Abstract
Context
Maintaining optimal glycemic control is an important goal of therapy in patients with diabetes mellitus. Patients of Hispanic ancestry have been shown to have high rates of diabetes and poor glycemic control (PGC). Although depression is common in adults with diabetes, its relationship to glycemic control remains unclear, especially among Hispanics.
Objective
To assess the association of depression with PGC in Hispanics.
Design
Data from a cross-sectional mental health survey in primary care were crosslinked to the hospital's computerized laboratory database.
Setting
Urban general medicine practice at a teaching hospital.
Patients
Two hundred and nine patients (mean [standard deviation] age, 57.1 [10.3] years; 68% females) with recent International Classification of Diseases, Ninth Revision (ICD-9) codes for diabetes mellitus, and 1 or more hemoglobin A1c (HbA1c) tests.
Main Outcome Measure
Probability of PGC (HbA1c≥8%).
Results
Probability for PGC steadily increased with severity of depression. Thirty-nine (55.7%) of the 70 patients with major depression had HbA1c≥8%, compared with 39/92 (42.4%) in the minimal to mild depression group, and 15/47 (31.9%) in the no depression group (Ptrend=.01; adjusted odds ratio, 3.27; 95% confidence interval, 1.23 to 8.64, for moderate or severe depression vs no depression). Only 29 (41.4%) of the patients with major depression received mental health treatment in the previous year.
Conclusions
In this primary care sample of Hispanic patients with diabetes, we found a significant association between increasing depression severity and PGC. Yet, less than one half of the patients with moderate or severe depression received mental health treatment in the previous year. Improving identification and treatment of depression in this high-risk population might have favorable effects on diabetic outcomes.
Keywords: glycemic control, depression, diabetes, hispanics, primary care
There is ample evidence that depressive disorders are more prevalent among adults with diabetes than in the general population.1–4 The relationship between depression and glycemic control in patients with diabetes, however, is less obvious.5 A meta-analysis found a small to moderate overall association between depression and hyperglycemia in both type 1 and type 2 diabetes.6 A closer look at the 27 individual studies reveals that about half of them were negative, and that publication bias, a particular threat to the validity of meta-analyses of observational studies,7 could not be ruled out.
We identified 9 studies that were published (in English) after this meta-analysis. Four studies reported an association between depression severity and hemoglobin A1c (HbA1c) limited to patients with type 1 diabetes8,9; males with type 2 diabetes10; and individuals <65 years of age.11 Gary et al.12 found that depressive symptoms were marginally associated with suboptimal levels of HbA1c among African Americans with type 2 diabetes. Singh et al.13 reported an association between depression and HbA1c in Pima Indians, but did not adjust for socioeconomic status. In contrast, Ciechanowski et al.14 did not demonstrate an association between severity of depressive symptoms and glycemic control in a large sample (N=367) of patients with diabetes; Kruse et al.15 found that people with diabetes and affective disorders were more likely to have adequate glycemic control; and a more recent study reported somewhat lower HbA1c levels in patients with type 2 diabetes and major mood disorders (mostly depression) compared with primary care diabetic patients without serious mental illness.16 In addition, although a recent systematic review and meta-analysis of randomized controlled psychological interventions in type 2 diabetes concluded that there is long-term improvement in glycemic control among patients who receive psychological therapies,17 randomized clinical trials aimed at depressed diabetic patients do not support a direct or significant effect of treatment for depression on glycemic control.18–22
Most patients with diabetes are treated in primary rather than specialty care settings. There are likely important differences between these two populations, such as severity of the disorder, physicians' characteristics, magnitude of competing demands on physicians' time and other resources, and physicians' ability to obtain mental health services for their patients.23–25
Hispanic individuals compose the fastest growing minority group in the United States. Hispanics have been shown to have high rates of diabetes26–29 and are more likely to have poor glycemic control (PGC).30–32 Diabetes ranks fifth among the leading causes of death in people of Hispanic origin.33 Hispanic patients were also shown to be less likely to have regular source of medical care, to undergo screening, to use preventive services, to be referred to a specialist,29 or to receive appropriate treatment.34 Moreover, predictors of depression and anxiety,35 as well as self-care behavior and family support,36,37 differ between Hispanic and European-American patients with diabetes. There is also evidence that, as compared with white depressed patients, Hispanic depressed patients are significantly less likely to receive antidepressant medications.38
We sought to examine the relationship between depression and glycemic control in a sizable systematic sample of Hispanic, urban primary care patients.
METHODS
Setting
These data derive from a general medicine practice–based study that was conducted at the Associates in Internal Medicine (AIM), the faculty and resident group practice of the Division of General Medicine at the College of Physicians and Surgeons of Columbia University. The practice serves approximately 18,000 patient visits each year. Most patients are low-income, Medicaid-covered, Hispanic individuals of Caribbean origin.
Sample Selection
Study subjects were drawn from a larger study of mental disorders in primary care, conducted between October 19, 1998 and April 15, 1999, and described in detail elsewhere.39 Briefly, the study population consisted of systematically sampled consecutive adult primary care patients with scheduled physician appointments. Research assistants were randomly assigned daily to 2 or 3 of the clinic's 5 waiting rooms. Within each waiting room, patients were approached one at a time according to their seat location, following a pattern determined in advance by the investigators. If a patient refused to participate or did not meet eligibility criteria, the closest patient sitting to the right was approached next. Eligible patients included those who were between 18 and 70 years of age, who made at least 1 previous visit to the clinic, and could speak and understand English or Spanish. Patients were excluded from the study if their current general health status prohibited completion of survey forms or if they were assessed as highly suicidal. A total of 1,264 patients met study eligibility criteria, and 1,005 (79.5%) consented to participate. Study participants were slightly younger than eligible nonparticipants.
Patients of Hispanic ancestry with type 1 and type 2 diabetes mellitus or diabetes-related complications, according to International Classification of Diseases, Ninth Revision (ICD-9) codes 250.0–250.940 (excluding gestational diabetes) during a 1-year time frame before and after the study evaluation,41 were identified from an automated database (n=231). Laboratory measurements were obtained via crosslinkage to the hospital's computerized clinical information system.
To confirm the accuracy of the coding system, we used casual plasma glucose concentration ≥140 mg/dl (7.8 mmol/L) or at least a mildly elevated HbA1c level (>6.2%) during the defined time frame, in the presence of an ICD-9 code for diabetes, as “gold standard.” In order to assess potential false negatives, that is, patients with diabetes who were not coded as such, we used casual plasma glucose concentration ≥200 mg/dl (11.1 mmol/L) or, alternatively, casual plasma glucose concentration ≥140 mg/dl (7.8 mmol/L) and HbA1c >6.2% as “gold standard.” Based on these values, we found that in our dataset an ICD-9 code for diabetes mellitus was highly sensitive (87.2%), specific (96.7%), and reliable (κ=0.85).
We limited the analytic sample to those 209 (90.5%) patients who had at least 1 HbA1c measurement recorded in the hospital's laboratory data system during 1998, 1999, and January to September of 2000. Patients who had at least 1 HbA1c test were somewhat older (57 vs 52 years; P=.03) and were somewhat more likely to report poor or fair (vs good, very good, or excellent) physical health (67% vs 50%; P=.11). There were no differences in gender, level of education or income, depression, anxiety, or substance use disorders. For patients with more than 1 test, the HbA1c value recorded closest to the date of the study interview was used. The median for the time interval between the test and the study interview was 36 days. During the study period, total glycohemoglobin levels were also measured, and those values have been converted to HbA1c for this analysis using a standard nomogram (Robin S. Goland, MD, written communication, December 20, 2000). All the glycosylated hemoglobin assays were performed at the Columbia Presbyterian Medical Center's laboratory, using National Glycohemoglobin Standardization Program (NGSP)–certified methods.42
Psychological Measurements
Current (past month) major depression, panic disorder, and generalized anxiety disorder, and past year substance (alcohol and drug) abuse/dependence were measured with the validated Patient Health Questionnaire (PHQ),43,44 the self-report version of the PRIME-MD (Primary Care Evaluation of Mental Disorders).45 There is a good agreement between the PHQ diagnoses and those of independent mental health professionals (for the diagnosis of any 1 or more PHQ disorder, κ=0.65; overall accuracy, 85%; sensitivity, 75%; specificity, 90%).43 The Spanish version of the PHQ has been validated in general hospital patients.44 Continuous scores for depression were created on the basis of the number and severity of symptoms reported by the patient in each domain. Severity score ranges from 0 (not at all) to 3 (nearly every day) for each item. This resulted in a range of possible scores 0 to 27, with higher scores indicating greater severity.46 Anxiety was defined as having either panic or generalized anxiety disorder (or both). Continuous score for the anxiety disorders was created using computation similar to that used for depression. This resulted in a range of 0 to 29 of possible scores for anxiety disorders.
At study intake, patients completed a 5-point, self-rated, overall physical health scale (1=poor, 2=fair, 3=good, 4=very good, 5=excellent). In the data analysis, this measure was collapsed into 2 categories, the first consisting of excellent, very good, and good; the second of fair and poor. This was done to allow for a more meaningful interpretation of this measurement. Patients were also asked about past year and lifetime professional mental health treatment.
Spanish versions of the PHQ and all other study forms were used for patients who preferred Spanish. A bilingual team of trained mental health professionals conducted all the assessments via face-to-face interviews. The study protocol was approved by the Institutional Review Board, and all study participants gave informed consent.
Data Analysis
Comparisons between patients with at least 1 glycosylated hemoglobin test and those with none were conducted with t tests for continuous variables, χ2 tests for categorical variables, and the Wilcoxon rank-sum test for ordinal data (education and income).
In order to assess the validity of the psychological measures, depression and anxiety scores were correlated with mental health status, measured by the Mental Component Score of the Medical Outcomes Study 36-item Short-Form Health Survey (SF-36), a generic, highly valid, and reliable health status measure,47 which was available for 53 (25%) of the sample. Higher scores on the SF-36 denote better mental health. The high Pearson correlation coefficients (r=−.78, P<.0001 for depression; r=−.69, P<.0001 for anxiety) support the validity of the depression and anxiety measures in our sample.
The total analytic sample was then grouped into 3 categories based on PHQ depression scores, to allow for a general, nonlinear relation between depression severity and PGC. The PHQ score ranges for depression severity were as follows: 0 to 1 points indicating no depression; 2 to 11 points, minimal or mild depression; and ≥12 points, moderate or severe depression. We chose a score of 12 points as the cut point for determining major depression because of its optimal operating characteristics, that is, high specificity (92%) combined with minimal tradeoff in sensitivity (83%) for the diagnosis of major depression.46 We defined the 0 to 1 category as “no depression” to ensure that the reference group does not include cases of depression, because a total score of 2 or more on the first two PHQ-9 items (depressed mood and anhedonia) demonstrated a sensitivity of 82% and specificity of 80% for any depressive disorder.48 First, we compared sociodemographic and psychological characteristics across the 3 depression groups using χ2 tests for categorical variables, one-way analysis of variance (ANOVA) for continuous variables, and the nonparametric Kruskal-Wallis test for ordinal (income and education) and skewed (number of primary care visits) data. Next, we examined whether the probability of PGC (defined as HbA1c≥8%49) was different for each depression category, compared to the reference category of no depression. Logistic regression was used to compute unadjusted and adjusted odds ratios (OR) and 95% confidence intervals (CIs). Wald tests were used to compute corresponding χ2 statistics and P values. Sociodemographic and psychological variables that were found to be associated with depression status at P<.2550 (age, gender, income level, education level, marital status [married or living with someone vs not], anxiety disorders, and self-rated physical health) were included as covariates in the adjusted model. We used the Cochran-Armitage trend test to test for a gradient (dose-response) effect, that is, whether the probability of PGC increased with depression severity.51 In a secondary analysis, we followed the same analytic approach to assess for association between anxiety and glycemic control, with depression added as a covariate, and to assess the relationship between depression and glycemic control in non-Hispanic patients.
All tests were two-tailed, and statistical significance (α) was set at .05. Statistical analysis was performed using SAS statistical software (SAS Institute Inc., Cary, NC).
RESULTS
Seventy (33.5%) patients had current moderate or severe depression, as measured by the PHQ. The demographic characteristics and current psychological measurements of the 3 study groups are presented in Table 1 and 2. Patients with moderate to severe depression were mostly women (80%), with low income, and more likely to be living alone. They had higher prevalence of anxiety disorders, and were more likely to report poor/fair self-rated physical health.
Table 1.
Sociodemographic Characteristics of Adult Hispanic Primary Care Patients with Diabetes, by Severity of Depression (N=209)
| Characteristics | No Depression | Minimal to Mild Depression | Moderate to Severe Depression | P Value |
|---|---|---|---|---|
| n=47 | n=92 | n=70 | ||
| Mean age, y (SD) | 61.3 (7.1) | 56.0 (11.7) | 55.7 (9.5) | .006 |
| Gender, female, n (%) | 22 (46.8) | 64 (69.6) | 56 (80.0) | .0007 |
| Preferred language, Spanish, n (%) | 41 (87.2) | 82 (89.1) | 65 (92.9) | .58 |
| Married/living with partner, n (%) | 21 (44.7) | 31 (33.7) | 16 (22.9) | .04 |
| Education levels | .10* | |||
| Less than high school, n (%) | 30 (63.8) | 73 (79.4) | 55 (78.6) | … |
| High school, n (%) | 9 (19.2) | 9 (9.8) | 8 (11.4) | … |
| Some college, n (%) | 4 (8.5) | 6 (6.5) | 6 (8.6) | … |
| ≥4 years of college, n (%) | 4 (8.5) | 4 (4.3) | 1 (1.4) | … |
| Income (annual, per household) | .07* | |||
| <$11,999, n (%) | 39 (83.0) | 86 (93.5) | 66 (94.3) | … |
| $12,000–$17,999, n (%) | 4 (8.5) | 4 (4.4) | 1 (1.4) | … |
| $18,000–$35,999, n (%) | 2 (4.3) | 0 | 3 (4.3) | … |
| ≥$36,000, n (%) | 2 (4.3) | 2 (2.2) | 0 | … |
Omnibus P value derived from Kruskal-Wallis test
SD, standard deviation
Table 2.
Depression Scores, Current Mental Disorders,* Self-rated Health, and Primary Care Visits of Adult Hispanic Primary Care Patients with Diabetes, by Severity of Depression
| Variables | No Depression | Minimal to Mild Depression | Moderate to Severe Depression | P Value |
|---|---|---|---|---|
| n=47 | n=92 | n=70 | ||
| Depression score, mean (SD) | 0.4 (0.5) | 6.3 (2.8) | 17.0 (4.4) | <.0001 |
| Anxiety disorders, n (%) | 0 | 6 (6.5) | 32 (45.7) | <.0001 |
| Alcohol and/or drug use disorder, n (%) | 2 (4.3) | 7 (7.6) | 7 (10.0) | .26 |
| Poor/fair physical health,†n (%) | 16 (34.0) | 63 (68.5) | 61 (87.1) | <.0001 |
| Primary care visits, per year, mean‡ (SD) | 7.8 (4.9) | 8.2 (5.5) | 9.2 (6.0) | .37 |
Past month prevalence based on the Patient Health Questionnaire (PHQ) version of the Primary Care Evaluation of Mental Disorders (PRIME-MD)
Anxiety disorders include generalized anxiety disorder and panic disorder
Self-rated; versus good, very good, or excellent.
Per 6 months before to 6 months after the study interview. P value derived from Kruskal-Wallis test
SD, standard deviation
Our main analysis is presented in Table 3. We found a steady increase in the probability of PGC with advancing categories of depression severity. In the adjusted model, likelihood for PGC was more than 3-fold higher among patients with moderate to severe depression compared to patients without depression. A test for trend, based on these depression categories, yielded a statistically significant result (P=.01), supporting a significant, monotonic, dose-response relationship between depression severity and PGC. We also compared PGC between the moderate to severe depression group and the minimal to mild depression group. The adjusted OR for this comparison was 2.16 (95% CI, 1.03 to 4.50; P=.04).
Table 3.
Poor Glycemic Control (HbA1c≥8.0%) by Depression Severity*
| No Depression | Minimal to Mild Depression | Moderate to Severe Depression | P Value | |
|---|---|---|---|---|
| n=47 | n=92 | n=70 | ||
| Poor glycemic control | ||||
| N (%) | 15 (31.9) | 39 (42.4) | 39 (55.7) | .01† |
| Unadjusted OR (95% CI) | reference | 1.57 (0.75 to 3.29) | 2.68 (1.24 to 5.82) | .01‡ |
| Adjusted OR (95% CI)§ | reference | 1.51 (0.67 to 3.42) | 3.27 (1.23 to 8.64) | .02‡ |
For patients with more than 1 HbA1c test available in the dataset, the value closest to the administration of the study's questionnaire was used in the analysis. The “no depression” group is the reference category.
Calculated with the Cochran-Armitage trend test (Z=−2.59).
Calculated by Wald χ2 tests for the comparison between the moderate to severe and no depression groups.
Adjusted for age, gender, household income, education level, marital status, presence of current anxiety disorders, and self-perceived physical health.
Results did not change when anxiety score rather than presence of current anxiety disorders was included as a covariate.
OR, odds ratio; CI, confidence interval.
Thirty-eight (18.2%) patients had current anxiety disorder: 25 (12%) had generalized anxiety disorder; 5 (2.4%) had panic disorder; and 8 (3.8%) had both. Anxiety was not associated with PGC when modeled as a categorical yes/no variable (unadjusted OR, 1.15; 95% CI, 0.57 to 2.33) or as continuous score (unadjusted OR, 1.01; 95% CI, 0.98 to 1.05). Including depression as a covariate did not change the results (data not presented).
We did not find an association between depression and PGC among the 86 non-Hispanic (74 blacks, 12 whites) diabetes patients: unadjusted OR, 0.90; 95% CI, 0.23 to 3.58 for the comparison between the moderate to severe depression and the no depression groups.
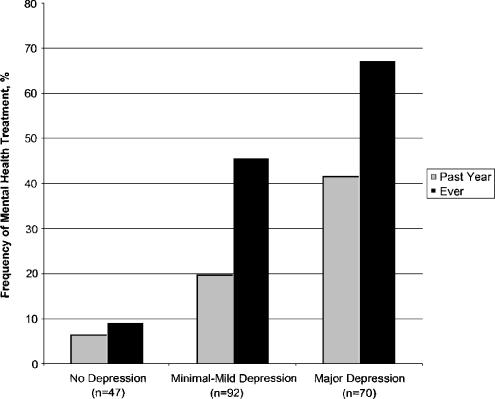
Only 29 (41.4%) of the patients with moderate or severe depression received mental health treatment in the previous year, compared with 18 (19.6%) of those with minimal to mild depression and 3 (6.4%) of those without depression (Fig. 1).
FIGURE 1.
Mental health treatment by depression severity (N=209).
DISCUSSION
Maintaining optimal glycemic control is a widely accepted goal of therapy in patients with diabetes. The American Diabetes Association (ADA) guidelines encourage physicians to set a goal of maintaining HbA1c levels at <8%.49 We found a significant and robust, dose-dependant association between increasing depression severity and PGC in a systematic sample of Hispanic, low-income, primary care patients. More specifically, we demonstrated about a 3-fold increase in the likelihood of PGC (HbA1c≥8%) among patients with diabetes who have PHQ scores corresponding with major depression, compared to those without depression. This finding was specific to Hispanics. Consistent with some of the previous reports, we did not find an association between anxiety and glycemic control.52
Although we cannot be sure of the direction of causation in a cross-sectional analysis, it is reasonable to assume, given prospective data from other studies in diabetes patients53,54 and the well-established observation that major depression usually begins in adolescence or early adulthood,55 that depression influences diabetes and glycemic control, rather than vice versa. Furthermore, if PGC causes depression, we would expect this relationship to be mediated at least in part by low self-perceived physical health,56,57 a valid measure among Latinos living in the United States for 10 years or more58 and a strong predictor of diabetes-related mortality,59 and to become attenuated following adjustment for perceived physical health. Our analysis does not support this hypothesis.
Considering the usually transient nature of depression in diabetic adults,60 a relative advantage of using cross-sectional data in the investigation of the relationship between depression and HbA1c is that current depression may be more causally relevant to current or recent glycemic control than past depressive episodes; potentially biasing factors, such as selective loss to follow-up or nonrandom changes in the treatment of diabetes, are practically eliminated.61
Several plausible behavioral and biological mechanisms might account for the association between depression and PGC. Depression is a risk factor for noncompliance with medical treatment recommendations, including medications, diet, and health behavior62,63; some patients with depression have increased cortisol production and insulin resistance64,65; and serotonin concentrations and catecholamine levels, both altered in depression, might affect glucose regulation.3
A potential limitation of our study is lack of data on several possible confounders and mediators, namely, body mass index, a risk factor for diabetes that might be associated with depression,66 use of psychotropic drugs, and cigarette smoking. The two latter possible confounders, however, are not likely to account for our finding. Although atypical antipsychotic drugs were shown to be associated with hyperglycemia,67 outpatients with depression are rarely treated with these drugs.68 Rather, depressed primary care patients are likely to be treated with antidepressants, commonly serotonin reuptake inhibitors,69 which often suppress appetite and may cause some degree of weight loss. Therefore, not adjusting for use of antidepressants might have resulted in underestimation of the effect of depression on glycemic control. Finally, patients with depression are more likely to smoke than nondepressed patients,70 and smoking has been independently associated with slightly (0.08%) higher HbA1c levels.71 Thus, although smoking might, theoretically, act as a confounder in our data, it is unlikely to account fully for the observed association.
Our assessment of depression was based on self-report of symptoms by means of face-to-face interviews using a validated instrument, not on the more accurate clinical diagnostic interview.72 However, we demonstrated a strong correlation between depression and the Mental Component Score of the SF-36, supporting the validity of our assessment.
Finally, our sampling strategy, by which frequent attendees were more likely to be sampled than less frequent, presumably less depressed and better controlled diabetes patients, might have introduced selection bias. Nevertheless, in our dataset, patients with moderate or severe depression did not make significantly more visits to the practice (P=0.37; see Table 2), and thus were not more likely to be sampled for the study.
There are important clinical and public health implications to our finding. As rates of diabetes, especially among Hispanics,26,28 continue to increase, it is important for clinicians caring for patients with diabetes to be aware of the association between depression and PGC that might mediate the reported association between depression and diabetes complications,73 which are more common among Hispanics.74–76
Hispanic primary care patients were shown to be less likely to receive appropriate care for depression compared with white patients.77 The finding that fewer than one half of the patients with moderate or severe depression in our sample reported receiving mental health treatment in the previous year probably also indicates underrecognition of major depression in this population. Recognition of depression in symptomatic diabetes patients is particularly difficult, because of overlap in physical (e.g., weight loss and fatigue) or cognitive (e.g., trouble concentrating) symptoms. Our findings suggest that identification and adequate treatment of depression in this understudied, high-risk population of Hispanic primary care patients might have favorable effects on diabetic outcomes.78 Relatively simple and safe pharmacological and psychological interventions are effective in treating depression in primary care,79,80 and specific recommendations for treatment of depression in patients with diabetes are available.81–83 Whether such interventions improve glycemic control in this population awaits additional prospective controlled research.
Acknowledgments
This study was supported by investigator-initiated grants from Upjohn, Kalamazoo, MI (Dr. Weissman); by training grant 5T32-MH13043 from the National Institute of Mental Health, Rockville, MD (Dr. Gross); and by grant P30-AG15294 from the National Institute of Aging, Bethesda, MD (Drs. Shea and Lantigua).
We thank Priya Wickramaratne, PhD for statistical advice and review.
REFERENCES
- 1.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24:1069–78. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 2.Gavard JA, Lustman PJ, Clouse RE. Prevalence of depression in adults with diabetes. An epidemiological evaluation. Diabetes Care. 1993;16:1167–78. doi: 10.2337/diacare.16.8.1167. [DOI] [PubMed] [Google Scholar]
- 3.Lustman PJ, Griffith LS, Clouse RE. Depression in adults with diabetes. Semin Clin Neuropsychiatry. 1997;2:15–23. doi: 10.1053/SCNP00200015. [DOI] [PubMed] [Google Scholar]
- 4.Wells KB, Golding JM, Burnam MA. Psychiatric disorder in a sample of the general population with and without chronic medical conditions. Am J Psychiatry. 1988;145:976–81. doi: 10.1176/ajp.145.8.976. [DOI] [PubMed] [Google Scholar]
- 5.Talbot F, Nouwen A. A review of the relationship between depression and diabetes in adults: is there a link? Diabetes Care. 2000;23:1556–62. doi: 10.2337/diacare.23.10.1556. [DOI] [PubMed] [Google Scholar]
- 6.Lustman PJ, Anderson RJ, Freedland KE, De Groot M, Carney RM, Clouse RE. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care. 2000;23:934–42. doi: 10.2337/diacare.23.7.934. [DOI] [PubMed] [Google Scholar]
- 7.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 8.Ciechanowski PS, Katon WJ, Russo JE, Hirsch IB. The relationship of depressive symptoms to symptom reporting, self-care and glucose control in diabetes. Gen Hosp Psychiatry. 2003;25:246–52. doi: 10.1016/s0163-8343(03)00055-0. [DOI] [PubMed] [Google Scholar]
- 9.Van Tilburg MA, McCaskill CC, Lane JD, et al. Depressed mood is a factor in glycemic control in type 1 diabetes. Psychosom Med. 2001;63:551–5. doi: 10.1097/00006842-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Lloyd CE, Dyer PH, Barnett AH. Prevalence of symptoms of depression and anxiety in a diabetes clinic population. Diabet Med. 2000;17:198–202. doi: 10.1046/j.1464-5491.2000.00260.x. [DOI] [PubMed] [Google Scholar]
- 11.Katon W, von Korff M, Ciechanowski P, et al. Behavioral and clinical factors associated with depression among individuals with diabetes. Diabetes Care. 2004;27:914–20. doi: 10.2337/diacare.27.4.914. [DOI] [PubMed] [Google Scholar]
- 12.Gary TL, Crum RM, Cooper-Patrick L, Ford D, Brancati FL. Depressive symptoms and metabolic control in African-Americans with type 2 diabetes. Diabetes Care. 2000;23:23–9. doi: 10.2337/diacare.23.1.23. [DOI] [PubMed] [Google Scholar]
- 13.Singh PK, Looker HC, Hanson RL, Krakoff J, Bennett PH, Knowler WC. Depression, diabetes, and glycemic control in Pima Indians. Diabetes Care. 2004;27:618–9. doi: 10.2337/diacare.27.2.618-a. [DOI] [PubMed] [Google Scholar]
- 14.Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med. 2000;160:3278–85. doi: 10.1001/archinte.160.21.3278. [DOI] [PubMed] [Google Scholar]
- 15.Kruse J, Schmitz N, Thefeld W. On the association between diabetes and mental disorders in a community sample: results from the German National Health Interview and Examination Survey. Diabetes Care. 2003;26:1841–6. doi: 10.2337/diacare.26.6.1841. [DOI] [PubMed] [Google Scholar]
- 16.Dixon LB, Kreyenbuhl JA, Dickerson FB, et al. A comparison of type 2 diabetes outcomes among persons with and without severe mental illnesses. Psychiatr Serv. 2004;55:892–900. doi: 10.1176/appi.ps.55.8.892. [DOI] [PubMed] [Google Scholar]
- 17.Ismail K, Winkley K, Rabe-Hesketh S. Systematic review and meta-analysis of randomised controlled trials of psychological interventions to improve glycaemic control in patients with type 2 diabetes. Lancet. 2004;363:1589–97. doi: 10.1016/S0140-6736(04)16202-8. [DOI] [PubMed] [Google Scholar]
- 18.Lustman PJ, Griffith LS, Clouse RE, et al. Effects of nortriptyline on depression and glycemic control in diabetes: results of a double-blind, placebo-controlled trial. Psychosom Med. 1997;59:241–50. doi: 10.1097/00006842-199705000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Lustman PJ, Griffith LS, Freedland KE, Kissel SS, Clouse RE. Cognitive behavior therapy for depression in type 2 diabetes mellitus. A randomized, controlled trial. Ann Intern Med. 1998;129:613–21. doi: 10.7326/0003-4819-129-8-199810150-00005. [DOI] [PubMed] [Google Scholar]
- 20.Lustman PJ, Freedland KE, Griffith LS, Clouse RE. Fluoxetine for depression in diabetes: a randomized double-blind placebo-controlled trial. Diabetes Care. 2000;23:618–23. doi: 10.2337/diacare.23.5.618. [DOI] [PubMed] [Google Scholar]
- 21.Williams JW, Jr, Katon W, Lin EH, et al. The effectiveness of depression care management on diabetes-related outcomes in older patients. Ann Intern Med. 2004;140:1015–24. doi: 10.7326/0003-4819-140-12-200406150-00012. [DOI] [PubMed] [Google Scholar]
- 22.Lustman PJ, Clouse RE. Treatment of depression in diabetes: impact on mood and medical outcome. J Psychosom Res. 2002;53:917–24. doi: 10.1016/s0022-3999(02)00416-6. [DOI] [PubMed] [Google Scholar]
- 23.Grumbach K. Primary care in the United States—the best of times, the worst of times. N Engl J Med. 1999;341:2008–10. doi: 10.1056/NEJM199912233412611. [DOI] [PubMed] [Google Scholar]
- 24.Rost K, Nutting P, Smith J, Coyne JC, Cooper-Patrick L, Rubenstein L. The role of competing demands in the treatment provided primary care patients with major depression. Arch Fam Med. 2000;9:150–4. doi: 10.1001/archfami.9.2.150. [DOI] [PubMed] [Google Scholar]
- 25.Trude S, Stoddard JJ. Referral gridlock: primary care physicians and mental health services. J Gen Intern Med. 2003;18:442–9. doi: 10.1046/j.1525-1497.2003.30216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA. 2001;286:1195–200. doi: 10.1001/jama.286.10.1195. [DOI] [PubMed] [Google Scholar]
- 27.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–9. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 28.Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290:1884–90. doi: 10.1001/jama.290.14.1884. [DOI] [PubMed] [Google Scholar]
- 29.Hispanic health in the United States. Council on Scientific Affairs. JAMA. 1991;265:248–52. [PubMed] [Google Scholar]
- 30.Harris MI, Eastman RC, Cowie CC, Flegal KM, Eberhardt MS. Racial and ethnic differences in glycemic control of adults with type 2 diabetes. Diabetes Care. 1999;22:403–8. doi: 10.2337/diacare.22.3.403. [DOI] [PubMed] [Google Scholar]
- 31.Tucker KL, Bermudez OI, Castaneda C. Type 2 diabetes is prevalent and poorly controlled among Hispanic elders of Caribbean origin. Am J Public Health. 2000;90:1288–93. doi: 10.2105/ajph.90.8.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carter JS, Pugh JA, Monterrosa A. Non-insulin-dependent diabetes mellitus in minorities in the United States. Ann Intern Med. 1996;125:221–32. doi: 10.7326/0003-4819-125-3-199608010-00011. [DOI] [PubMed] [Google Scholar]
- 33.Anderson R. National Vital Statistics Reports. Hyattsville, MD: National Center for Health Statistics; 2001. Deaths: Leading Causes for 1999. [Google Scholar]
- 34.Sudano JJ, Jr, Baker DW. Antihypertensive medication use in Hispanic adults: a comparison with black adults and white adults. Med Care. 2001;39:575–87. doi: 10.1097/00005650-200106000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Fisher L, Chesla CA, Mullan JT, Skaff MM, Kanter RA. Contributors to depression in Latino and European-American patients with type 2 diabetes. Diabetes Care. 2001;24:1751–7. doi: 10.2337/diacare.24.10.1751. [DOI] [PubMed] [Google Scholar]
- 36.Fisher L, Chesla CA, Skaff MA, et al. Disease management status: a typology of Latino and Euro-American patients with type 2 diabetes. Behav Med. 2000;26:53–66. doi: 10.1080/08964280009595752. [DOI] [PubMed] [Google Scholar]
- 37.Fisher L, Chesla CA, Skaff MM, et al. The family and disease management in Hispanic and European-American patients with type 2 diabetes. Diabetes Care. 2000;23:267–72. doi: 10.2337/diacare.23.3.267. [DOI] [PubMed] [Google Scholar]
- 38.Sclar DA, Robison LM, Skaer TL, Galin RS. Ethnicity and the prescribing of antidepressant pharmacotherapy: 1992–1995. Harv Rev Psychiatry. 1999;7:29–36. doi: 10.1093/hrp/7.1.29. [DOI] [PubMed] [Google Scholar]
- 39.Olfson M, Shea S, Feder A, et al. Prevalence of anxiety, depression, and substance use disorders in an urban general medicine practice. Arch Fam Med. 2000;9:876–83. doi: 10.1001/archfami.9.9.876. [DOI] [PubMed] [Google Scholar]
- 40. International Classification of Diseases, Ninth Revision, Clinical Modification. Washington, DC: Public Health Service, U.S. Dept of Health and Human Services; 1996.
- 41.Hebert PL, Geiss LS, Tierney EF, Engelgau MM, Yawn BP, McBean AM. Identifying persons with diabetes using Medicare claims data. Am J Med Qual. 1999;14:270–7. doi: 10.1177/106286069901400607. [DOI] [PubMed] [Google Scholar]
- 42.Goldstein DE, Little RR, Lorenz RA, et al. Tests of glycemia in diabetes. Diabetes Care. 2004;27:1761–73. doi: 10.2337/diacare.27.7.1761. [DOI] [PubMed] [Google Scholar]
- 43.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282:1737–44. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 44.Diez-Quevedo C, Rangil T, Sanchez-Planell L, Kroenke K, Spitzer RL. Validation and utility of the patient health questionnaire in diagnosing mental disorders in 1003 general hospital Spanish inpatients. Psychosom Med. 2001;63:679–86. doi: 10.1097/00006842-200107000-00021. [DOI] [PubMed] [Google Scholar]
- 45.Spitzer RL, Williams JB, Kroenke K, et al. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA. 1994;272:1749–56. [PubMed] [Google Scholar]
- 46.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–63. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 48.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41:1284–92. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- 49.American Diabetes Association. Standards of medical care for patients with diabetes mellitus. Diabetes Care. 2005;28(suppl 1):S4–S36. [PubMed] [Google Scholar]
- 50.Hosmer DW, Lemeshow S. Wiley Series in Probability and Statistics. New York, NY: Wiley; 2000. Applied Logistic Regression; p. xii, 373. [Google Scholar]
- 51.Chuang-Stein G, Agresti A. A review of tests for detecting a monotone dose-response relationship with ordinal response data. Stat Med. 1997;16:2599–618. doi: 10.1002/(sici)1097-0258(19971130)16:22<2599::aid-sim734>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 52.Anderson RJ, Grigsby AB, Freedland KE, et al. Anxiety and poor glycemic control: a meta-analytic review of the literature. Int J Psychiatry Med. 2002;32:235–47. doi: 10.2190/KLGD-4H8D-4RYL-TWQ8. [DOI] [PubMed] [Google Scholar]
- 53.Carnethon MR, Kinder LS, Fair JM, Stafford RS, Fortmann SP. Symptoms of depression as a risk factor for incident diabetes: findings from the National Health and Nutrition Examination Epidemiologic Follow-up Study, 1971–1992. Am J Epidemiol. 2003;158:416–23. doi: 10.1093/aje/kwg172. [DOI] [PubMed] [Google Scholar]
- 54.Eaton WW, Armenian H, Gallo J, Pratt L, Ford DE. Depression and risk for onset of type II diabetes. A prospective population-based study. Diabetes Care. 1996;19:1097–102. doi: 10.2337/diacare.19.10.1097. [DOI] [PubMed] [Google Scholar]
- 55.Weissman MM. Juvenile-onset major depression includes childhood-and adolescent-onset depression and may be heterogeneous. Arch Gen Psychiatry. 2002;59:223–4. doi: 10.1001/archpsyc.59.3.223. [DOI] [PubMed] [Google Scholar]
- 56.Bailey BJ. Mediators of depression in adults with diabetes. Clin Nurs Res. 1996;5:28–42. doi: 10.1177/105477389600500104. [DOI] [PubMed] [Google Scholar]
- 57.Egede LE, Zheng D. Independent factors associated with major depressive disorder in a national sample of individuals with diabetes. Diabetes Care. 2003;26:104–11. doi: 10.2337/diacare.26.1.104. [DOI] [PubMed] [Google Scholar]
- 58.Finch BK, Hummer RA, Reindl M, Vega WA. Validity of self-rated health among Latino(a)s. Am J Epidemiol. 2002;155:755–9. doi: 10.1093/aje/155.8.755. [DOI] [PubMed] [Google Scholar]
- 59.Benjamins MR, Hummer RA, Eberstein IW, Nam CB. Self-reported health and adult mortality risk: an analysis of cause-specific mortality. Soc Sci Med. 2004;59:1297–306. doi: 10.1016/j.socscimed.2003.01.001. [DOI] [PubMed] [Google Scholar]
- 60.Peyrot M, Rubin RR. Persistence of depressive symptoms in diabetic adults. Diabetes Care. 1999;22:448–52. doi: 10.2337/diacare.22.3.448. [DOI] [PubMed] [Google Scholar]
- 61.Louis TA, Robins J, Dockery DW, Spiro A, III, Ware JH. Explaining discrepancies between longitudinal and cross-sectional models. J Chronic Dis. 1986;39:831–9. doi: 10.1016/0021-9681(86)90085-8. [DOI] [PubMed] [Google Scholar]
- 62.McKellar JD, Humphreys K, Piette JD. Depression increases diabetes symptoms by complicating patients' self-care adherence. Diabetes Educ. 2004;30:485–92. doi: 10.1177/014572170403000320. [DOI] [PubMed] [Google Scholar]
- 63.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101–7. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 64.Nathan RS, Sachar EJ, Asnis GM, Halbreich U, Halpern FS. Relative insulin insensitivity and cortisol secretion in depressed patients. Psychiatry Res. 1981;4:291–300. doi: 10.1016/0165-1781(81)90031-7. [DOI] [PubMed] [Google Scholar]
- 65.Winokur A, Maislin G, Phillips JL, Amsterdam JD. Insulin resistance after oral glucose tolerance testing in patients with major depression. Am J Psychiatry. 1988;145:325–30. doi: 10.1176/ajp.145.3.325. [DOI] [PubMed] [Google Scholar]
- 66.Onyike CU, Crum RM, Lee HB, Lyketsos CG, Eaton WW. Is obesity associated with major depression? Results from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2003;158:1139–47. doi: 10.1093/aje/kwg275. [DOI] [PubMed] [Google Scholar]
- 67.Luna B, Feinglos MN. Drug-induced hyperglycemia. JAMA. 2001;286:1945–8. doi: 10.1001/jama.286.16.1945. [DOI] [PubMed] [Google Scholar]
- 68.Olfson M, Marcus SC, Druss B, Elinson L, Tanielian T, Pincus HA. National trends in the outpatient treatment of depression. JAMA. 2002;287:203–9. doi: 10.1001/jama.287.2.203. [DOI] [PubMed] [Google Scholar]
- 69.Sleath BL, Rubin RH, Huston SA. Antidepressant prescribing to Hispanic and non-Hispanic white patients in primary care. Ann Pharmacother. 2001;35:419–23. doi: 10.1345/aph.10245. [DOI] [PubMed] [Google Scholar]
- 70.Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: a population-based prevalence study. JAMA. 2000;284:2606–10. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- 71.Sargeant LA, Khaw KT, Bingham S, et al. Cigarette smoking and glycaemia: the EPIC-Norfolk Study. European Prospective Investigation into Cancer. Int J Epidemiol. 2001;30:547–54. doi: 10.1093/ije/30.3.547. [DOI] [PubMed] [Google Scholar]
- 72.Eaton WW, Neufeld K, Chen LS, Cai G. A comparison of self-report and clinical diagnostic interviews for depression: diagnostic interview schedule and schedules for clinical assessment in neuropsychiatry in the Baltimore epidemiologic catchment area follow-up. Arch Gen Psychiatry. 2000;57:217–22. doi: 10.1001/archpsyc.57.3.217. [DOI] [PubMed] [Google Scholar]
- 73.De Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: a meta-analysis. Psychosom Med. 2001;63:619–30. doi: 10.1097/00006842-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 74.Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV. Ethnic disparities in diabetic complications in an insured population. JAMA. 2002;287:2519–27. doi: 10.1001/jama.287.19.2519. [DOI] [PubMed] [Google Scholar]
- 75.Lavery LA, Ashry HR, Van Houtum W, Pugh JA, Harkless LB, Basu S. Variation in the incidence and proportion of diabetes-related amputations in minorities. Diabetes Care. 1996;19:48–52. doi: 10.2337/diacare.19.1.48. [DOI] [PubMed] [Google Scholar]
- 76.Varma R, Torres M, Pena F, Klein R, Azen SP. Prevalence of diabetic retinopathy in adult Latinos: the Los Angeles Latino eye study. Ophthalmology. 2004;111:1298–306. doi: 10.1016/j.ophtha.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 77.Miranda J, Cooper LA. Disparities in care for depression among primary care patients. J Gen Intern Med. 2004;19:120–6. doi: 10.1111/j.1525-1497.2004.30272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eaton WW. Epidemiologic evidence on the comorbidity of depression and diabetes. J Psychosom Res. 2002;53:903–6. doi: 10.1016/s0022-3999(02)00302-1. [DOI] [PubMed] [Google Scholar]
- 79.Schulberg HC, Katon W, Simon GE, Rush AJ. Treating major depression in primary care practice: an update of the Agency for Health Care Policy and Research Practice Guidelines. Arch Gen Psychiatry. 1998;55:1121–7. doi: 10.1001/archpsyc.55.12.1121. [DOI] [PubMed] [Google Scholar]
- 80.Whooley MA, Simon GE. Managing depression in medical outpatients. N Engl J Med. 2000;343:1942–50. doi: 10.1056/NEJM200012283432607. [DOI] [PubMed] [Google Scholar]
- 81.Katon W, Von Korff M, Lin E, et al. Improving primary care treatment of depression among patients with diabetes mellitus: the design of the pathways study. Gen Hosp Psychiatry. 2003;25:158–68. doi: 10.1016/s0163-8343(03)00013-6. [DOI] [PubMed] [Google Scholar]
- 82.Jacobson AM. The psychological care of patients with insulin-dependent diabetes mellitus. N Engl J Med. 1996;334:1249–53. doi: 10.1056/NEJM199605093341907. [DOI] [PubMed] [Google Scholar]
- 83.Rubin RR, Ciechanowski P, Egede LE, Lin EH, Lustman PJ. Recognizing and treating depression in patients with diabetes. Curr Diab Rep. 2004;4:119–25. doi: 10.1007/s11892-004-0067-8. [DOI] [PubMed] [Google Scholar]