Abstract
Candida dubliniensis is a recently described Candida species associated with oral candidiasis in human immunodeficiency virus (HIV)-infected patients and patients with AIDS. The majority of C. dubliniensis clinical isolates tested to date are susceptible to the commonly used antifungal drugs, including fluconazole, ketoconazole, itraconazole, and amphotericin B. However, the appearance of fluconazole-resistant C. dubliniensis strains in this patient group is increasing. Histatins are a family of basic histidine-rich proteins present in human saliva which have therapeutic potential in the treatment of oral candidiasis. The mechanism of action of histatin is distinct from that of commonly used azole and polyene drugs. Characterization of the antifungal activity of histatin has mainly been carried out using C. albicans but it is also effective in killing C. glabrata and C. krusei. Here we report that C. dubliniensis is also susceptible to killing by histatin 3. The concentration of histatin 3 giving 50% killing (the IC50 value) ranged from 0.043 to 0.196 mg/ml among different strains of C. dubliniensis. The least-susceptible C. dubliniensis strain, P9224, was found to internalize histatin at a lower rate than the C. albicans reference strain CA132A. The dissociation constant (Kd) for the least-susceptible strain (C. dubliniensis 9224) was ninefold higher than that for the C. albicans reference strain. These results suggest that histatin 3 may have potential as an effective antifungal agent, particularly in the treatment of oral candidiasis in HIV-infected patients and patients with AIDS in which resistance to the commonly used antifungal drug fluconazole has emerged.
Histatins are a family of low-molecular-weight, cationic, histidine-rich peptides that are found in human saliva and which have potent and broad-spectrum antifungal activity. A number of histatin peptides have now been identified in saliva. Histatin 1 and histatin 3 are the gene products of the HIS1 and HIS2 genes, respectively, while histatin 2 and histatins 4 to 12 are their proteolytic cleavage products. One of the first in vivo observations on the role of histatin was that the concentration of histatins was greatly reduced in a group of human immunodeficiency virus (HIV)-infected patients with oral candidiasis (16). Clinical observations in other groups suggest that patients with inherently low levels of salivary histatins are predisposed to oral carriage of yeasts; however, the expression of histatins may be upregulated in response to actual candidal infection (2, 13). Histatin 5 is the most potent of the histatins in killing the blastospore and germinated forms of Candida albicans, followed by histatin 3, although histatin 3 is the most efficient in inhibiting germination of blastoconidia (37). Characterization of the antifungal activity of the histatins has been performed mainly with Candida albicans, the most common and the most pathogenic oral Candida species (5). However, histatins are also active against other yeasts and fungi—including Candida glabrata, Candida krusei, Saccharomyces cerevisiae, and Cryptococcus neoformans (11, 28, 29, 35, 37)—and some bacterial species—including Streptococcus mutans, Porphyromonas gingivalis, and Actinobacillus actinomycetemcomitans (18, 22). The mechanism of action of histatin, though not yet fully elucidated, involves pathways distinct from those involved in the mechanisms of action of commonly used antifungal drugs, such as polyenes and azole antifungals. Importantly, histatin 5 has been found to be active against amphotericin-resistant and azole-resistant Candida isolates and species (12). The current proposal on the sequence of events leading to histatin-mediated cell death is as follows: (i) binding of histatin to a fungal membrane receptor, (ii) translocation of histatin across the membrane, (iii) release of histatin into the intracellular compartment, (iv) interaction of histatin with cellular targets, and (iv) release of ATP (7, 11, 15, 39)
Candida dubliniensis is a recently described Candida species associated with oral colonization and infection in HIV-infected patients and patients with AIDS (36). More recently, it has been associated with oral carriage and infection in HIV-negative individuals and has also been recovered from a variety of specimens from nonoral sites, including the vagina, the respiratory tract, urine, sputum, feces, and blood (3, 4, 19, 20, 24, 27, 32, 36; D. Marriott, M. Laxton, and J. Harkness, Letter, Emerg. Infect. Dis. 7:479, 2001). C. dubliniensis is phylogenetically closely related to C. albicans and has a worldwide distribution (6). The majority of C. dubliniensis isolates studied to date are susceptible to commonly used azole and polyene antifungal drugs, including ketoconazole, fluconazole, itraconazole and amphotericin B (14, 17, 20, 21, 25). However, resistance to fluconazole has been reported in clinical isolates (20), and studies have shown that a stable fluconazole-resistance phenotype associated with up-regulation of multidrug transporters can be generated following sequential exposure of C. dubliniensis isolates to increasing fluconazole concentrations in vitro (20). Azole antifungal drug resistance has been reported widely in C. albicans and other Candida species (8, 24, 38). The emergence of resistant fungal strains and the availability of only limited types of antifungal agents for patient treatment have created a need for new broad-spectrum, nontoxic antifungals, and therefore, the histatin peptide may be a promising candidate. While the susceptibility of C. albicans to histatin 5 and histatin 3 is well documented, the susceptibility of C. dubliniensis to histatin has not been studied. In this study we examined the susceptibility of 11 C. dubliniensis clinical isolates and derivatives to histatin 3 and found a range of susceptibilities to histatin 3 among the strains tested. The binding and internalization of histatin 3 in these organisms was also investigated to determine whether these factors might contribute to the altered susceptibilities found.
MATERIALS AND METHODS
Preparation of 14C-labeled and fluorescein-labeled histatin.
Histatin 3 (DSH AKR HHG YKR KFH EKH HSH RGY RSN YLY DN) was synthesized by Albachem, University of Edinburgh, Edinburgh, Scotland) and purified by high-performance liquid chromatography. The peptide was radiolabeled by reductive methylation using the method of Xu et al. (39). Radiolabeled histatin was stored in aliquots at −20°C. The specific activity of the [14C]histatin was 5.36 Ci/mol. Fluorescent histatin 3 was made by Albachem using a 5,6-carboxyfluorescein conjugate at the N terminus of the peptide. It has been shown previously that the candidacidal activity of labeled histatin, either radiolabeled or fluorescently labeled, is not significantly affected by the labeling reaction (15, 39).
Candidacidal assay of histatin 3.
The candidacidal assay used was a modification of the method previously described by Xu et al. (39). C. dubliniensis strains were grown on potato dextrose agar (PDA) (Oxoid, Dorset, United Kingdom) plates at 30οC for 48 h. A single colony was inoculated into 10 ml of yeast extract-peptone-dextrose (YEPD) medium and grown overnight at 30°C in a shaking incubator at 200 × rpm. Cells were washed with 0.9% (wt/vol) NaCl, counted using a hemocytometer, and resuspended at 106 cells/ml. Killing assays were performed in 100 μl of 10 mM potassium phosphate buffer, pH 7.4, and contained 105 cells and a range of histatin 3 concentrations (0 to 100 μM). The reaction was incubated for 1 h at 37°C with vigorous mixing. The mixture was then diluted 1/10 by adding 900 μl of 0.9% (wt/vol) NaCl, and 100 μl of each dilution was spread onto PDA plates, which was followed by incubation at 30οC for 48 h. The number of single colonies on each plate was then counted. Killing activity was calculated as the number of CFU on test plates as a percentage of CFU on control plates (cells incubated in the absence of histatin). The 50% inhibitory concentration (IC50) determined was the concentration of histatin required to reduce the number of CFU by 50% and was determined visually from plots of killing activity versus histatin concentration.
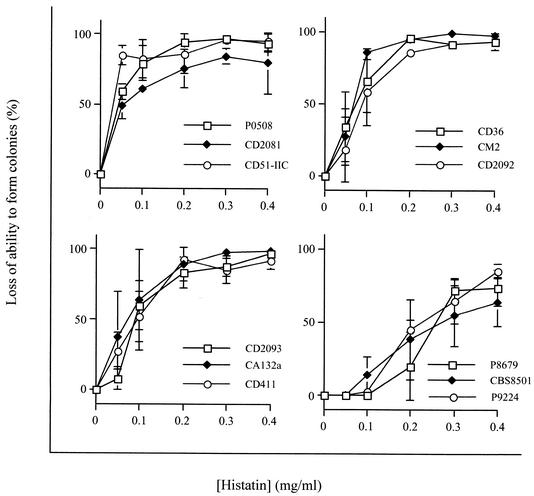
Candidacidal assays were performed with 11 strains of C. dubliniensis and the C. albicans reference strain CA132A (Table 1). Candidacidal assays were performed on at least three separate occasions and the mean and standard errors were calculated for each strain.
TABLE 1.
Histatin IC50s determined for a range of C. dubliniensis strains from different sources
| Yeast | Body site | Country of isolation | HIV status of patient | IC50 (mg/ml)
|
Source or reference | |
|---|---|---|---|---|---|---|
| Mean ± SDa (n) | Range | |||||
| C. dubliniensis | ||||||
| P0508 | Blood | Israel | − | 0.043 ± 0.016 (6) | 0.024-0.079 | This study |
| CD2081 | Oral cavity | Ireland | − | 0.055 ± 0.018 (3) | 0.040-0.082 | This study |
| CD51-IICb | Oral cavity | Ireland | + | 0.057 ± 0.032 (6) | 0.022-0.112 | 20 |
| CD36c | Oral cavity | Ireland | + | 0.072 ± 0.033 (3) | 0.048-0.119 | 36 |
| CM2d | Oral cavity | Australia | + | 0.075 ± 0.015 (5) | 0.054-0.099 | 20 |
| CD2092 | Oral | France | + | 0.092 ± 0.036 (3) | 0.050-0.140 | This study |
| CD2093 | Oral | France | + | 0.095 ± 0.019 (3) | 0.076-0.122 | This study |
| CD411 | Oral cavity | Ireland | + | 0.103 ± 0.026 (3) | 0.065-0.145 | 26 |
| P8679 | Retina | Israel | − | 0.190 ± 0.060 (6) | 0.130-0.079 | This study |
| CBS8501 | Blood | Holland | − | 0.191 ± 0.115 (6) | 0.088-0.440 | 19 |
| P9224 | Cornea | Israel | − | 0.196 ± 0.022 (6) | 0.170-0.230 | This study |
| C. albicans 132A | Oral cavity | Ireland | + | 0.096 ± 0.049 (6) | 0.028-0.152 | 9 |
Values shown are mean averages of several independent experiments (n) ± standard deviations. IC50 was determined as described in Materials and Methods.
CD51-IIC is an in vitro-generated fluconazole-resistant derivative of C. dubliniensis clinical isolate CD51-II, which is fluconazole susceptible.
CD36 is the C. dubliniensis type strain (36).
CM2 is a fluconazole-resistant clinical isolate; all the other isolates tested were fluconazole susceptible.
Determination of Kd.
Overnight cultures of C. albicans 132A or C. dubliniensis were grown from 48-h-old PDA plates in YEPD medium, and cell counts were estimated using a hemocytometer. Cultures were diluted to 2.5 × 10 8 cells/ml and washed, before resuspending in 250 μl of 10 mM potassium phosphate buffer. The reaction mixture contained 2 × 10 7 cells and a range of concentrations (0.5 to 20 μM) of 14C-labeled histatin 3 (specific radioactivity, 5.36 Ci/mol) in a total volume of 200 μl. The mixture was then incubated at 37°C for 30 min with constant shaking (200 rpm). Following centrifugation (13,000 × g, 5 min, 4°C), cells were washed twice with phosphate-buffered saline and resuspended in 10 mM potassium phosphate buffer, pH 7.4. The histatin-bound pellet was transferred to a scintillation vial containing 4 ml of scintillation fluid (Ecoscint; National Diagnostics, Atlanta, Ga.) and counted in a liquid scintillation counter. Nonspecific binding was found to be negligible, as determined by adding a 10-fold excess of unlabeled histatin in the assay. Preliminary assays were performed to confirm that binding was linear with time. Binding assays were performed in duplicate on three separate occasions. The MACCURVEFIT software program, version 1.2, for Apple Macintosh computers (Kevin Raner Software, Mt. Waverley, Victoria, Australia) was used to determine the dissociation constant, Kd, from saturation curves using the equation f(x) = (a × x)/(b + x).
Visualization of histatin binding using fluorescein-labeled histatin 3.
A modification of the method previously described by Xu et al. (39) was used for visualization of histatin binding. Freshly grown C. albicans or C. dubliniensis cells (3.4 × 10 6 cells/ml) were incubated with 12 nmol of carboxyfluorescein-labeled histatin in a total volume of 1 ml of potassium phosphate buffer, 10 mM, pH 7.4. Cells were incubated for 15, 45, and 90 min, centrifuged at 13,000 × g for 5 min, washed twice with 0.9% (wt/vol) NaCl and resuspended in 100 μl of distilled water. Cell suspensions were then spotted onto glass microscope slides, flame fixed quickly over a Bunsen burner, and air-dried in the dark. A drop of mounting medium (Sigma-Aldrich Chemical Company, Tallaght, Dublin, Ireland) was placed over the sample before covering with a glass coverslip. Slides were viewed under the 100× oil immersion lens of a Nikon (Eclipse ɛ600) microscope with a fluorescent light source (Nikon Super High Power Mercury Lamp). Fluorescent micrographs were taken at a set exposure time of 5 s.
RESULTS
Susceptibility testing of C. dubliniensis strains.
The C. dubliniensis strains studied are listed in Table 1. The group included clinical isolates from HIV-infected and non-HIV-infected individuals, recovered from several body sites, representing various worldwide locations, and including fluconazole-resistant strains. Histatin 3 susceptibility curves for each strain tested are shown in Fig. 1. IC50s were determined from susceptibility curves and the values determined are shown in Table 1. All strains were susceptible to killing by histatin 3, but differential susceptibility to histatin 3 was found among the strains tested. The strains can be broadly divided into three categories based on the range of IC50s found, namely, low-IC50, high-IC50, and intermediate-IC50 strains. Based on the small number of strains studied, it was not possible to correlate susceptibility to histatin 3 with any one global location or site of isolation. It is notable, however, that strains which showed decreased susceptibility to histatin 3 were from HIV-negative individuals, whereas the majority of those isolated from HIV-positive individuals fell into the low- or intermediate-IC50 category, which suggests that the histatins may have potential in the treatment of HIV-related candidal infection.
FIG. 1.
Candidacidal activity of histatin 3 against various C. dubliniensis strains and C. albicans CA132A. Results are means ± standard deviations (error bars) of three independent assays. Where error bars are not visible, they are smaller than the data symbol.
Since the antimicrobial activity of peptides may be influenced by the growth phase of the cells, we determined the affect of growth phase on the susceptibility to histatin 3 of C. albicans strain 132A and the most- and least-susceptible C. dubliniensis strains (C. dubliniensis P0508 and C. dubliniensis P9224). Cells in logarithmic (5 h, 37°C) and stationary (18 h, 37°C) phase of growth were compared for their susceptibilities to histatin, and the resulting IC 50s are shown in Table 2. The results show that, in each case, logarithmic-phase cells are more susceptible to histatin killing. However, the C. dubliniensis strain P9224 is the least susceptible to histatin in either phase of growth compared to C. dubliniensis P0508 or C. albicans 132A.
TABLE 2.
Comparison of histatin susceptibility of yeast cells grown to different phases
| Yeast | Mean IC50 (mg/ml) ± SD
|
|
|---|---|---|
| Logarithmic phase (n = 3) | Stationary phase (n = 6) | |
| C. albicans 132A | 0.014 ± 0.0004 | 0.096 ± 0.049 |
| C. dubliniensis P0508 | 0.024 ± 0.011 | 0.043 ± 0.016 |
| C. dubliniensis P9224 | 0.071 ± 0.017 | 0.196 ± 0.022 |
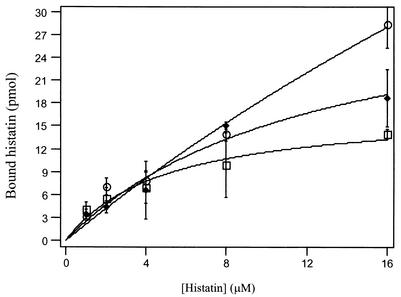
In C. albicans species, the binding of histatin 3 to a specific receptor on the fungal cell membrane is critical in the mechanism of killing, and it has been shown that binding is both temperature and salt concentration dependent (39). To investigate whether alterations in the binding of histatin to its receptor might contribute to the susceptibility to histatin, binding assays were performed for the C. dubliniensis strain which gave the lowest IC 50 (C. dubliniensis P0508; 0.043 mg/ml) and highest IC50 (C. dubliniensis P9224; 0.196 mg/ml) compared to the reference C. albicans (CA132A; 0.096 mg/ml), representing the intermediate IC50 range. The C. albicans reference strain CA132A was chosen as the control because the interaction of C. albicans with histatin has been extensively characterized and its susceptibility to histatin 3 (Table 1) and binding characteristics (data not shown) did not differ significantly from those of the C. dubliniensis reference strain CD36. Figure 2 shows the binding of [14C]histatin 3 to cells of C. albicans CA132A and C. dubliniensis P0508 and P9224 as a function of the concentration of histatin in the assay medium. Both C. albicans CA132A and C. dubliniensis P0508 were shown to approach saturation at a concentration of about 12 μM histatin 3; however, the less-susceptible C. dubliniensis strain P9224 did not appear to saturate within the concentration range tested. The maximum concentration of [14C]histatin that could be used in the assay was limited by the concentration of the stock solution. The dissociation constant (Kd) of histatin 3, at 37°C and pH 7.4, for each strain was determined from the binding plots using the MACCURVEFIT program for Apple Macintosh computers and were as follows (means ± standard deviations): C. albicans strain CA132A, 7.29 ± 2.04 μM; C. dubliniensis strain P0508, 21.1 ± 7.5 μM. Although C. dubliniensis strain P9224 did not reach saturation under these conditions, the estimated Kd was 67.5 ± 12.02 μM (n = 3).
FIG. 2.
Binding of 14C-labeled histatin to CA132A (□), P0508 (⧫), P9224 (○). A total of 107 cells/ml were incubated for 15 min with 0 to 16 μM [14C]histatin 3. Assays were performed in 10 mM potassium phosphate buffer, pH 7.4. Data points shown are the means ± standard deviations (error bars) of duplicate determinations from a representative binding assay.
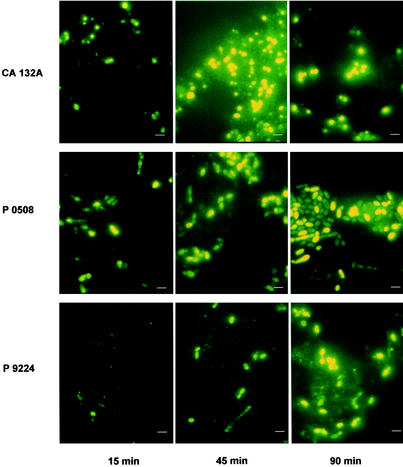
Fluorescent histatin 3 was used to visualize the time course of histatin 3 internalization in the three Candida strains examined (C. albicans CA132A and C. dubliniensis P0508 and P9224). Cells from each strain were incubated with 12 μM fluorescent histatin for 15, 45, and 90 min, and the resulting micrographs are shown in Fig. 3. After 15 min of incubation histatin had accumulated in some cells of C. albicans CA132A and C. dubliniensis P0508, but C. dubliniensis P9224 exhibited significantly less internalization of histatin at that time point. After 45 min, accumulation of peptide in C. albicans CA132A and C. dubliniensis strain P0508 was observed in almost all cells, but in the case of C. dubliniensis P9224 only a small population (10% approximately) of cells had taken up the peptide. After 90 min of incubation with fluorescent histatin 3, the majority of C. albicans CA132A and C. dubliniensis P0508 cells were heavily stained, whereas the staining of C. dubliniensis P9224 cells was more patchy, with some cells appearing full of histatin, while others showed little uptake of the peptide. The binding and internalization pattern found here is typical of the pattern seen in C. albicans by other workers using fluorescein-labeled histatin 3 or histatin 5 (11, 15, 39). The characteristic initial binding of the labeled peptide to a discrete area of the cell membrane is clearly visible at the 15-min time point before it accumulates within the cell at the later time points. The morphology of the cells of C. dubliniensis P0508 differed from that of the two other strains tested. Cells appeared oval in shape rather than showing the rounded appearance of C. albicans CA132A and C. dubliniensis P9224 cells. Furthermore, at the 90-min time point, two types of cells could be distinguished in C. dubliniensis P0508, which significantly differed in their intensities of staining (lightly stained and heavily stained cells). This phenomenon was not apparent in C. albicans CA132A or C. dubliniensis P9224 cells (Fig. 3).
FIG. 3.
Binding and internalization of fluorescently labeled histatin to C. albicans and C. dubliniensis strains. Cells from C. albicans CA132A and C. dubliniensis P0508 and P9224 were incubated with 12 μM fluorescently labeled histatin at 37°C for the periods indicated. The number of cells exposed in each case was 3.4 × 106 cells/ml. Micrographs are for a film exposure time of 5 s. Scale bars, 3 μm.
DISCUSSION
Over the last decade there has been an increase in the prevalence of fungal infections and in the range of infecting organisms, particularly in HIV-positive and immunocompromised patients (1, 30). However, there remains only a limited number of available antifungal agents. C. dubliniensis is a newly described Candida species which has a global distribution and is primarily associated with oral candidiasis in HIV-infected patients and patients with AIDS (36). While the majority of C. dubliniensis clinical isolates tested are susceptible to azole drugs such as fluconazole, ketoconazole, and itraconazole (14, 20, 25), azole-resistant clinical isolates have previously been reported (20). Therefore, there is now a need for new antifungal agents and a renewed interest in the use of host-derived antifungal agents, such as histatin.
Currently there are no reports in the literature on the susceptibility of Candida dubliniensis to histatin 3. We have shown in this study that histatin 3 is a potent inhibitor of C. dubliniensis and that within the C. dubliniensis species there is relatively wide variation in histatin susceptibility among different strains, ranging from IC 50 0.043 to 0.196 mg/ml. Importantly, the fluconazole-resistant C. dubliniensis clinical isolate CM2 and the in vitro-generated fluconazole-resistant C. dubliniensis derivative CD51-IIC were susceptible to histatin 3 killing (IC 50 = 0.075 and 0.057 mg/ml, respectively). In both of these C. dubliniensis strains, fluconazole resistance has been associated with overexpression of the MDR1 gene (20). This gene encodes the major facilitator protein Mdr1p, which plays a role in reducing the intracellular fluconazole concentration by a process of active drug efflux (20). The finding that these C. dubliniensis strains are susceptible to histatin, highlights the potential usefulness of histatin 3 as an effective alternative antifungal therapy.
Based on the small number of C. dubliniensis strains studied, it is difficult to correlate the variation in susceptibility to histatin with any particular anatomical site of isolation or global location or with the HIV status of the individuals from which the isolates were originally recovered. Recently, C. dubliniensis has been subdivided into four distinct genotypes based on comparative nucleotide sequence analysis of the internal transcribed spacer region of the ribosomal gene cluster and on DNA fingerprint analysis using the C. dubliniensis-specific Cd25 hybridization probe (10). The majority of genotype 1 isolates were recovered from HIV-infected individuals, whereas the majority of genotype 2 to 4 isolates were recovered from HIV-negative individuals (P < 0.001). It will be interesting to determine whether differences in histatin 3 susceptibility are associated with different genotypes.
The mechanism of action of histatin, though not yet fully elucidated, is different from that of the azole antifungal drugs, which disrupt ergosterol biosynthesis. In C. albicans, histatins exert their candidacidal activity by binding to a specific fungal membrane receptor (7), followed by internalization and interaction with intracellular targets, believed to be located in the mitochondria (11). Non-C. albicans Candida species including C. glabrata, C. krusei, C. tropicalis, C. guilliermondii, and C. parapsilosis have all been shown to be susceptible to histatin-mediated killing (23, 31). However, the mechanism of the antifungal activity of histatin has, to date, only been studied in C. albicans. The data presented in this study suggest that the mechanism of histatin 3-mediated killing in C. dubliniensis follows a similar mechanism involving the same targets, though confocal images would be useful to verify the fate of bound histatin 3. In support of this hypothesis, it has been shown that another yeast, S. cerevisiae, both is susceptible to histatin 5 and expresses a binding protein with a similar size to that in C. albicans, indicating that the antifungal mechanisms are similar (7). The variations in susceptibility of C. dubliniensis strains to histatin 3 found in the present study may reflect an alteration in binding to the histatin-binding protein, or in the interaction of histatin with cellular targets. In the present study, the higher dissociation constant (67.5 μM) determined for C. dubliniensis P9224 (the least-susceptible strain tested) suggests that histatin 3 has reduced affinity for this strain compared to the most susceptible C. dubliniensis P0508 (21.1 μM) or the reference C. albicans strain CA132A (7.29 μM). The reduced affinity of C. dubliniensis P9224 for histatin 3 may reflect a decrease in the number of binding sites or an alteration in the binding site itself in this isolate compared to the more susceptible isolates. The slower progress of histatin internalization in the C. dubliniensis strain P9224, compared to either the most-susceptible C. dubliniensis strain P0508 or the intermediately susceptible C. albicans strain CA132A, also suggests that there is a deficit in internalization of the peptide in C. dubliniensis P9224. Alternatively, less susceptible strains may exhibit differences in the expression of efflux pumps, similar to that described for fluconazole resistance in C. albicans and C. dubliniensis involving the ATP-binding cassette transporters Cdr1p and Cdr2p and the major facilitator protein Mdr1p (20, 33, 34). We cannot exclude the possibility that the histatin peptide is being pumped out of the cells of the less-susceptible C. dubliniensis strain P9224 by some unidentified transport system or is rapidly degraded at the surface. In this study, efflux of fluorescent peptide following incubation with fluorescent histatin could not be visualized as the cells were washed to remove unbound fluorescent histatin.
C. dubliniensis strain P0508 was found to be more susceptible to histatin 3 than the C. albicans reference strain CA132A (IC50 = 0.043 mg/ml, compared to 0.096 mg/ml). However, the increased susceptibility could not be attributed to greater binding affinity (Kd = 21.1 μM) (Fig. 2) or to increased rates of accumulation (Fig. 3). It is interesting that the cells of this strain showed two distinct staining intensities after 90 min of incubation with fluorescent histatin. It is possible that these two staining patterns represent different levels of expression of some yet unidentified protein, which is important in the accumulation or internal targeting of histatin. It is likely that at the 90-min time point a fully stained cell indicates that cell death has occurred and the cell has become fully saturated with the peptide. This pattern suggests that within C. dubliniensis strain P0508 there are individuals cells with different susceptibilities to histatin. Variations in fluconazole susceptibility have been previously reported among clonal isolates of C. dubliniensis recovered from the same oral specimen. In a recent study, by Gee et al. (10), of 15 clonal isolates recovered from the same clonal specimen from the oral cavity of an Irish AIDS patient, 4 isolates exhibited reduced susceptibility to fluconazole (MIC, 16 μg/ml) whereas the other 11 were fluconazole susceptible (MIC ≤ 1 μg/ml). Candidacidal assays which record loss of colony-forming cells would not necessarily reveal subpopulations of cells that had different susceptibilities to histatin 3. It would be important to investigate whether this variation in histatin internalization phenotype occurs in other susceptible C. albicans or C. dubliniensis strains, as this effect has not been reported previously in C. albicans.
The therapeutic potential of histatin is now becoming evident, particularly because the mechanism of its antifungal activity involves pathways distinct from that of the more commonly used azole drugs which affect ergosterol biosynthesis. The data presented here suggest that in C. dubliniensis, variations in susceptibility to histatin may involve alterations in the binding or internalization of histatin. The role of the specific histatin-binding protein in histatin-mediated killing of Candida species may be studied more directly by constructing knockout strains or by overexpressing the protein in mammalian cells, which are inherently resistant to histatin-mediated killing and in which the binding activity is undetectable (7). Identification and characterization of the specific histatin-binding protein and intracellular targets of histatin, which are now being undertaken in this laboratory, will be critical if the full potential of histatin as an antifungal is to be exploited. In this study we have shown that a number of C. dubliniensis isolates are susceptible to histatin 3 killing. It has been shown that histatin 5 kills many of the Candida species associated with oral candidiasis (37), and further characterization of the range of organisms susceptible to histatin 5 and histatin 3 killing is ongoing. In particular, it is important to ascertain whether the mechanism of histatin-mediated killing is the same for all susceptible Candida species or whether small differences in the pathway may affect histatin susceptibility.
This knowledge, together with a deeper understanding of the pathways involved in its mechanism of killing, may represent a way forward in the use of histatin-based drugs, alone or in addition to azole drugs, in the treatment of oral candidiasis and mucosal disease.
Acknowledgments
This work was supported by the School of Dental Sciences, Dublin Dental Hospital and the Health Research Board of Ireland, grant no. RP 119-2002.
REFERENCES
- 1.Beck-Sague, C. M., W. R. Jarwis, et al. 1993. Secular trends in the epidemiology of nosocomial fungal infection in the United States, 1980-1990. J. Infect. Dis. 167:1247-1251. [DOI] [PubMed] [Google Scholar]
- 2.Bercier, J. G., I. Al-Hashimi, N. Haghighat, T. D. Rees, and F. G. Oppenheim. 1999. Salivary histatins in patients with recurrent oral candidiasis J. Oral Pathol. Med. 28:26-29. [DOI] [PubMed] [Google Scholar]
- 3.Boyle, B. M., D. J. Sullivan, C. Forkin, F. Mulcahy, C. T., Keane, and D. C. Coleman. 2002. Candida dubliniensis candidaemia in an HIV-positive patient in Ireland. Int. J. STD AIDS 13:55-57. [DOI] [PubMed] [Google Scholar]
- 4.Brandt, M. E., L. H. Harrison, M. Pass, A. N. Sofair, S. Huie, R. K. Li, C. J. Morrison, D. W. Warnock, and R. A. Hajjeh. 2000. Candida dubliniensis fungemia: the first four cases in North America. Emerg. Infect. Dis. 6:46-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calderone, R. A., and W. A. Fonzi. 2001. Virulence factors of Candida albicans. Trends Microbiol. 9:327-335. [DOI] [PubMed] [Google Scholar]
- 6.Coleman, D., D. Sullivan, B. Harrington, K. Haynes, M. Henman, D. Shanley, D. Bennett, G. Moran, C. McCreary, L O'Neill. 1997. Molecular and phenotypic analysis of Candida dubliniensis: a recently identified species linked with oral candidosis in HIV-infected and AIDS patients. Oral Dis. 3(Suppl. 1):S96-101. [DOI] [PubMed] [Google Scholar]
- 7.Edgerton, M., S. E Koshlukova, T. E. Lo, B. G. Chrzan, R. M. Straubinger, and P. A. Raj. 1998. Candidacidal activity of salivary histatins. Identification of a histatin 5 binding protein on Candida albicans. J. Biol. Chem. 273:20438-20447. [DOI] [PubMed] [Google Scholar]
- 8.Fung-Tomc, J. C., T. C. White, B. Minassian, E. Huczko, and D. P. Bonner. 1999. In vitro antifungal activity of BMS-207147 and itraconazole against yeast strains that are non-susceptible to fluconazole. Diagn. Microbiol. Infect. Dis. 35:163-167. [DOI] [PubMed] [Google Scholar]
- 9.Gallagher, P. J., D. E. Bennett, M. C. Henman, R. J. Russell, S. R. Flint, D. B. Shanley, and D. C. Coleman. 1992. Reduced azole susceptibility of oral isolates of Candida albicans from HIV-positive patients and a derivative exhibiting colony morphology variation. J. Gen. Microbiol. 138:1901-1911. [DOI] [PubMed] [Google Scholar]
- 10.Gee, S., S. Joly, D. R. Soll., J. F. G. M. Meis, P. E. Verweij, I. Polacheck, D. J. Sullivan, and D. C. Coleman. 2002. Identification of four distinct genotypes of Candida dubliniensis and detection of microevolution in vitro and in vivo. J. Clin. Microbiol. 40:556-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helmerhorst, E. J., P. Breeuwer, W. van't Hof, E. Walgreen-Weterings, L. C. Oomen, E. C. Veerman, A. V. Amerongen, and T. Abee. 1999. The cellular target of histatin 5 on Candida albicans is the energized mitochondrion. J. Biol. Chem. 274:7286-7291. [DOI] [PubMed] [Google Scholar]
- 12.Helmerhorst, E. J., I. M. Reijnders, W. van't Hof, I. Simoons-Smit, E. C. Veerman, and A. V. Amerongen. 1999. Amphotericin B- and fluconazole-resistant Candida spp., Aspergillus fumigatus, and other newly emerging pathogenic fungi are susceptible to basic antifungal peptides. Antimicrob. Agents Chemother. 43:702-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jainkittivong, A., D. A. Johnson and C-K. Yeh. 1998. The relationship between salivary histatin levels and oral yeast carriage. Oral Microbiol. Immunol. 13:181-187. [DOI] [PubMed] [Google Scholar]
- 14.Kirkpatrick, W. R., S. G. Reyankar, R. K. Mcatee, J. L. Lopez-Ribot, A. W. Fothergill, D. I. McCarthy, S. E. Sanche, R. A. Cantu, M. G. Rinaldi, and T. F. Patterson. 1998. Detection of Candida dubliniensis in oropharyngeal samples from human immunodeficiency virus-infected patients in North America by primary CHROMagar CANDIDA screening and susceptibility testing of isolates. J. Clin. Microbiol. 36:3007-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koshlukova, S. E., M. W. B. Araujo, D. Baev, and M. Edgerton. 2000. Released ATP is an extracellular cytotoxic mediator in salivary histatin 5-induced killing of Candida albicans. Infect. Immun. 68:6848-6856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lal, K., J. J. Pollock, R. P. Santarpia III, H. M. Heller, H. W. Kaufman, J. Fuhrer, and R. T. Steigbigel. 1992. Pilot study comparing the salivary cationic protein concentrations in healthy adults and AIDS patients: correlation with antifungal activity. J. Acquir. Immune Defic. Syndr. 5:904-914. [PubMed] [Google Scholar]
- 17.Löffler, J., S. L. Kelly, H. Hebart, U. Schumacher, C. Lass-Florl, and H. Einsele. 1997. Molecular analysis of cyp51 from fluconazole-resistant Candida albicans strains. FEMS Microbiol. Lett. 151:263-268. [DOI] [PubMed] [Google Scholar]
- 18.MacKay, D. J., L. Denepitiya, V. J. Iacono, S. B. Krost, and J. J. Pollock. 1984. Growth inhibitory and bacteriocidal effects of human parotid salivary histidine-rich polypeptides on Streptococcus mutans. Infect. Immun. 44:695-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meis, J. F., M. Ruhnke, B. E. De Pauw, F. C. Odds, W. Siegert, and P. E. Verweij. 1999. Candida dubliniensis candidemia in patients with chemotherapy-induced neutropenia and bone marrow transplantation. Emerg. Infect. Dis. 5:150-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moran, G. P., D. Sanglard, S. M. Donnelly, D. B. Shanley, D. J. Sullivan, and D. C. Coleman. 1998. Identification and expression of multidrug transporters responsible for fluconazole resistance in Candida dubliniensis. Antimicrob. Agents Chemother. 42:1819-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moran, G. P., D. J. Sullivan, M. C. Henman, C. E. McCreary, B. J. Harrington, D. B. Shanley, and D. C. Coleman. 1997. Antifungal drug susceptibilities of oral Candida dubliniensis isolates from human immunodeficiency virus (HIV)-infected and non-HIV-infected subjects and generation of stable fluconazole-resistant derivatives in vitro. Antimicrob. Agents Chemother. 41:617-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murakami, Y., H. Nagata, A. Amano, M. Takagaki, S. Shizukuishi, A. Tsunemitsu, and S. Aimoto. 1991. Inhibitory effects of human salivary histatins and lysozyme on coaggregation between Porphyromonas gingivalis and Streptococcus mitis. Infect. Immun. 59:3284-3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nikawa, H., C. Jin, H. Fukushima, S. Makihira, and T. Hamada. 2001. Antifungal activity of histatin-5 against non-albicans Candida species. Oral Microbiol. Immunol. 16:250-252. [DOI] [PubMed] [Google Scholar]
- 24.Nolte, F. S., T. Parkinson, D. J. Falconer, S. Dix, J. Williams, C. Gilmore, R. Geller, and J. R. Wingard. 1997. Isolation and characterization of fluconazole- and amphotericin B-resistant Candida albicans from blood of two patients with leukemia. Antimicrob. Agents Chemother. 4:196-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfaller, M. A., S. A. Messer, S. Dee, S. Joly, C. Pujol, D. J. Sullivan, D. C. Coleman, and D. R. Soll. 1999. In vitro susceptibilities of Candida dubliniensis isolates tested against the new triazole and echinocandin antifungal agents. J. Clin. Microbiol. 37:870-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinjon, E., D. J. Sullivan, L. Salkin, D. B. Shanley, and D. C. Coleman. 1998. Simple, inexpensive, reliable method for differentiation of Candida dubliniensis from Candida albicans. J. Clin. Microbiol. 36:2093-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polacheck, I. J. Strahilevitz, D. Sullivan, S. Donnelly, I. F. Salkin, and D. C. Coleman. 2000. Recovery of Candida dubliniensis from non-human immunodeficiency virus-infected patients in Israel. J. Clin. Microbiol. 38:170-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pollock, J. J., L. Denepitiya, B. J. MacKay, and V. J. Iacono. 1984. Fungistatic and fungicidal activity of human parotid salivary histidine-rich polypeptides on Candida albicans. Infect. Immun. 45:610-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rayhan, R., L. Xu, R. P. Santarpia III, C. A Tylenda, and J. J. Pollock. 1992. Antifungal activities of salivary histidine-rich polypeptides against Candida albicans and other oral yeast isolates. Oral Microbiol. Immunol. 7:51-52. [DOI] [PubMed] [Google Scholar]
- 30.Rocco, T. R. S. E. Reinert, and H. H. Simms. 2000. Effects of fluconazole administration in critically ill patients: analysis of bacterial and fungal resistance. Arch. Surg. 135:160-165. [DOI] [PubMed] [Google Scholar]
- 31.Rothstein, D. M. P. Spacciapoli, L. T. Tran, T. Xu, F. D. Roberts, M. Dalla Serra, D. K. Buxton, F. G. Oppenheim, and P. Friden. 2001. Anticandida activity is retained in P-113, a 12-amino-acid fragment of histatin 5. Antimicrob Agents Chemother. 45:1367-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salesa, R., M. D. Moragues, R. Sota, J. Peman, G. Quindos, and J. Ponton. 2001. Specific antibody response in a patient with Candida dubliniensis fungemia. Rev. Iberoam. Micol. 18:42-44. [PubMed] [Google Scholar]
- 33.Sanglard, D., F. Ischer, M. Monod, and J. Bille. 1997. Cloning of Candida albicans genes conferring resistance to azole antifungal agents: characterization of CDR2, a new multidrug ABC transporter gene. Microbiology 143:405-416. [DOI] [PubMed] [Google Scholar]
- 34.Sanglard, D., F. Ischer, M. Monod, and J. Bille. 1996. Susceptibilities of Candida albicans multidrug transporter mutants to various antifungal agents and other metabolic inhibitors. Antimicrob. Agents Chemother. 40:2300-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Situ, H., and L. A. Bobek. 2000. In vitro assessment of antifungal therapeutic potential of salivary histatin 5, two variants of histatin 5 and salivary mucin (MUC7) Domain 1. Antimicrob. Agents Chemother. 44:1485-1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sullivan, D. J., T. J. Westerneng, K. A. Haynes, D. E. Bennett, and D. C. Coleman. 1995. Candida dubliniensis sp. nov.: phenotypic and molecular characterization of a novel species associated with oral candidosis in HIV-infected individuals. Microbiology 41:1507-1521. [DOI] [PubMed] [Google Scholar]
- 37.Tsai. H., and L. A. Bobek. 1997. Studies of the mechanism of human salivary histatin 5 candidacidal activity with histatin 5 variants and azole-sensitive and -resistant Candida species. Antimicrob. Agents Chemother. 41:2224-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White, T. C., K. A. Marr, and R. A. Bowden. 1998. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin. Microbiol. Rev. 11:382-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu, Y., I. Ambudkar, H. Yamagishi, W. Swaim, T. J. Walsh, and B. C. O'Connell. 1999. Histatin 3-mediated killing of Candida albicans: effects of extracellular salt concentration on binding and internalization. Antimicrob. Agents Chemother. 43:2256-2262. [DOI] [PMC free article] [PubMed] [Google Scholar]