical vesicles exists with both GEM- and non–GEM-associated transmembrane proteins. Alternatively, it is also possible that functional GEMs are required as a barrier to exclude specific proteins that use a different apical route. The evidence in the present study showing that, in addition to the effect on HA and gD1-DAF transport, the reduction of MAL levels in MDCK cells produces a decrease in apical transport of p75NTR, a transmembrane protein excluded from GEMs, and in the bulk of apical membrane proteins, indicates not only that MAL is necessary for the apical transport of a limited set of proteins but also that it is a general element of the machinery for the overall delivery of apical membrane protein in MDCK cells.
Both PLAP (Benting et al., 1999) and HA (Figure 4) are found associated with lipid rafts in FRT cells. Transport of PLAP to the apical surface in these cells is sensitive to treatment with fumonisin B1 but resistant to cholesterol sequestration with methyl-β-cyclodextrin (Lipardi et al., 2000). On the other hand, we have shown in this work that apical transport of HA in FRT cells is sensitive to cholesterol depletion. Thus, it appears that at least two different pathways of transport exist for apical proteins present in rafts: one sensitive to fumonisin B1 and the other dependent on normal levels of cholesterol. A third pathway, insensitive to both fumonisin B1 treatment and cholesterol depletion, has been proposed for proteins, such as p75NTR, that are not associated with GEMs (Lipardi et al., 2000). The requirement of MAL for apical transport of PLAP, HA, p75NTR, DPPIV, and the overall apical membrane protein in FRT cells indicates that MAL is a common element of the machinery for apical delivery of membrane proteins in this cell line.
Curiously, whereas MAL depletion caused partial missorting of exogenous HA, p75NTR, and gD1-DAF to the basolateral surface in MDCK cells, no increase in basolateral delivery was observed when the apical transport of the bulk of endogenous membrane proteins or endogenous DPPIV was examined. This indicates that the missorting observed is probably due to overexpression of the reporter molecules or, more unlikely, to the existence of sorting features in all these molecules that make them respond to MAL depletion in a manner different from that of most endogenous membrane proteins. In addition, a greater reduction in apical delivery was observed for the endogenous proteins compared with that of the exogenous ones. The finding that apical endogenous membrane proteins are not missorted to the basolateral surface when apical transport is reduced by MAL depletion implies that these proteins are retained intracellularly under these conditions. This observation is consistent with the dramatic accumulation of the endogenous transmembrane protein gp114 in the Golgi observed in MDCK II cells with reduced levels of MAL (Cheong et al., 1999). Collectively, the findings presented herein support our previous proposal of MAL as responsible for the formation and trafficking of apical transport vesicles in polarized epithelial cells (Puertollano et al., 1997; Puertollano and Alonso, 1999) and highlight MAL as a central component of the integral protein machinery for apical transport.
ACKNOWLEDGMENTS
We express our gratitude to Dr. E. Rodriguez-Boulan for generosity and advice. This work was supported by grants from the Dirección General de Enseñanza Superior (PM96-0004) and the Comunidad de Madrid (08.3/0020/1998). An institutional grant from the Fundación Ramón Areces to Centro de Biología Molecular “Severo Ochoa” is also acknowledged. F.M.-B, R.P., and J.M. are recipients of fellowships from the Comunidad de Madrid.
Abbreviations used:
- d
dog
- DPPIV
dipeptidyl peptidase IV
- FRT
Fischer rat thyroid
- GEM
glycolipid- and cholesterol-enriched membrane
- GPI
glycosylphosphatidylinositol
- h
human
- HA
hemagglutinin
- MDCK
Madin–Darby canine kidney
- NTR
neurotrophin receptor
- PLAP
placental alkaline phosphatase
- r
rat
- sulfo-NHS-biotin
sulfo-N-hydroxyl-succinimido-biotin
- sulfo-SHPP
sulfo-N-hydroxyl-succinimido-phenyl-propionate
REFERENCES
- Alonso MA, Weissman SM. cDNA cloning and sequence of MAL, a hydrophobic protein associated with human T-cell differentiation. Proc Natl Acad Sci USA. 1987;84:1997–2001. doi: 10.1073/pnas.84.7.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benting J, Rietveld A, Ansorge I, Simons K. Acyl and alkyl chain length of GPI-anchors is critical for raft association in vitro. FEBS Lett. 1999;462:47–50. doi: 10.1016/s0014-5793(99)01501-x. [DOI] [PubMed] [Google Scholar]
- Brown DA, Crise B, Rose JK. Mechanism of membrane anchoring affects polarized expression of two proteins in MDCK cells. Science. 1989;245:1499–1501. doi: 10.1126/science.2571189. [DOI] [PubMed] [Google Scholar]
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Cheong KH, Zacchetti D, Schneeberger EE, Simons K. VIP17/MAL, a lipid raft-associated protein, is involved in apical transport in MDCK cells. Proc Natl Acad Sci USA. 1999;96:6241–6248. doi: 10.1073/pnas.96.11.6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler K, Simons K. The role of N-glycans in the secretory pathway. Cell. 1995;81:309–312. doi: 10.1016/0092-8674(95)90380-1. [DOI] [PubMed] [Google Scholar]
- Graichen R, Lösch A, Appel D, Koch-Brandt C. Glycolipid-independent sorting of a secretory glycoprotein to the apical surface of polarized epithelial cells. J Biol Chem. 1996;271:15854–15857. doi: 10.1074/jbc.271.27.15854. [DOI] [PubMed] [Google Scholar]
- Keller P, Simons K. Cholesterol is required for surface transport of influenza virus hemagglutinin. J Cell Biol. 1998;140:1357–1367. doi: 10.1083/jcb.140.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T, Fiedler K, Madison DL, Krueger W H, Pfeiffer SE. Cloning and characterization of MVP17: a developmentally regulated myelin protein in oligodendrocytes. J Neurosci Res. 1995;42:413–422. doi: 10.1002/jnr.490420316. [DOI] [PubMed] [Google Scholar]
- Lin S, Naim HY, Rodriguez AC, Roth MG. Mutations in the middle of the transmembrane domain reverse the polarity of transport of the influenza virus hemagglutinin in MDCK epithelial cells. J Cell Biol. 1998;142:51–57. doi: 10.1083/jcb.142.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipardi C, Nitsch L, Zurzolo C. Detergent-insoluble GPI-anchored. proteins are apically sorted in Fischer rat thyroid cells, but interference with cholesterol or sphingolipids differentially affects detergent insolubility and apical sorting. Mol Biol Cell. 2000;11:531–542. doi: 10.1091/mbc.11.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti MP, Caras IW, Davitz MA, Rodriguez-Boulan E. A glycophospholipid membrane anchor acts as an apical targeting signal in polarized epithelial cells. J Cell Biol. 1989;109:2145–2156. doi: 10.1083/jcb.109.5.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti MP, Caras IW, Gilbert T, Hanzel D, Rodriguez-Boulan E. Vectorial apical delivery and slow endocytosis of a glycolipid-anchored fusion protein in transfected MDCK cells. Proc Natl Acad Sci USA. 1990;87:7419–7423. doi: 10.1073/pnas.87.19.7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Belmonte F, Kremer L, Albar JP, Marazuela M, Alonso MA. Expression of the MAL gene in the thyroid: the MAL proteolipid, a component of glycolipid-enriched membranes, is apically distributed in thyroid follicles. Endocrinology. 1998;139:2077–2084. doi: 10.1210/endo.139.4.5875. [DOI] [PubMed] [Google Scholar]
- Matter K, Mellman I. Mechanisms of cell polarity: sorting and transport in epithelial cells. Curr Opin Cell Biol. 1994;6:545–554. doi: 10.1016/0955-0674(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Millán J, Puertollano R, Fan L, Alonso MA. Caveolin and MAL, two protein components of internal detergent-insoluble membranes, are in distinct lipid microenvironments in MDCK cells. Biochem Biophys Res Commun. 1997;233:707–712. doi: 10.1006/bbrc.1997.6530. [DOI] [PubMed] [Google Scholar]
- Puertollano R, Alonso MA. A short peptide motif at the carboxyl terminus is required for incorporation of the integral membrane MAL protein to glycolipid-enriched membranes. J Biol Chem. 1998;233:12740–12745. doi: 10.1074/jbc.273.21.12740. [DOI] [PubMed] [Google Scholar]
- Puertollano R, Alonso MA. MAL, an integral element of the apical sorting machinery, is an itinerant protein that cycles between the trans-Golgi network and the plasma membrane. Mol Biol Cell. 1999;10:3435–3447. doi: 10.1091/mbc.10.10.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puertollano R, Li S, Lisanti MP, Alonso MA. Recombinant expression of the MAL proteolipid, a component of glycolipid-enriched membrane microdomains, induces the formation of vesicular structures in insect cells. J Biol Chem. 1997;272:18311–18315. doi: 10.1074/jbc.272.29.18311. [DOI] [PubMed] [Google Scholar]
- Puertollano R, Martín-Belmonte F, Millán J, de Marco MC, Albar JP, Kremer L, Alonso MA. The MAL proteolipid is necessary for normal apical transport and accurate sorting of the influenza virus hemagglutinin in Madin-Darby canine kidney cells. J Cell Biol. 1999;145:141–145. doi: 10.1083/jcb.145.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sargiacomo M, Lisanti M, Graeve L, Le Bivic A, Rodriguez-Boulan E. Integral and peripheral protein composition of the apical and basolateral membrane domains in MDCK cells. J Membr Biol. 1989;107:277–286. doi: 10.1007/BF01871942. [DOI] [PubMed] [Google Scholar]
- Sargiacomo M, Sudol M, Tang Z, Lisanti MP. Signal transducing molecules and glycosyl-phophatidylinositol-linked proteins form a caveolin-rich insoluble complex in MDCK cells. J Cell Biol. 1993;122:789–807. doi: 10.1083/jcb.122.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiffele P, Roth MG, Simons K. Interaction of influenza virus hemagglutinin with sphingolipid-cholesterol membrane domains via its transmembrane domain. EMBO J. 1998;16:5501–5508. doi: 10.1093/emboj/16.18.5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiffele P, Simons K. N-glycans as apical sorting signals in epithelial cells. Nature. 1995;378:96–98. doi: 10.1038/378096a0. [DOI] [PubMed] [Google Scholar]
- Simons K, Wandinger-Ness A. Polarized sorting in epithelia. Cell. 1990;62:207–210. doi: 10.1016/0092-8674(90)90357-k. [DOI] [PubMed] [Google Scholar]
- Skibbens JE, Roth MG, Matlin KS. Differential extractability of influenza virus hemagglutinin during intracellular transport in polarized epithelial cells and nonpolar fibroblasts. J Cell Biol. 1989;108:821–832. doi: 10.1083/jcb.108.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandinger-Ness A, Bennett MK, Antony C, Simons K. Distinct transport vesicles mediate the delivery of plasma membrane proteins to the apical and basolateral domains of MDCK cells. J Cell Biol. 1990;111:987–1000. doi: 10.1083/jcb.111.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman C, Le Gall AH, Baldwin AN, Monlauzeur L, Le Bivic A, Rodriguez-Boulan E. The O-glycosylated stalk domain is required for apical sorting of neurotrophin receptors in polarized MDCK cells. J Cell Biol. 1997;139:929–940. doi: 10.1083/jcb.139.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacchetti D, Peranen J, Murata M, Fiedler K, Simons K. VIP17/MAL, a proteolipid in apical transport vesicles. FEBS Lett. 1995;377:465–469. doi: 10.1016/0014-5793(95)01396-2. [DOI] [PubMed] [Google Scholar]
- Zurzolo C, Lisanti MP, Caras IW, Nitsch L, Rodriguez-Boulan E. Glycosylphosphatidylinositol-anchored proteins are preferentially targeted to the basolateral surface in Fischer rat thyroid epithelial cells. J Cell Biol. 1993;121:1031–1039. doi: 10.1083/jcb.121.5.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurzolo C, van't Hof W, van Meer G, Rodriguez-Boulan E. VIP21/caveolin, glycosphingolipid clusters and the sorting of glycosylphophatidylinositol-anchored proteins in epithelial cells. EMBO J. 1994;13:42–53. doi: 10.1002/j.1460-2075.1994.tb06233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]