Abstract
OBJECTIVE
To determine how well a single question of self-reported erectile dysfunction compares to a gold standard clinical urologic examination.
DESIGN AND SETTING
Clinical validation study nested within the Massachusetts Male Aging Study (MMAS), which is an observational cohort study of aging and health in a population-based random sample of men.
MEASUREMENT
During an in-person interview, men were asked to respond to a single-question self-report of erectile dysfunction. A subsample of MMAS participants was then subjected to a clinical urologic examination to obtain a clinical diagnosis of erectile dysfunction.
PARTICIPANTS
One hundred thirty-nine men 55 to 85 years of age from the MMAS.
RESULTS
Complete data were available from 137 men. Erectile dysfunction (ED) measured by self-report and independent urologic examination were strongly correlated (Spearman r=.80). Receiver operating curve analysis showed that the self-reported ED item accurately predicts the clinician-diagnosed ED (area under the curve [AUC]=0.888). Stratum-specific likelihood ratios (95% confidence intervals) for self-reports predicting the gold standard were: no ED=0.11 (0.06 to 0.22), minimal ED=1.48 (0.67 to 3.26), moderate ED=8.57 (1.21 to 60.65), and complete ED=12.69 (1.81 to 88.79). These data indicate that men diagnosed with ED by urologic examination can be distinguished from men not diagnosed with ED by urologic examination if the respondent self-reported no, moderate, or complete ED.
CONCLUSION
Our single-question self-report accurately identifies men with clinically diagnosed ED, and may be useful as a referral screening tool in both research studies and general practice settings.
Keywords: erectile dysfunction, screening, measurement, epidemiology, men
Erectile dysfunction (ED), defined as the inability to achieve or maintain an erection sufficient for satisfactory sexual performance,1 is estimated to affect millions of American men.2,3 Although not a life-threatening condition, ED is common among aging men, especially those with comorbid chronic conditions (e.g., diabetes and cardiovascular disease (CVD)). Some have suggested that it may be a biomarker for occult diabetes,4,5 CVD,6–9 and lower urinary tract infections.10–12 ED has been shown to have a profound effect on the quality of life of aging men.13–15 Given these observations, ED should not simply be ignored and dismissed as a normal consequence of aging.
Approaches to measuring ED have evolved over the past few decades. Early methods (e.g., nocturnal penile tumescence) were performed in a clinical setting and were quite invasive. These methods are clearly not appropriate for screening or use in observational epidemiologic studies. Researchers have recently moved toward questionnaire-based determination of erectile function. Three commonly used questionnaire-based instruments are the 11-item Brief Male Sexual Function Inventory (BMSFI),16 the 15-item International Index of Erectile Function (IIEF),17 and a 13-item composite scale developed in the Massachusetts Male Aging Study (MMAS).18 Given the sensitive nature of the questions contained in these instruments, item nonresponse is likely and missing data could impede classification of study subjects on ED status.
Following others who have developed single-question self-reports for measuring depression19 and personal safety,20 we developed a single-question self-assessment for ED21 based on the National Institutes of Health (NIH) consensus definition1 (Table 1). We have previously shown that self-administration of this single question correlates well with the IIEF and the BMSFI, and that the proportion with missing data for the single question (9%) was similar to that for the BMSFI (8%) and lower than that for the IIEF (18%).21 In addition to addressing the problem of missing data, the single question may have advantages for use in clinic-based screening and in large, population-based studies in which ED is one of many outcomes of interest (e.g., Behavioral Risk Factor Surveillance Survey [BRFSS], National Health and Nutrition Examination Survey [NHANES], National Health Interview Survey [NHIS]).
Table 1.
Single-question Self-report of Erectile Dysfunction, Massachusetts Male Aging Study
| Erectile dysfunction (sometimes called impotence) means being unable to get and keep an erection that is rigid enough for satisfactory sexual activity. | |
| Not impotent | Always able to get and keep an erection good enough for sexual intercourse |
| Minimally impotent | Usually able to get and keep an erection good enough for sexual intercourse |
| Moderately impotent | Sometimes able to get and keep an erection good enough for sexual intercourse |
| Completely impotent | Never able to get and keep an erection good enough for sexual intercourse |
While both the IIEF and BMSFI are widely used, neither is considered a gold standard for assessing ED. In order to further test the validity of the single-question self-assessment of ED, we conducted a validation substudy to compare directly the MMAS single question (interviewer administered as part of a general health status inventory) with a standardized clinical urologic examination in a subsample of 139 men from the MMAS cohort.
METHODS
The MMAS is an observational cohort study of health and aging in a population-based random sample of men. The design has been well-described elsewhere.3,22,23 As part of the in-person interview, respondents were queried about erectile function with a single question. Table 1 presents the ED single question as it was presented to respondents in the MMAS.
The substudy was described to all participants at the conclusion of the in-person interview, and each was offered the opportunity to participate. They were told that participation was strictly voluntary and independent of their participation in the main study. Those who participated in the substudy received a remuneration of $100. Upon agreeing to participate, men underwent a complete urologic examination with a single sexual medicine and urologic expert (IG) who was blinded with respect to self-reported ED status obtained in the MMAS survey.
The standardized, semistructured clinical exam was based on the “Process of Care Model for ED,” evidence-based guidelines in the management of ED that were developed by a multidisciplinary panel of experts to examine practice standards and entailed a sexual, psychological, and medical history.24 The components of this approach have been accepted by the U.S.24,25 and international26 urologic community.
The examination itself was composed of 4 main components (see Table 2): a detailed sexual history, medical history, psychosocial history, and a physical examination. Included in the sexual history were questions about the subject's ability to have ever achieved a rigid, sustained, and spontaneous erection either upon awakening, during masturbation, or with sexual intercourse. An arbitrary axial rigidity scale of the erection was established by comparing the subject's erectile rigidity to the rigidity of a metal stapler. Participants were asked first to provide retrospective accounts on these issues as young adults and then asked about their current status. They were asked whether there had been any consistent and persistent changes in the quality (spontaneity, rigidity, or maintenance) of their erections, and if so, when during their aging process they noticed these changes. They were also asked to speculate on the reason (surgery, medical illness, medication use, relationship changes, psychological conditions, etc.) for any changes in their erectile function. Finally, subjects were asked whether they had seen any health care professionals for treatment of any sexual problems and whether they had ever received treatment for sexual problems.
Table 2.
Components of the Urologic Examination for the Diagnosis of Erectile Dysfunction, Massachusetts Male Aging Study
| Sexual history | Establish whether the subject ever experienced consistent rigid, spontaneous, appropriately lasting, painless, and straight penile erections in the morning or with sexual activity |
| If indicated, establish approximately when in the subject's life he experienced consistent and persistent changes in rigidity, spontaneity, or duration of the erection in the morning or with sexual activity | |
| If indicated, establish approximately when in the subject's life he experienced consistent and persistent changes in curvature, shortening, or pain associated with penile erection | |
| Establish whether there are other sexual problems such as diminished or excessive interest; early, late, or absent ejaculation; changes in orgasm intensity; or changes in sexual satisfaction | |
| Establish whether the subject's partner has any sexual problems | |
| Assess the degrees of distress that the subject has to any sexual difficulties if present | |
| Medical history | Establish current general health and past medical history, especially association with vascular risk factors such as hypertension, hypercholesterolemia, cigarette smoking, coronary artery disease, etc. |
| Determine current medications | |
| Establish any psychiatric or psychological history | |
| Determine whether the subject's partner has medical health issues | |
| Assess the degree of distress that the subject has to any medical difficulties if present | |
| Psychosocial history | Establish current and past mood and mental health, including traumas and losses |
| Identify nature and duration of current relationship | |
| Determine partner's mood and mental health | |
| Assess the level of distress that the subject has and psychological difficulties if present | |
| Physical examination | Assess for balanitis, phimosis, penile masses, and presence of dorsal neurovascular bundle thickening |
| Measure stretched penile length | |
| Assess for scrotal masses, testicular size, and tissue consistency |
All subjects underwent a physical examination restricted to the genital area. Scrotal contents were examined and testicular size estimated. A detailed assessment for the presence and severity of Peyronie's disease and dorsal neurovascular bundle thickening was performed. Neither objective measurements of penile tumescence nor specific laboratory tests were utilized.
The ultimate goal of the MMAS substudy clinical examination was to determine: 1) whether the respondent had ED, and 2) if so, the extent (minimal, moderate, or complete) of the dysfunction. Presence or absence of ED was based on the process described above. Key elements in the assignment of ED included the persistence of the ED complaint for a period of at least 3 months, the ability to both initiate and sustain the erectile response during adequate sexual stimulation, and finally achieving satisfactory sexual performance. When ED was identified, it was classified according to severity—mild ED consists of a slight, intermittent, or irregular loss of penile rigidity, while moderate and complete ED pertain to an increasing degree of loss of penile rigidity and its accompanying adverse effect on erectile functional capabilities.
Statistical Analysis
In all analyses, the dichotomous outcome variable is the presence or absence of ED diagnosed by urologic examination. We used the receiver operating characteristic (ROC) curve to quantify how well the self-reported ED item predicted ED by urologic examination. The area under the curve (AUC, also known as the c-statistic) was used as an indication of the overall accuracy of the single question for predicting the response. An AUC of 1.0 indicates perfect accuracy, while an AUC of 0.5 indicates a nondiscriminating test.
In order to further test the accuracy of the self-reported single item, we computed stratum-specific likelihood ratios (SSLR).27 The SSLR is the probability of a given test result when the disease is present, divided by the probability of the same test result when the disease is absent. SSLRs summarize how many times more (or less) likely persons with the disease are to have a particular test result than persons without the disease. In this sense, SSLRs can be interpreted in the same manner as risk ratios, and confidence intervals can be computed accordingly.
RESULTS
Clinical urologic examinations were performed on 139 respondents; 2 men were not assigned a diagnosis of ED during the clinical urologic examination, thus yielding an effective sample size of N=137. The 137 men ranged in age from 55 to 85 years (mean=67.1 years, SD=7.4 years). The majority of respondents were white, married, of relatively high socioeconomic status, and employed (see Table 3).
Table 3.
Selected Characteristics of MMAS T3 Participants by Substudy Participation, Massachusetts Male Aging Study (MMAS) (N=853)
| T1 Variable | Not in Substudy (n=716) | In Substudy (n=137) | P Value* | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Age decade | .2133 | ||||
| 55–64 | 284 | 39.7 | 61 | 44.5 | |
| 65–74 | 246 | 34.4 | 50 | 36.5 | |
| 75–85 | 186 | 26.0 | 26 | 19.0 | |
| Race | .8454 | ||||
| White | 687 | 96.8 | 133 | 97.1 | |
| Other | 23 | 3.2 | 4 | 2.9 | |
| Marital status | .4545 | ||||
| Never married | 64 | 9.0 | 12 | 8.8 | |
| Currently married | 516 | 72.3 | 94 | 68.6 | |
| Divorced/separated | 70 | 9.8 | 19 | 13.9 | |
| Widowed | 56 | 7.8 | 9 | 6.6 | |
| Living with male partner | 8 | 1.1 | 3 | 2.2 | |
| Employed | 351 | 49.2 | 72 | 52.6 | .4666 |
| Education | .1192 | ||||
| Less than high school | 49 | 6.9 | 9 | 6.6 | |
| High school graduate | 118 | 16.5 | 16 | 11.7 | |
| Some college | 170 | 23.8 | 23 | 16.8 | |
| Bachelor's degree | 101 | 14.2 | 24 | 17.5 | |
| Advanced degree | 276 | 38.7 | 65 | 47.5 | |
| Annual household income | .1292 | ||||
| <$50,000 | 235 | 34.6 | 37 | 27.6 | |
| $50,000–$79,999 | 164 | 24.1 | 40 | 29.9 | |
| $80,000–$99,999 | 88 | 12.9 | 24 | 17.9 | |
| ≥$100,000 | 193 | 28.4 | 33 | 24.6 | |
P value based on χ2 test of independence
A total of 104 of the 137 (75.9%) participants were found to have ED by urologic examination. The prevalence of ED by self-report was nearly identical (75.2%). The self-reported prevalence of ED in substudy participants was significantly higher than that observed in men not participating in the substudy (63.5%, P=.0083). There were no differences in the distribution of demographic variables between substudy participants and nonparticipants (see Table 3). ED by self-report and clinical urologic examination were strongly correlated (Spearman r=.80). Crossclassification of ED by urologic examination variable with the self-reported ED variable showed that within each self-reported ED category, concordant cells comprised the largest proportion of individuals; the level of ED determined by urologic examination matched the self-reported level in 75.8%, 66.7%, 39.3%, and 69.1% of men self-reporting no, minimal, moderate, and complete ED, respectively. A higher percentage of cases were classified as “complete” by urologic exam (40.1%) than by self-report (29.9%).
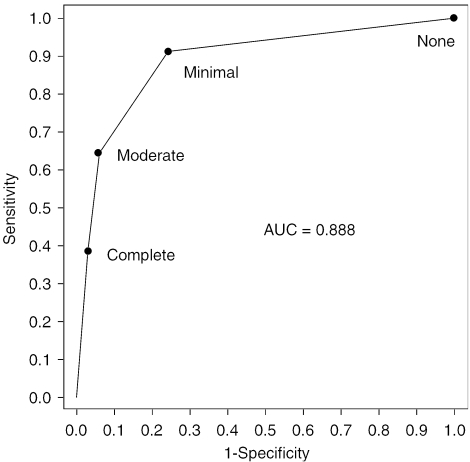
The results of the ROC analysis, with ED by urologic examination as the response and self-reported ED as the predictor, are displayed in Figure 1. The AUC is 0.888, indicating that the self-reported ED item provides a significant improvement in predicting the response compared with chance (0.5). Based on the ROC curve, the cut point on the self-reported ED item which provided a good balance between sensitivity and specificity is the minimal category. By defining as having ED anyone reporting minimal, moderate, or complete ED, the associated sensitivity and specificity are 91.3% and 75.8%, respectively.
FIGURE 1.
Receiver operating characteristic (ROC) curve for the self-reported erectile dysfunction (ED) item predicting ED by urologic examination. Labeled points on the ROC curve indicate cut points. Massachusetts Male Aging Study (MMAS) (N=137).
The results of the SSLR analysis are displayed in Table 4. Men diagnosed with ED by urologic examination were more likely to screen positive on the self-reported ED item. Likelihood ratios were particularly high for self-reports of moderate and complete ED, with men diagnosed with ED by urologic examination being 8.57 and 12.69 times more likely than men not diagnosed with ED by urologic examination to report moderate and complete ED, respectively.
Table 4.
Stratum-specific Likelihood Ratios for Self-reported Erectile Dysfunction Predicting Erectile Dysfunction by Urologic Examination, Massachusetts Male Aging Study (N=137)
| Self-reported ED | ED by Urologic Examination | Likelihood Ratio* | 95% Confidence Interval | |||
|---|---|---|---|---|---|---|
| Yes | No | |||||
| N | % | N | % | |||
| None | 9 | 8.7 | 25 | 75.8 | 0.11 | 0.06 to 0.22 |
| Minimal | 28 | 26.9 | 6 | 18.2 | 1.48 | 0.67 to 3.26 |
| Moderate | 27 | 26.0 | 1 | 3.0 | 8.57 | 1.21 to 60.65 |
| Complete | 40 | 38.5 | 1 | 3.0 | 12.69 | 1.81 to 88.79 |
| Total | 104 | 100.0 | 33 | 100.0 | ||
Likelihood ratio (i.e., SSLR) computed by the formula, where x1 is the number of men in a particular self-reported ED stratum with ED by urologic examination, n1 is the total number with ED by urologic examination, x0 is the number of men in the same self-reported ED stratum without ED by urologic examination, and n0 is the total number of men without ED by urologic evaluation
ED, erectile dysfunction; SSLR, stratum-specific likelihood ratio
DISCUSSION
In this study, we found that a self-report of ED predicts clinically diagnosed ED with reasonable accuracy, as indicated by AUC=0.888. The ROC analysis shows that using minimal as the cut point for the self-reported ED item in this population provides a good balance between sensitivity (91.3%) and specificity (75.8%). The SSLR analysis extends the results of the ROC analysis by taking advantage of the polytomous nature of the self-reported ED item, showing a clear dose-response increase in likelihood ratios with increasing severity of self-reported ED. The advantage of the SSLR approach is that unlike traditional screening measures such as positive and negative predictive value, results obtained with this method can be applied to populations with a different prevalence of ED. The cut points chosen by clinicians and investigators using the single question might vary between minimal and moderate depending on how sensitive or specific they would like their case definition to be.
Limitations to the current study should be acknowledged. There are two limitations related to selection bias that are noteworthy. First, by the nature of the study and sampling design (random sample of men in the Boston metropolitan area begun in the mid-1980s), participants in the ED substudy were almost all white and relatively affluent. Thus, these data are not necessarily applicable to more sociodemographically diverse populations. Additional research in such populations is clearly needed. Second, there was a significant difference in the prevalence of self-reported ED among substudy participants and nonparticipants (63.5% vs 75.9%, P=.0083). It is not clear whether or how this difference in prevalence might have affected the operating characteristics of the self-reported single item. The distributions of demographic variables did not differ by substudy participation. A third possible limitation lies in the method used to assess the gold standard. Although subjects underwent a comprehensive urologic evaluation, it was based solely on questions about erectile functioning and did not include any measurements of penile tumescence.
Our findings concerning the validity of self-reported ED (when compared with a gold standard urologic examination) have important implications for clinical practice and research. Until quite recently, ED was often dismissed as an unavoidable consequence of aging. However, given its potential as a “sentinel event,” primary care physicians are now encouraged to take ED seriously.28 Yet there are numerous obstacles to considering male sexuality in a general medical encounter. These include shorter medical encounters,29,30 reluctance on the part of older men to discuss issues of sexuality,31 and lack of training in the diagnosis and management of sexual difficulties among clinicians most likely to hear complaints about sexual difficulties (i.e., general practitioners). Use of the MMAS ED single question could address these obstacles. A validated screening instrument that is quickly administered and approximates a clinical examination would be of great value in such time-constrained medical practices. Administration of the single question, prefaced with a statement about the importance of sexual health to overall health, might make the patient feel more comfortable discussing such issues.28 Overcoming these obstacles will increase the likelihood of detecting ED at a routine office visit, thereby providing better care for older men.
Our findings also have implications for researchers. A single question concerning ED could be added cost-efficiently to large ongoing national and international epidemiologic surveys to provide needed information concerning the prevalence of ED in specific population groups. Furthermore, the single question should reduce translational difficulties associated with multi-item scales, making it useful for crossnational comparative epidemiologic studies. Finally, ED status could be added as a secondary endpoint/outcome to clinical trials focusing on new therapies (e.g., cholesterol-lowering agents and hormone replacement for aging men), as well as community interventions. The inclusion of a single ED question in such studies would provide information useful for the primary and secondary prevention of ED.32
Acknowledgments
This work was supported by the National Institute of Diabetes and Digestive and Kidney Disorders (grant DK44995).
References
- 1.NIH Consensus Conference. Impotence. (Speakers included McKinlay JB, The prevalence and demographics of impotence.) J Am Med Assoc. 1993;270:83–90. Also published as NIH Consensus Statement. 1992;10:1–31. [Google Scholar]
- 2.Feldman HA, Goldstein I, McKinlay JB, Hatzichristou DG, Krane RJ. Impotence and its medical and psychosocial correlates in men aged 40–70. results of the Massachusetts Male Aging Study J Urol. 1994;151:54–61. doi: 10.1016/s0022-5347(17)34871-1. [DOI] [PubMed] [Google Scholar]
- 3.Johannes CB, Araujo AB, Feldman HA, Derby CA, Kleinman KP, McKinlay JB. Incidence of erectile dysfunction in men ages 40–69. longitudinal results from the Massachusetts Male Aging Study J Urol. 2000;163:460–463. [PubMed] [Google Scholar]
- 4.Sairam K, Kulinskaya E, Boustead GB, Hanbury DC, McNicholas TA. Prevalence of undiagnosed diabetes mellitus in male erectile dysfunction. BJU Int. 2001;88:68–71. doi: 10.1046/j.1464-410x.2001.02260.x. [DOI] [PubMed] [Google Scholar]
- 5.Sasayama S, Ishii N, Ishikura F, et al. Men's Health Study. epidemiology of erectile dysfunction and cardiovascular disease Circ J. 2003;67:656–659. doi: 10.1253/circj.67.656. [DOI] [PubMed] [Google Scholar]
- 6.McKinlay JB. The worldwide prevalence and epidemiology of erectile dysfunction. Int J Impot Res. 2000;12(suppl 4):S6–S11. doi: 10.1038/sj.ijir.3900567. [DOI] [PubMed] [Google Scholar]
- 7.Speel TG, van Langen H, Meuleman EJ. The risk of coronary heart disease in men with erectile dysfunction. Eur Urol. 2003;44:366–370. doi: 10.1016/s0302-2838(03)00304-x. [DOI] [PubMed] [Google Scholar]
- 8.Kirby M, Jackson G, Betteridge J, Friedli K. Is erectile dysfunction a marker for cardiovascular disease? Int J Clin Pract. 2001;55:614–618. [PubMed] [Google Scholar]
- 9.Rosen R, Altwein J, Boyle P, et al. Lower urinary tract symptoms and male sexual dysfunction. the multinational study of the aging male (MSAM-7) Eur Urol. 2003;44:637–649. doi: 10.1016/j.eururo.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 10.Boyle P, Robertson C, Mazzetta C, et al. UrEpik Study Group. The association between lower urinary tract symptoms and erectile dysfunction in four centers. the UrEpik study BJU Int. 2003;92:719–725. doi: 10.1046/j.1464-410x.2003.04459.x. [DOI] [PubMed] [Google Scholar]
- 11.Braun MH, Sommer F, Haupt G, Mathers MJ, Reinfenrath B, Engelmann UH. Lower urinary tract symptoms and erectile dysfunction. co-morbidity or typical “Aging Male” symptoms? Results of the “Cologne Male Survey Eur Urol. 2003;44:588–594. doi: 10.1016/s0302-2838(03)00358-0. [DOI] [PubMed] [Google Scholar]
- 12.Levine LA, Kloner RA. Importance of asking questions about erectile dysfunction. Am J Cardiol. 2000;86:1210–1213. doi: 10.1016/s0002-9149(00)01204-2. [DOI] [PubMed] [Google Scholar]
- 13.Krane RJ, Goldstein I, Sáenz de Tejada I. Impotence. N Engl J Med. 1989;321:1648–1659. doi: 10.1056/NEJM198912143212406. [DOI] [PubMed] [Google Scholar]
- 14.Guest JF, Das Gupta R. Health-related quality of life in a UK-based population of men with erectile dysfunction. Pharmacoeconomics. 2002;20:109–117. doi: 10.2165/00019053-200220020-00004. [DOI] [PubMed] [Google Scholar]
- 15.Sanchez-Cruz JJ, Cabrera-Leon A, Martin-Morales A, Fernandez A, Burgos R, Rejas J. Male erectile dysfunction and health-related quality of life. Eur Urol. 2003;44:245–253. doi: 10.1016/s0302-2838(03)00215-x. [DOI] [PubMed] [Google Scholar]
- 16.O'Leary MP, Lenderking WR, Barber B, Sagnier PP, Guess HA, Barry MJ. A brief male sexual function inventory for urology. Urology. 1995;46:697–706. doi: 10.1016/S0090-4295(99)80304-5. [DOI] [PubMed] [Google Scholar]
- 17.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The International Index of Erectile Function (IIEF) a multidimensional scale for assessment of erectile dysfunction Urology. 1997;49:822–830. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 18.Kleinman KP, Feldman HA, Johannes CB, McKinlay JB. A new surrogate variable for erectile dysfunction status in the Massachusetts Male Aging Study. J Clin Epidemiol. 2000;53:71–78. doi: 10.1016/s0895-4356(99)00150-x. [DOI] [PubMed] [Google Scholar]
- 19.Whooley MA, Avins AL, Miranda J, Browner WS. Case-finding instruments for depression. Two questions are as good as many. J Gen Intern Med. 1997;12:439–45. doi: 10.1046/j.1525-1497.1997.00076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peralta RL, Fleming MF. Screening for intimate partner violence in a primary care setting. the validity of “feeling safe at home” and prevalence results J Am Board Fam Pract. 2003;16:525–532. doi: 10.3122/jabfm.16.6.525. [DOI] [PubMed] [Google Scholar]
- 21.Derby CA, Araujo AB, Johannes CB, Feldman HA, McKinlay JB. Measurement of erectile dysfunction in population-based studies. the use of a single question self-assessment in the Massachusetts Male Aging Study Int J Impot Res. 2000;12:197–204. doi: 10.1038/sj.ijir.3900542. [DOI] [PubMed] [Google Scholar]
- 22.O'Donnell AB, Araujo AB, McKinlay JB. The health of normally aging men. the Massachusetts Male Aging Study (1987–2004) Exp Gerontol. 2004;39:975–984. doi: 10.1016/j.exger.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 23.McKinlay JB, Feldman HA. Age-related variation in sexual activity and interest in normal men. In: Rossi A, editor. results from the Massachusetts Male Aging Study In ed Sexuality Across the Life Course: Proceedings of the MacArthur Foundation Research Network on Successful Mid-life Development. Chicago, IL: University of Chicago Press; 1994. pp. 261–85. [Google Scholar]
- 24.Rosen RC, Goldstein I, Heiman J, et al. The process of care model for evaluation and treatment of erectile dysfunction. Int J Impot Res. 1999;11:59–74. doi: 10.1038/sj.ijir.3900411. [DOI] [PubMed] [Google Scholar]
- 25.Lizza EF, Rosen RC. Definition and classification of erectile dysfunction. report of the Nomenclature Committee of the International Society of Impotence Research Int J Impot Res. 1999;11:141–143. doi: 10.1038/sj.ijir.3900396. [DOI] [PubMed] [Google Scholar]
- 26.Jardin A, Wagner G, Khoury S, et al. Recommendations of the 1st International Consultation on Erectile Dysfunction. In: Jardin A, Wagner G, Khoury S, Giuliano F, Padma-Nathan H, Rosen RC, et al., editors. In: eds. Erectile Dysfunction: 1st International Consultation on Erectile Dysfunction. Oxford: Health Publication Ltd.; 2000. pp. 711–26. [Google Scholar]
- 27.Deeks JJ, Altman DG. Diagnostic tests. likelihood ratios BMJ. 2004;329:168–169. doi: 10.1136/bmj.329.7458.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trude S. So much to do, so little time. physician capacity constraints, 1997–2001 Track Rep. 2003;8:1–4. [PubMed] [Google Scholar]
- 29.Linzer M, Konrad TR, Douglas J, et al. Managed care, time pressure, and physician job satisfaction: results from the Physician Worklife Study. J Gen Intern Med. 2000;15:441–450. doi: 10.1046/j.1525-1497.2000.05239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marwick C. Survey says patients expect little physician help on sex. JAMA. 1999;281:2173–2174. doi: 10.1001/jama.281.23.2173. [DOI] [PubMed] [Google Scholar]
- 31.Amory JK, Watts NB, Easley KA, et al. Exogenous testosterone or testosterone with finasteride increases bone mineral density in older men with low serum testosterone. J Clin Endocrinol Metab. 2004;89:503–510. doi: 10.1210/jc.2003-031110. [DOI] [PubMed] [Google Scholar]
- 32.Derby CA, Mohr B, Goldstein I, Feldman HA, Johannes CB, McKinlay JB. Modifiable risk factors and erectile dysfunction. can lifestyle changes modify risk? Urology. 2000;56:302–306. doi: 10.1016/s0090-4295(00)00614-2. [DOI] [PubMed] [Google Scholar]