Abstract
CONTEXT
Optimal ages of breast cancer screening cessation remain uncertain.
OBJECTIVE
To evaluate screening policies based on age and quartiles of life expectancy (LE).
DESIGN AND POPULATION
We used a stochastic model with proxies of age-dependent biology to evaluate the incremental U.S. societal costs and benefits of biennial screening from age 50 until age 70, 79, or lifetime.
MAIN OUTCOME MEASURES
Discounted incremental costs per life years saved (LYS).
RESULTS
Lifetime screening is expensive ($151,434 per LYS) if women have treatment and survival comparable to clinical trials (idealized); stopping at age 79 costs $82,063 per LYS. This latter result corresponds to costs associated with an LE of 9.5 years at age 79, a value expected for 75% of 79-year-olds, about 50% of 80-year-olds, and 25% of 85-year-olds. Using actual treatment and survival patterns, screening benefits are greater, and lifetime screening of all women might be considered ($114,905 per LYS), especially for women in the top 25% of LE for their age ($50,643 per LYS, life expectancy of∼7 years at age 90).
CONCLUSIONS
If all women receive idealized treatment, the benefits of mammography beyond age 79 are too low relative to their costs to justify continued screening. However, if treatment is not ideal, extending screening beyond age 79 could be considered, especially for women in the top 25% of life expectancy for their age.
Keywords: screening, cost–effectiveness, breast neoplasms, elderly
Breast cancer is largely a disease of old age,1 with almost 50% of new cases and nearly two thirds of deaths occurring among the 13% of the female population that is aged 65 or more.2 By the year 2030, 1 in 5 women will be 65 years of age or older (hereinafter referred to as “older women”).3 This “graying of America”4 is expected to result in a large increase in the absolute number of breast cancer cases among older women.5
However, the benefits, costs,6,7 and potential harms8 of breast cancer screening for these older women9 and the optimal ages of screening cessation6 remain uncertain, owing to the interplay of competing age-related factors, including comorbidity,10–12 mammography sensitivity,13,14 tumor characteristics,1,15–17 deviations from treatment guidelines,18 and morbidity associated with breast cancer and its treatments.19,20
There is limited research addressing selected components of the balance between screening benefits, harms, and costs. For instance, Rich and Black used average age-specific mortality and estimated that 80-year-olds who stop screening would forego a maximum of 5 days of life.21 However, this represents an average of days lost for women who develop breast cancer and the vast majority of women who do not, and does not include costs. In another study, Walter and Covinsky refined this approach by estimating benefits based on life expectancy distributions.22 Kerlikowske et al.6 developed a rigorous model to estimate costs and benefits by level of risk, but did not consider age-related variations in tumor biology or underlying health status.
In this paper, we used a simulation model that incorporates differences in tumor biology and emulates the effects of “physiological age,” or life expectancy to address the question of optimal timing of cessation of screening in older populations.
METHODS
We used an event-driven continuous time Monte Carlo simulation model23 of the natural history of breast cancer to evaluate the costs, harms, and benefits associated with differing ages of screening cessation. Beginning at age 50, we compare biennial screening over the entire life span versus cessation of screening at age 70 or 79 years using average population mortality. We also evaluate outcomes using life expectancy as a proxy for physiologic age. We calculate incremental cost-effectiveness ratios, where the additional costs of a strategy, divided by the added savings in life years, are compared to the next least expensive effective strategy.24 We do not include a “no screening” strategy because current recommendations include screening for women age 50 to 70. Investments in screening programs yield future savings in costs and lives. Discounting adjusts these future costs and outcomes to current values. Thus, all costs and effects are discounted at 3%.24
Each strategy is examined by generating 250,000 simulated patients, a sample size chosen to ensure that the standard error of the difference in estimated population life expectancy between screening strategies would be less than 2 days. Finally, we simulated the life history of the “same” woman under all 3 screening strategies (stop at age 70, 79, screen for a lifetime). This “matched” approach decreases the standard errors and improves the precision of estimation of the differences between the strategies and maximizes computer resource efficiency.25
Model Overview
The model begins with a hypothetical cohort of women aged 50 and randomly assigns dates of death, preclinical and symptomatic breast cancer incidence, and uptake of first and subsequent screening mammograms. Women who are destined to get breast cancer are assigned a date at which symptomatic illness will present (clinical presentation). The stage of clinical presentation is selected randomly from age-specific distributions in unscreened women. If the tumor gets screen detected before this time (a true positive screen), a new stage is calculated using Bayes' theorem with a prior distribution taken from screened breast cancer incidence rates and a conditional distribution calculated from the rates of progression through the stages (in situ, local, regional, and distant). When women develop breast cancer, they are also randomly assigned an estrogen receptor (ER) status. Treatment is randomly selected based on current patterns of care, given age, stage at presentation, and ER status. Survival is randomly assigned based on age, stage, ER status, and treatment. We use survival obtainable in clinical trials as our base case; general breast cancer population survival as reported to the Surveillance, Epidemiology, and End Results (SEER) registry is used in sensitivity analyses. For women who do not develop cancer, the probability of a false positive mammogram is based on the age-specific specificity of mammography and the number of mammograms received between age 50 and the date of death or cessation of screening.
Model Assumptions
We used differences in the distribution of ER status as a proxy for age-related differences in prognostic tumor markers.15,16,26 We make the simplifying assumption that lobular carcinoma in situ (LCIS) and ductal carcinoma in situ (DCIS) have the same history and survival. Because the survival data we used were based on 15 years of clinical trial follow-up, we assumed that women with carcinoma in situ that was destined to progress to invasive disease or those with local stage who survived for 15 years without recurrence would have survival after that time that was similar to their age–matched non–breast cancer cohort. Survival for women with local disease who were alive with recurrent disease at 15 years, or women with regional disease surviving 15 years was estimated using a declining exponential approximation of life expectancy.27,28 Given the truncation of observations for many women with distant disease, survival beyond the period of observation was estimated using SEER data.29 We also assumed that screen and clinically detected cancers have the same survival, all other factors being equal. Finally, we assumed that all women comply with treatment.
Model Parameters
To estimate the probability of all costs and events in the model, we reviewed the medical literature to abstract the best data applicable to U.S. women.30 All parameters are summarized in Tables 115,29,31–40 and 2.41–51
Table 1.
Parameters Used to Estimate the Natural History of Breast Cancer in Older Women
| Parameter | Estimate | Source | |||
|---|---|---|---|---|---|
| In Situ % | Local (%) | Regional (%) | Distant % | ||
| Stage distribution for screen-detected breast cancer cases, 1995–2001* | |||||
| Age, y | |||||
| 50–54 | 27 | 45 | 23.5 | 4.5 | Breast Cancer |
| 55–59 | 21 | 50 | 24 | 4 | Surveillance |
| 60–64 | 24 | 52 | 20.5 | 4.5 | Consortium, |
| 65–69 | 22 | 54 | 20.5 | 4.5 | personal written |
| 70–74 | 18 | 56 | 21 | 5 | communication |
| 75–79 | 18 | 58 | 18.5 | 6.5 | 2002, Diane Miglioretti, PhD, and31 |
| 80–84 | 17 | 59 | 17.5 | 6.5 | |
| 85+ | 14 | 64 | 14.5 | 7.5 | |
| Stage distribution for non–screen-detected breast cancer cases, 1975–1979* | |||||
| Age, y | 29 | ||||
| 50–54 | 6.0 | 45.0 | 41.7 | 7.3 | |
| 55–59 | 3.9 | 44.0 | 42.8 | 9.3 | |
| 60–64 | 3.7 | 45.6 | 40.2 | 10.5 | |
| 65–69 | 3.3 | 46.66 | 38.8 | 11.2 | |
| 70–74 | 3.3 | 47.9 | 37.8 | 11.0 | |
| 75–79 | 3.0 | 49.5 | 36.8 | 10.7 | |
| 80–84 | 2.4 | 48.4 | 37.1 | 12.1 | |
| 85+ | 1.9 | 42.0 | 39.7 | 16.4 | |
| Annual transition probabilities | Mean (SD) | 32–34 |
|---|---|---|
| P(DCIS-DCIS) | 0.714 (0.452) | |
| P(DCIS-Local) | 0.286 (0.452) | |
| P(Local-Local) | 0.828 (0.377) | |
| P(Local-Regional) | 0.172 (0.377) | |
| P(Regional-Regional) | 0.916 (0.201) | |
| P(Regional-Distant) | 0.084 (0.201) | |
| P(Distant-Distant) | 1 (0) | |
| Dwell time by age, y | 35,36 | |
| 50–59 | 2.1 | |
| 60–69 | 3 | |
| 70+ | 4.7 | |
| Mammography sensitivity | (%) | 37,38 |
| First screen | ||
| 50–59 | 93.6 | |
| 60–69 | 94.1 | |
| 70+ | 91.2 | |
| Subsequent screens | ||
| <50 | 76.5 | |
| 50+ | 73.8 | |
| Mammography specificity | 37,38 | |
| First screen | ||
| 50–59 | 92.9 | |
| 60–69 | 92.6 | |
| 70+ | 93.4 | |
| Subsequent screens | ||
| <50 | 98.1 | |
| 50+ | 98.2 |
| Mammography use: percent of all women 65+having reported mammogram in past 2 years | Median % (Low–High) | 39 |
|---|---|---|
| 73.7 (56.5–83.6) | ||
| Estrogen receptor positivity | 15 | |
| by age, y | ||
| 50–64 | 72.0% (95% CI, 76% to 77%) | |
| 65–79 | 82.0% (95% CI, 78% to 86%) |
| Distribution of local treatment for women diagnosed with breast cancer between 1993–1997 | BCS (%) | BCS+RT (%) | MST (%) | 29 |
|---|---|---|---|---|
| Age, y | ||||
| DCIS | ||||
| 50–54 | 34.0 | 38.8 | 27,2 | |
| 55–59 | 34.4 | 35.9 | 29.7 | |
| 60–64 | 31.2 | 36.0 | 32.8 | |
| 65–69 | 32.2 | 35.2 | 32.5 | |
| 70–74 | 26.9 | 37.3 | 35.8 | |
| 75–79 | 21.2 | 43.3 | 35.5 | |
| 80–84 | 17.1 | 52.7 | 30.2 | |
| 85+ | 9.4 | 62.9 | 27.7 | |
| Local | ||||
| 50–54 | 12.1 | 54.1 | 33.8 | |
| 55–59 | 10.9 | 51.1 | 38.0 | |
| 60–64 | 10.2 | 47.8 | 42.1 | |
| 65–69 | 10.5 | 43.6 | 45.9 | |
| 70–74 | 12.0 | 41.2 | 46.8 | |
| 75–79 | 17.1 | 34.3 | 48.6 | |
| 80–84 | 26.4 | 24.8 | 48.8 | |
| 85+ | 45.5 | 11.6 | 42.8 | |
| Regional | ||||
| 50–54 | 11.4 | 29.5 | 59.1 | |
| 55–59 | 9.3 | 27.5 | 63.2 | |
| 60–64 | 9.4 | 26.7 | 63.9 | |
| 65–69 | 7.1 | 22.3 | 70.6 | |
| 70–74 | 6.1 | 21.0 | 72.9 | |
| 75–79 | 6.2 | 18.2 | 75.6 | |
| 80–84 | 8.7 | 12.4 | 79.0 | |
| 85+ | 17.7 | 5.5 | 76.8 | |
| Distant | ||||
| 50–54 | 17.4 | 12.2 | 70.4 | |
| 55–59 | 16.7 | 13.6 | 69.7 | |
| 60–64 | 22.0 | 15.0 | 63.0 | |
| 65–69 | 26.5 | 12.3 | 61.3 | |
| 70–74 | 21.7 | 13.0 | 65.4 | |
| 75–79 | 25.3 | 9.8 | 64.9 | |
| 80–84 | 37.0 | 11.6 | 51.4 | |
| 85+ | 29.2 | 11.3 | 59.4 |
| Systemic treatment distribution by age and stage | (%) | 40 | |||
|---|---|---|---|---|---|
| Chemo | Tamoxifen | Both | Neither | ||
| Local | |||||
| Age 50–64 | 13.6 | 40.1 | 10.9 | 35.3 | |
| Age≥65 | 2.0 | 48.7 | 0.3 | 49.0 | |
| Regional/distant | |||||
| Age 50–64 | 26.6 | 18.8 | 42.3 | 12.3 | |
| Age≥65 | 11.4 | 45.0 | 23.4 | 20.2 | |
Women reported as unknown stage were distributed as follows: 50% as regional and 50% as distant based on survival.
Chemo, chemotherapy; BCS, breast-conserving therapy; MST, mastectomy; RT, radiation therapy; DCIS, ductal carcinoma in situ.
Table 2.
Costs of Breast Cancer Care, Year 2000 Dollars*
| Parameter | Estimate | Source |
| Mammography† | $82.51 | 41,42 |
| Total cost of an abnormal mammogram | $95.35 | 42,43 |
| Cost of treatment by phase and stage, mean±SD | 44 and SEER Medicare Data |
| BCS | BCS+RAD | MRM | |||||
|---|---|---|---|---|---|---|---|
| Ages 65–74 | Ages 75+ | Ages 65–74 | Ages 75+ | Ages 65–74 | Ages 75+ | ||
| Initial care‡ | |||||||
| DCIS | $2,398 | $1,759 | $7,304 | $6,949 | $6,074 | $5,818 | |
| ±$166 | ±$169 | ±$222 | ±$498 | ±$138 | ±$184 | ||
| Local | $6,329 | $3,905 | $8,810 | $7,936 | $6,475 | $6,340 | |
| ±$202 | ±$110 | ±$88 | ±$114 | ±$65 | ±$64 | ||
| Regional | $8,792 | $6,758 | $10,802 | $10,224 | $8,629 | $7,647 | |
| ±$411 | ±$384 | ±$230 | ±$396 | ±$98 | ±$111 | ||
| Distant | $11,682 | $6,413 | $23,691 | $15,241 | $9,540 | $8,810 | |
| ±$809 | ±$656 | ±$2,345 | ±$1,287 | ±$406 | ±$459 | ||
| Continuing care§ | |||||||
| DCIS | $600 | $1,128 | $708 | $120 | $444 | $564 | |
| ±$120 | ±$156 | ±$144 | ±$312 | ±$96 | ±$168 | ||
| Local | $1,140 | $792 | $528 | $636 | $756 | $612 | |
| ±$120 | ±$72 | ±$60 | ±$84 | ±$36 | ±$36 | ||
| Regional | $936 | $996 | $1,068 | $444 | $1,296 | $912 | |
| ±$300 | ±$192 | ±$120 | ±$192 | ±$48 | ±$48 | ||
| Distant | $4,272 | $3,732 | $4,572 | $2,424 | $2,796 | $1620 | |
| ±$756 | ±$708 | ±$804 | ±$660 | ±$336 | ±$288 | ||
| Terminal phase∥ | |||||||
| DCIS | $35,360 | $21,229 | $22,646 | $22,666 | $25,296 | $17,930 | |
| ±$2,317 | $1,647 | ±$5,830 | ±$9,383 | ±$2,504 | ±$1,866 | ||
| Local | $19,031 | $14,966 | $26,656 | $19,051 | $26,526 | $16,912 | |
| ±$1,408 | $650 | ±$1,345 | ±$1,054 | ±$656 | ±$341 | ||
| Regional | $22,712 | $14,572 | $28,012 | $20,087 | $23,126 | $17,229 | |
| ±$1,686 | $901 | ±$1,403 | ±$1,510 | ±$405 | ±$289 | ||
| Distant | $19,949 | $17,363 | $32,011 | $17,491 | $21,406 | $19,962 | |
| ±$1,180 | $1,128 | ±$1,690 | ±$1,324 | ±$992 | ±$681 | ||
| Monthly patient time costs (travel, treatment)¶ | 45–47 | ||||
|---|---|---|---|---|---|
| BCS | BCS+RT | Mastectomy | Chemotherapy | ||
| Initial phase | |||||
| All stages | $26/month | $91 | $61 | $39 | |
| Continuing phase | |||||
| DCIS/local | $5.08/year | $5.08 | $5.08 | $5.08 | |
| Regional/distant | $6.33/year | $6.33 | $6.33 | $6.33 | |
| Terminal phase | |||||
| All stages | $10/month | $10 | $10 | $10 | |
| Cost of tamoxifen over 5-year period# | Medical care cost | 48 | |||
| $6,352 | |||||
| Cost of adjuvant chemotherapy for initial treatment# | Medical care cost | ||||
| $4,725 | 48 |
All costs updated to 2000 using the medical care component of the consumer price index.49
Average among women who generally are not diagnosed with cancer.
The initial phase of care includes all costs incurred by breast cancer patients for the 12-month period following the date of diagnosis (e.g., initial diagnostic evaluation and staging, hospitalizations and surgery, and any adjuvant chemotherapy, medical visits, and laboratory procedures).44,50
The continuing care phase includes all costs incurred by breast cancer patients after the initial phase up to the 12 months prior to death (e.g., medical visits for surveillance, treatment of recurrences, hospitalizations, mammograms, and laboratory procedures, etc).44,50
Terminal care costs refer to all costs incurred by breast cancer patients in the last 12 months of life (e.g., hospitalizations, chemotherapy, laboratory procedures, and medical visits).44,50
Patient time costs were calculated based on estimated time valued at current average U.S. wage rates, even though many older women are not in the workforce.51
Because Medicare does not cover tamoxifen, we added these costs for estrogen receptor–positive women. Also, because rates of nonhormonal chemotherapy are low in older women, we added these costs to the initial care SEER-Medicare phase-specific costs for women who receive this therapy in the simulation.
BCS, breast-conserving treatment; RAD, radiation therapy; MRM, modified radical mastectomy; DCIS, ductal carcinoma in situ.
Disease Natural History
Age-specific incidence rates were estimated from SEER data.29 Stage distributions for screened women were estimated using 1995–2001 data (written personal communication, Diane Miglioretti, PhD, 2002).31 SEER data from 1975 to 1979 were used to approximate stage distribution in the absence of screening.29 Probability of disease progression between stages was estimated using data from screening trials and simulating stage distributions in screened settings.32–34 Dwell times (time in the stage before transitions occur) were assumed to be age dependent.35,36
Screening
We used age–specific test characteristics in women not using hormone replacement therapy.37,38 Screening rates for older women were estimated based on Behavioral Risk Factor Surveillance Survey data.39
Diagnosis and Treatment
Women with an abnormal mammogram underwent a diagnostic evaluation. Women with cancer underwent assessment of ER status and stage. Therapy was selected based on current patterns.29,40
Life Expectancy
The average annual probability of non–breast cancer death at each age was abstracted from life tables (“chronological age”).52 In addition, to capture the within age variability in survival, we developed a synthetic life curve based on quartiles of life expectancy. These quartiles roughly correlate with self-reported general health22,52,53 and are a proxy for “physiologic age.” For women with breast cancer, we used pooled data from 13 National Surgical Adjuvant Breast and Bowel Project (NSABP) trials among approximately 20,000 breast cancer patients to estimate 15-year survival by stage, treatment, age, and ER status.54–66 Women participating in trials, especially those with regional disease, generally have higher than average survival (“idealized treatment and survival”),67 giving resulted biased against screening. That is, the better treatment is, the lower the benefits of down staging disease via screening. We tested alternative community survival rates as reported to SEER in sensitivity analyses.29
Utilities
We presented our base case results using life years saved (LYS). 6 We examined the impact of estimated utilities in sensitivity analyses: without cancer .95, treatment for DCIS .87, treatment for local and regional disease .84, treatment for distant disease .55, surviving cancer .93, and living with metastatic disease as .55. Utilities for treatment were applied for 1 year after diagnosis.
Costs
We included medical (consumable supplies, personnel, laboratory, and procedure costs) and nonmedical (patient time costs) direct costs in year 2000 dollars (Table 2).49 Mammography costs were based on a microcosting approach using Medicare data for the components.42,68 The costs of cancer diagnosis and initial treatment, continuing care (including recurrences), and terminal care were estimated from linked SEER–Medicare reimbursement data from 1990 to 1999.44,50 We assumed that Medicare reimbursements closely approximate societal costs.68
Sensitivity Analyses
We varied individual parameters and combinations of parameters over reasonable ranges to examine the robustness of the model results under a variety of realistic conditions.
Model Validation
The technical programming accuracy was verified using “pseudo-input” designed to test the model under hypothetical conditions in which the results should be obvious. Our scientific advisors reviewed face and clinical validity.
Role of the Funding Source
The funding agencies had no role in data analysis, interpretation of results, or decisions to publish the results.
RESULTS
Over a lifetime, the model estimates that women have a 7% chance of having cancer diagnosed. At baseline screening rates of 73%, a program of biennial screening beginning at age 50 results in each woman undergoing an average of 15 mammograms in her lifetime, with a 66% cumulative risk of having a false positive screening result in this period.
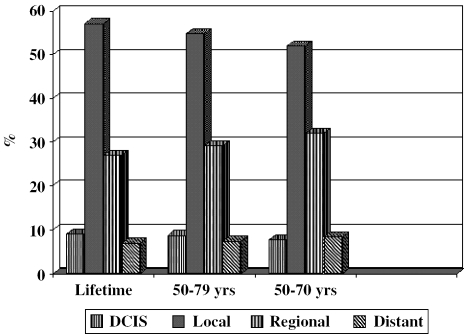
Regular biennial screening from age 50 to age 70 detects 90.9% of all lifetime cases, and continuing to age 79 captures 94.2% of these cases. Extending screening to age 79 or for a lifetime results in a larger proportion of cases being diagnosed as carcinoma in situ and local stage than more advanced stages (Fig. 1). Screening does not result in significant overdiagnosis (not shown).
FIGURE 1.
Stage distribution of screen-detected cancers by screening strategy. Stage distribution with screening from age 50 to different ages of cessation. The longer the screening period, the greater the proportion of cases detected as in situ and local stage.
Compared to stopping screening at age 70, extending screening to age 79 saves 2.4 additional days of life per woman (undiscounted) for the entire population. Among women destined to develop breast cancer, screening to age 79 saves an additional 24.9 days of life per woman. The incremental cost of this added screening is $82,063 per LYS, compared to stopping screening at age 70. This result is obtained in a population where the average life expectancy is 9.5 years at age 79.
Further extending screening from age 79 to lifetime screening saves proportionally fewer days: 1.1 additional days across the population and 12 added days for cases, and is very expensive for the remaining increases in life saved ($151,434 per LYS) (Table 3).
Table 3.
Incremental Cost-effectiveness of Biennial Breast Cancer Screening in Older Women by Chronological Age*
| Strategy | Number of Mammograms | Life Expectancy (Years) | Incremental Life Expectancy (Years) | Costs | Incremental Costs | Incremental Costs per LYS |
|---|---|---|---|---|---|---|
| 50–70 y | 9.4 | 19.453 | — | $2,291.945068 | — | — |
| 50–79 y | 12.5 | 19.455 | .002373432 | $2,488.173584 | $194.771484 | $82,063 |
| Lifetime | 15.1 | 19.456 | .000924076 | $2,628.110107 | $139.936523 | $151,434 |
Costs and effects discounted at 3%. Each strategy is considered compared to the next least expensive approach.
LYS, life years saved.
These results are largely dependent on competing mortality. Thus, if screening is limited to women in the top 25% of life expectancy, then cost-effectiveness improves, and the cost of screening to age 79 decreases to $57,934 per LYS in a population of women who have an average life expectancy of 14.6 years at age 79. Screening these women without an upper age limit costs $126,629 (Table 4).
Table 4.
Incremental Cost-effectiveness of Biennial Breast Cancer Screening in Older Women with Life Expectancies in the Top 25% (Physiologic Age)*
| Strategy | Life Expectancy (Years) | Incremental Life Expectancy (Years) | Costs | Incremental Costs | Incremental Costs per LYS |
|---|---|---|---|---|---|
| Costs per LYS | |||||
| 50–70 y | 23.493 | – | $3,327.632324 | – | – |
| 50–79 y | 23.079 | .006799338 | $3,721.545166 | $393.912842 | $57,934 |
| Lifetime | 23.072 | .003459292 | $4,159.591797 | $438.046631 | $126,629 |
Costs and effects discounted at 3%. Life expectancy based on health for screening women who are in the top 25% of life expectancies for their ages.Each strategy is considered compared to the next least expensive approach.
LYS, life years saved.
Sensitivity Analysis
Under certain circumstances, continuing to screen past chronological age 79 may be reasonable (Table 5). For example, if screening is targeted at women at high risk of cancer (twice the population average, roughly corresponding to having a first-degree relative with breast cancer),69 then screening to age 79, compared to age 70, costs substantially less than screening the entire population ($62,842 for high-risk vs $82,063 per LYS for all women), and screening over a lifetime approaches a more reasonable level ($112,094).
Table 5.
One-way Sensitivity Analyses: Incremental Cost-effectiveness of Breast Cancer Screening of Older Women Under Different Conditions*
| Variable | Incremental Costs per LYS† | Incremental Costs per LYS‡ |
|---|---|---|
| Screen Ages 50–79 Years | Screen Lifetime from 50 yrs | |
| Survival/treatment | ||
| Current (nonidealized) | $40,629 | $98,821 |
| Current and quality-adjusted | $71,756 | $114,915 |
| Screening interval annual | $118,762 | $301,173 |
| Discount rate 10% | $148,980 | $225,968 |
| Incidence rate (risk) | ||
| Twice average (high-risk) | $62,842 | $112,094 |
| Half average (low-risk) | $195,664 | $298,126 |
| Dwell times | ||
| Shorter than average | $103,939 | $190,788 |
| Longer than average | $155,865 | $368,801 |
| Quality-adjusted§ | – | – |
Costs and effects discounted at 3%. All parameters are the same as in the base case except for the one variable noted.
Compared to stopping screening at age 70 (i.e., compared to screening from age 50 to 70).
Compared to stopping screening at age 79 (i.e., compared to screening from age 50 to 79).
Costs are the same as in the base case using LYS. Quality-adjusted life expectancy for extending screening from age 70 to age 79 results in a loss of life expectancy due to the long lead time relative to absolute improvements in survival and disutility of undergoing cancer treatment and living with the knowledge of cancer over the period from screen detection until death (combination of lead time and extended absolute survival).
LYS, life years saved.
If treatment and survival is closer to that observed in SEER than in clinical trials, then the stage shifts observed with screening lead to greater savings in life years, especially for women diagnosed with regional cancer. In this situation, it becomes more cost-effective to continue screening until age 79 ($40,629 per LYS) than in the base case. If treatment and survival are similar to that observed in SEER and screening after age 70 is limited to women with the top 25% of life expectancies, then screening is very cost-effective, even without an upper age limit, costing $50,643 per LYS for lifetime screening compared to stopping at age 70.
If survival is quality adjusted, on average, screening should stop at age 70. This finding is the result of 2 facts: 1) being treated and living with the knowledge of cancer lowers quality of life in the years that are gained as “lead time,” or the earlier diagnosis of cancer without an improvement in survival, and 2) that lead time is fairly long relative to the true increase in life gained from screening. There are a few exceptions to this conclusion. Even if estimated quality-adjusted survival is considered, it remains cost-effective to screen all women until 79 if survival follows usual patterns of care (vs “idealized” care in RCT settings; $71,756 per quality-adjusted life year (QALY)). It may also be considered cost-effective to screen women with the highest life expectancies (top 25%) without an upper age limit when there is nonideal survival ($98,673 per QALY).
Changes in other parameters do not change the conclusions. Screening low-risk women (with one half the average risk) is very expensive. Screening annually is also very expensive and does not generate sufficient savings to justify screening beyond age 70. If women value present years of life to a much greater extent than future years (i.e., have a high discount rate for the future), then screening beyond age 70 is also extremely expensive (Table 5).
If breast cancer is a slower growing disease in older women than the average modeled in our base case, then there is less new disease that would become clinically apparent within women's lifetimes, so that screening has fewer benefits and the cost-effectiveness decreases. In this situation, our results indicate that screening should stop at age 70, or be performed at less frequent intervals. If, on the other hand, disease progresses more rapidly in older ages than the average modeled in our base case, then biennial screening will miss more cases and screening beyond age 70 is also not cost-effective (Table 5). In the latter situation, annual screening would become more cost-effective than with average base case dwell times (not shown). Varying the test sensitivity or costs does not change the conclusions and has minimal impact on the absolute cost-effectiveness ratios (not shown).
DISCUSSION
This is the first study to explicitly consider a proxy for women's physiologic age as well as age-specific disease natural history in making breast cancer screening decisions for older women. At a threshold of cost-effectiveness of $80,000, our results suggest that it is cost-effective to conduct biennial screening until age 79. If a threshold of $60,000 is considered cost-effective, then screening to age 79 is only cost-effective if limited to women with life expectancies in the top quartile for their ages. These conclusions are based on the assumption that all older women with breast cancer have survival comparable to that seen in clinical trials. If treatment patterns are not ideal and survival is lower, as is the case at present, then screening has greater benefits, and it might be appropriate to continue screening without an upper age limit.
The result that screening benefits are greatest in women with the longest life expectancy is intuitively obvious. However, clinicians often underestimate older women's life expectancy,70 and there is considerable heterogeneity in health and functioning.22 Using a threshold for cost-effectiveness of $80,000 per LYS, a figure many would consider reasonable by current standards for screening,6,71 it is cost-effective to screen women with a life expectancy of 9.5 years. This value can be expected for 75% of 79-year-olds, about 50% of 80-year-olds, and 25% of 85-year-olds.22 Therefore, one practical implication of our analysis is that simple methods to determine life expectancy in clinical settings could aid screening decisions for older women.27,28
Our results imply that prevailing treatment patterns (and the resultant survival) play a central role in screening decisions. However, optimal treatment of older women remains controversial, largely as a result of a paucity of primary data in this age group, with only 1% to 2% of older women treated in clinical trials,67 and only 30% to 40% receiving some chemotherapy.72,73 If such patterns continue, then our model suggests that screening may be beneficial beyond age 79, especially for the healthiest women.
If we could accurately triage women according to risk of developing breast cancer, then it is also reasonable to screen to age 79, and perhaps for life among high-risk women. This finding confirms results of an earlier model that showed that screening older high-risk women (based on bone mineral density, a proxy for estrogen exposure) was cost-effective until age 79, costing $66,773 per life year saved, compared to stopping at age 69. That result is very similar to our result of $62,843 per year of life saved for screening high-risk women (based on family history) to age 79 (vs stopping at age 70).
We found that from a societal perspective, screening is too expensive relative to its benefits to be offered on an annual basis to average-risk and average-health women after age 70. However, optimal intervals depend on disease biology, particularly the time from stages that are detectable preclinically to stages that present clinically with symptoms. For instance, if tumors grow more slowly in older women, with a preclinical detectable period of 6.2 years,74 then screening intervals might logically be extended from every 2 to every 3 to 5 years.
The benefits of screening older women have been noted in other studies75,76 and recent reviews of cost-effectiveness analyses have concluded that biennial screening after age 65 is generally cost-effective,77 especially in the absence of major comorbidity limiting life expectancy.78 This is very similar to our finding that cost-effectiveness is most favorable for women in the top 25% of life expectancy for their age group.
When caring for asymptomatic populations, it is important to consider the harms as well as the benefits of screening. If screening is extended from age 70 to 79, or for a lifetime, then women have an increased risk of having a false positive result.8 One way to decrease the number of false positive exams is to extend the screening interval among older women. This would decrease costs, and, if tumors are slow growing, still maintain benefits. Women who are screen detected live with cancer and treatment consequences for a longer period of time than if clinically diagnosed. If women value life as a breast cancer survivor less than life in their general health, then the cost per QALY increases and screening could result in a small loss of quality-adjusted years. If women are relieved to have their disease detected earlier through screening, even if they live longer with the knowledge of cancer, then screening will still be beneficial. Thus, women's values must be carefully weighed in all screening decisions.
Our analysis has several important strengths, including use of current standards for cost-effectiveness analyses,24 developing a paradigm for basing decisions on physiological as opposed to chronological age, estimation of age-specific tumor biology, a robust model, and assessment of the impact of uncertain parameters.
Despite these strengths, there are several issues that should be considered when interpreting our results. First, we used life expectancy corresponding to quartiles of health and age to measure the probability of death as a proxy for physiological age. Clearly, data that link direct measurement of physiological reserve to clinical assessments of health status and life expectancy would be invaluable in individual decision making. In the interim, using life expectancy based on individual health conditions or self-reported health, rather than average U.S. age-specific life expectancies, may be a better indicator than age per se for use in clinical practice.
Second, while our model goes beyond many prior models of screening, our estimates of age-specific tumor natural history are fairly crude and limited by the relative paucity of data for this age group. Also, within any age group, breast cancer is a heterogeneous disease with variability in aggressiveness and probability of disease progression.
Next, our results must also be considered in the context of the current controversies about the effectiveness of mammography.9,79 Our model does not make a direct assumption about the impact of mammography on breast cancer mortality. Rather, we rely on observed stage distributions among screened and unscreened older populations to calculate screening benefits. Also, within each stage, we assumed similar survival for mammographically detected and non-screen-detected cancers. This assumption biases results against screening benefits.
Fourth, we restricted the screening intervals evaluated to those currently under consideration or in use in the United States. If it becomes generally acceptable to extend screening intervals to every 3 to 5 years after a certain age, then our model could be used to estimate the potential costs savings relative to any losses in benefits.
In addition, we used Medicare reimbursements as the single best source of costs for all analyses, while our base survival estimates were derived from clinical trials. Costs in clinical trials may be higher or lower than average, depending on intensity and efficiency of care. At present, we are not aware of any data that compare treatment costs for older women off and on clinical trials. Our model also does not capture the effects of distress associated with a false positive screen,80,81 but given the transient nature of this adverse event, it is not likely to alter our conclusions. Finally, our results may only be generalizable to U.S. screening policies.
Breast cancer is largely a disease of old age. Older women are a rapidly growing segment of the U.S. population and will be very heterogeneous in their health and functioning. We recommend that policymakers and clinicians explicitly consider life expectancy, or “physiological age,” how aggressively older women with cancer will be treated, and women's preferences in making screening decisions affecting older women.
Acknowledgments
We acknowledge Joan Warren, Martin Brown, and Nicola Schussler for providing SEER-Medicare cost data; Marc Snyder and Deborah Conley from Kaiser Permanente Mid-Atlantic States for collaboration in collecting preliminary utility data, and Trina McClendon for manuscript preparation.
This work was supported by grants K05 CA96940 (JSM) and RO1 CA72908 (JSM, KRY, CBS) and cooperative agreement UO1-CA88293A from the National Cancer Institute (JSM, JC, WL, CBS).
Dr. Yabroff is currently at the National Cancer Institute, Division of Population Sciences.
Dr. Lawrence is currently at the Agency for HealthCare Research and Quality.
References
- 1.Ershler WB, Longo DL. Aging and cancer. issues of basic and clinical science J Natl Cancer Inst. 1997;89:1489–1497. doi: 10.1093/jnci/89.20.1489. [DOI] [PubMed] [Google Scholar]
- 2.Ries LAG, Kosary CL, Hankey BF, Edwards BK. SEER Cancer Statistic Review, 1973–1996. DHHS, NIH, NCI, Bethesda, MD:1999. [Google Scholar]
- 3.U.S. Bureau of the Census. Current Population Survey, July 2002. Available at: [Google Scholar]
- 4.Soldo BJ, Agree EM. America's Elderly. Washington, DC: Population Reference Bureau, Inc.; 1988. [PubMed] [Google Scholar]
- 5.Lash TL, Silliman RA. Prevalence of cancer. J Natl Cancer Inst. 1998;90:399–400. doi: 10.1093/jnci/90.5.399. [DOI] [PubMed] [Google Scholar]
- 6.Kerlikowske K, Salzmann P, Phillips KA, Cauley JA, Cummings S. Continuing screening mammography in women aged 70 to 79 years. Impact on life expectancy and cost-effectiveness. JAMA. 1999;282:2156–2163. doi: 10.1001/jama.282.22.2156. [DOI] [PubMed] [Google Scholar]
- 7.Eddy DM. Screening for breast cancer. Ann Intern Med. 1989;111:389–399. doi: 10.7326/0003-4819-111-5-389. [DOI] [PubMed] [Google Scholar]
- 8.Elmore JG, Barton MB, Moceri VM, Polk S, Arena PJ, Fletcher SW. Ten-year risk of false positive screening mammograms and clinical breast exams. N Engl J Med. 1998;338:1089–1096. doi: 10.1056/NEJM199804163381601. [DOI] [PubMed] [Google Scholar]
- 9.Gotzsche PC, Olsen O. Is screening for breast cancer with mammography justifiable? Lancet. 2000;355:129–134. doi: 10.1016/S0140-6736(99)06065-1. [DOI] [PubMed] [Google Scholar]
- 10.Greenfield S, Blanco DM, Slashoff RM, Ganz PA. Patterns of care related to age of breast cancer patients. JAMA. 1987;257:2766–2770. [PubMed] [Google Scholar]
- 11.Newschaffer CJ, Penberthy L, Desch CE, Retchin SM, Whittemore M. The effect of age and comorbidity in the treatment of elderly women with nonmetastatic breast cancer. Arch Intern Med. 1996;156:85–90. [PubMed] [Google Scholar]
- 12.Fried LP, Bandeen-Roche K, Kasper JD, Guralnik JM. Association of comorbidity with disability in older women. the Women's Health and Aging Study J Clin Epidemiol. 1999;52:27–37. doi: 10.1016/s0895-4356(98)00124-3. [DOI] [PubMed] [Google Scholar]
- 13.Brown ML. Economic considerations in breast cancer screening of older women. J Gerontol. 1992;47:51–58. [PubMed] [Google Scholar]
- 14.Kerlikowske K, Grady D, Barclay J, Sickles EA, Eaton A, Ernster V. Positive predictive value of screening mammography by age and family history of breast cancer. JAMA. 1994;271:982–983. [PubMed] [Google Scholar]
- 15.Gapstur SM, Dupuis J, Gann P, Collila S, Winchester DP. Hormone receptor status of breast tumors in black, Hispanic, and non-Hispanic white women. An analysis of 13,239 cases. Cancer. 1996;77:1465–1471. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1465::AID-CNCR7>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 16.Elledge RM, Clark GM, Chamness GC, Osborne CK. Tumor biologic factors and breast cancer prognosis among white, Hispanic, and black women in the United States. J Natl Cancer Inst. 1994;86:705–712. doi: 10.1093/jnci/86.9.705. [DOI] [PubMed] [Google Scholar]
- 17.Ershler WB, Balducci L. Treatment considerations for older patients with cancer. In Vivo. 1994;8:737–744. [PubMed] [Google Scholar]
- 18.Mandelblatt J, Kerner J, Hadley J, et al. Variations in breast cancer treatment in older Medicare beneficiaries. is it black or white? Cancer. 2002;95:1401–1414. doi: 10.1002/cncr.10825. [DOI] [PubMed] [Google Scholar]
- 19.Lyman GH, Lyman S, Balducci L, et al. Age and the risk of breast cancer recurrence. Cancer Control. 1996;3:421–427. [PubMed] [Google Scholar]
- 20.Extermann M, Balducci L, Lyman GH. What threshold for adjuvant therapy in older breast cancer patients? J Clin Oncol. 2000;18:1709–1717. doi: 10.1200/JCO.2000.18.8.1709. [DOI] [PubMed] [Google Scholar]
- 21.Rich JS, Black WC. When should we stop screening? Eff Clin Pract. 2000;3:78–84. [PubMed] [Google Scholar]
- 22.Walter LC, Covinsky KE. Cancer screening in elderly patients. a framework for individualized decision making JAMA. 2003;285:2750–2756. doi: 10.1001/jama.285.21.2750. [DOI] [PubMed] [Google Scholar]
- 23.Beck JR, Pauker SG. The Markov process in medical prognosis. Med Decis Making. 1983;3:419–458. doi: 10.1177/0272989X8300300403. [DOI] [PubMed] [Google Scholar]
- 24.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-effectiveness in Health and Medicine. New York, NY: Oxford University Press; 1996. [Google Scholar]
- 25.Rubenstein RY. Simulation and the Monte Carlo Method. New York, NY: John Wiley & Sons; 1981. [Google Scholar]
- 26.Eley JW, Hill HA, Chen VW, et al. Racial differences in survival from breast cancer. Results of the National Cancer Institute Black/White Cancer Survival Study. JAMA. 1994;272:947–954. doi: 10.1001/jama.272.12.947. [DOI] [PubMed] [Google Scholar]
- 27.Beck JR, Kassirer JP, Pauker SG. A convenient approximation of life expectancy (the “DEALE”). I. Validation of the method. Am J Med. 1982;73:883–888. doi: 10.1016/0002-9343(82)90786-0. [DOI] [PubMed] [Google Scholar]
- 28.Beck JR, Pauker SG, Gottlieb JE, Klein K, Kassirer JP. A convenient approximation of life expectancy (the “DEALE”). II. Use in medical decision-making. Am J Med. 1982;73:889–897. doi: 10.1016/0002-9343(82)90787-2. [DOI] [PubMed] [Google Scholar]
- 29.National Cancer Institute. SEER Public-use Database, 1973–2000. Available at: [Google Scholar]
- 30.Mandelblatt JS, Fryback DG, Weinstein MC, Russell LB, Gold MR. Assessing the effectiveness of health interventions for cost-effectiveness analysis. Panel on Cost-effectiveness in Health and Medicine. J Gen Intern Med. 1997;12:551–8. doi: 10.1046/j.1525-1497.1997.07107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ballard-Barbash R, Taplin SH, Yankaskas BC, et al. Breast Cancer Surveillance Consortium. a national mammography screening and outcomes database AJR Am J Roentgenol. 1997;169:1001–1008. doi: 10.2214/ajr.169.4.9308451. [DOI] [PubMed] [Google Scholar]
- 32.Shapiro S, Venet W, Strax P, Venet L. Periodic Screening for Breast Cancer: The Health Insurance Plan Project and Its Sequelae, 1963–86. Baltimore, MD: Johns Hopkins University Press; 1988. [Google Scholar]
- 33.Andersson I, Aspegren K, Janzon L, et al. Mammographic screening and mortality from breast cancer. the Malmo mammographic screening trial BMJ. 1988;297:943–948. doi: 10.1136/bmj.297.6654.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberts MM, Alexander FE, Anderson TJ, et al. Edinburgh trial of screening for breast cancer. mortality at seven years Lancet. 1990;335:241–246. doi: 10.1016/0140-6736(90)90066-e. [DOI] [PubMed] [Google Scholar]
- 35.Brekelmans CTM, Westers P, Faber JAJ, Peeters PHM, Collette HJA. Age specific sensitivity and sojourn time in a breast cancer screening programme (DOM) in the Netherlands. a comparison of different methods J Epidemiol Community Health. 1996;50:68–71. doi: 10.1136/jech.50.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paci E, Duffy SW. Modelling the analysis of breast cancer screening programmes. sensitivity, lead time and predictive values in the Florence district programme (1975–1986) Int J Epidemiol. 1991;20:852–858. doi: 10.1093/ije/20.4.852. [DOI] [PubMed] [Google Scholar]
- 37.Kerlikowske K, Grady D, Barclay J, Sickles EA, Ernster V. Likelihood ratios for modern screening mammography. Risk of breast cancer based on age and mammographic interpretation. JAMA. 1996;276:39–43. doi: 10.1001/jama.276.1.39. [DOI] [PubMed] [Google Scholar]
- 38.Carney PA, Miglioretti DL, Yankaskas BC, et al. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med. 2003;138:168–175. doi: 10.7326/0003-4819-138-3-200302040-00008. [DOI] [PubMed] [Google Scholar]
- 39. Morbidity and Mortality Weekly Report. Trends in self-reported use of mammograms (1989–1997) and Papanicolaou tests (1991–1997)—behavioral risk factor surveillance system 1999;38:1–22. [PubMed]
- 40.Harlan LC, Abrams J, Warren JL, Clegg L, Stevens J, Ballard-Barbash R. Adjuvant therapy for breast cancer. practice patterns of community physicians J Clin Oncol. 2002;20:1809–1817. doi: 10.1200/JCO.2002.07.052. [DOI] [PubMed] [Google Scholar]
- 41.Health Care Financing Administration. Medicare charges. Available at: [Google Scholar]
- 42.Schweitzer ME, French MT, Ullmann SG, McCoy CB. Cost effectiveness of detecting breast cancer in lower socioeconomic status African American and Hispanic women through mobile mammography services. Med Care Res Rev. 1998;55:99–115. doi: 10.1177/107755879805500106. [DOI] [PubMed] [Google Scholar]
- 43.Brown ML, Houn F. Quality assurance audits of community screening mammography practices. availability of active follow-up for data collection and outcome assessment AJR Am J Roentgenol. 2003;163:825–829. doi: 10.2214/ajr.163.4.8092017. [DOI] [PubMed] [Google Scholar]
- 44.Warren JL, Brown ML, Fay MP, Schussler N, Potosky AL, Riley GF. Costs of treatment for elderly women with early-stage breast cancer in fee-for-service settings. J Clin Oncol. 2002;20:307–316. doi: 10.1200/JCO.2002.20.1.307. [DOI] [PubMed] [Google Scholar]
- 45.Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project III, 1997. Available at: [Google Scholar]
- 46. National Center for Health Statistics, Centers for Disease Control and Prevention. National Health Interview Survey (NHIS). Cancer Prevention and Control Supplement, 1992. Available at: http://www.cdc.gov/nchs/nhis.htm Accessed July 2001.
- 47.Secker-Walker R, Vacek P, Hooper G, Plante D, Detsky A. Screening for breast cancer time, travel, and out-of-pocket expenses. J Natl Cancer Inst. 1999;91:702–8. doi: 10.1093/jnci/91.8.702. [DOI] [PubMed] [Google Scholar]
- 48. The Red Book. Medical Economics. Montclair, NJ: 2001.
- 49.Bureau of Labor Statistics 2000 [Google Scholar]
- 50.Brown ML, Riley GF, Schussler N, Etzioni R. Estimating health care costs related to cancer treatment from SEER-Medicare data. Med Care. 2002;40:104–17. doi: 10.1097/00005650-200208001-00014. [DOI] [PubMed] [Google Scholar]
- 51. Bureau of Labor Statistics. Available at: http://www.bls.census.gov/cps/ads/1999/sdata.htm.1999 Accessed July 2000.
- 52.National Center for Health Statistics. Available at: http://www.cdc.gov/nchswww/data/hp2k99.pdf 1999. Accessed July 2000.
- 53.National Center for Health Statistics. National Health Interview Survey. 2000. Available at: http://www.NCHS.gov Accessed July 2001.
- 54.Fisher B, Anderson S, Tan-Chiu E, et al. Tamoxifen and chemotherapy for axillary node-negative, estrogen receptor-negative breast cancer findings from National Surgical Adjuvant Breast and Bowel Project B-23. J Clin Oncol. 2001;19:931–42. doi: 10.1200/JCO.2001.19.4.931. [DOI] [PubMed] [Google Scholar]
- 55. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999;353:1993–2000. [DOI] [PubMed]
- 56.Fisher B, Anderson S, DeCillis A, et al. Further evaluation of intensified and increased total dose of cyclophosphamide for the treatment of primary breast cancer. findings from National Surgical Adjuvant Breast and Bowel Project B-25 J Clin Oncol. 1999;17:3374–3388. doi: 10.1200/JCO.1999.17.11.3374. [DOI] [PubMed] [Google Scholar]
- 57.Smith RE, Brown AM, Mamounas EP, et al. Randomized trial of 3-hour versus 24-hour infusion of high-dose paclitaxel in patients with metastatic or locally advanced breast cancer. National Surgical Adjuvant and Bowel Project Protocol B-26 J Clin Oncol. 1999;17:3403–3411. doi: 10.1200/JCO.1999.17.11.3403. [DOI] [PubMed] [Google Scholar]
- 58.Fisher B, Dignam J, Wolmark N, et al. Tamoxifen and chemotherapy for lymph node-negative, estrogen receptor-positive breast cancer. J Natl Cancer Inst. 1997;89:1673–1682. doi: 10.1093/jnci/89.22.1673. [DOI] [PubMed] [Google Scholar]
- 59.Fisher B, Anderson S, Wickerham DL, et al. Increased intensification and total dose of cyclophosphamide in a doxorubicin-cyclophosphamide regimen for the treatment of primary breast cancer. findings from National Surgical Adjuvant Breast and Bowel Project B-22 J Clin Oncol. 1997;15:1858–1869. doi: 10.1200/JCO.1997.15.5.1858. [DOI] [PubMed] [Google Scholar]
- 60.Fisher B, Dignam J, Mamounas EP, et al. Sequential methotrexate and fluorouracil for the treatment of node-negative breast cancer patients with estrogen receptor-negative tumors. eight-year results from National Surgical Adjuvant Breast and Bowel Project (NSABP) B-13 and first report of findings from NSABP B-19 comparing methotrexate and fluorouracil with conventional cyclophosphamide, methotrexate, and fluorouracil J Clin Oncol. 1996;14:1982–1992. doi: 10.1200/JCO.1996.14.7.1982. [DOI] [PubMed] [Google Scholar]
- 61.Fisher B, Redmond CF, Costantino J, et al. Lumpectomy compared with lumpectomy and radiation therapy for the treatment of intraductal breast cancer. N Engl J Med. 1993;328:1581–1586. doi: 10.1056/NEJM199306033282201. [DOI] [PubMed] [Google Scholar]
- 62.Fisher B, Brown AM, Dimitrov NV, et al. Two months of doxorubicin-cyclophosphamide with and without interval reinduction therapy compared with 6 months of cyclophosphamide, methotrexate, and fluorouracil in positive-node breast cancer patients with tamoxifen-nonresponsive tumors. results from the National Surgical Adjuvant Breast and Bowel Project B-15 J Clin Oncol. 1990;8:1483–1496. doi: 10.1200/JCO.1990.8.9.1483. [DOI] [PubMed] [Google Scholar]
- 63.Fisher B, Redmond C, Legault-Poisson S, et al. Postoperative chemotherapy and tamoxifen compared with tamoxifen alone in the treatment of positive-node breast cancer patients aged 50 years and older with tumors responsive to tamoxifen. results from the National Surgical Adjuvant Breast and Bowel Project B-16 J Clin Oncol. 1990;8:1005–1018. doi: 10.1200/JCO.1990.8.6.1005. [DOI] [PubMed] [Google Scholar]
- 64.Fisher B, Costantino J, Redmond C, et al. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med. 1989;320:479–484. doi: 10.1056/NEJM198902233200802. [DOI] [PubMed] [Google Scholar]
- 65.Fisher B, Redmond C, Dimitrov NV, et al. A randomized clinical trial evaluating sequential methotrexate and fluorouracil in the treatment of patients with node-negative breast cancer who have estrogen-receptor-negative tumors. N Engl J Med. 1989;320:473–478. doi: 10.1056/NEJM198902233200801. [DOI] [PubMed] [Google Scholar]
- 66.Fisher B, Redmond C, Fisher ER, Bauer M, Wolmark N, Wickerham DL. Ten-year results of a randomized clinical trial comparing radical mastectomy and total mastectomy with or without radiation. N Engl J Med. 1985;312:674–681. doi: 10.1056/NEJM198503143121102. [DOI] [PubMed] [Google Scholar]
- 67.Goodwin JS, Hunt WC, Humble CG, Key CR, Sanet JM. Cancer treatment protocols? Who gets chosen. Arch Intern Med. 1988;148:2258–2260. [PubMed] [Google Scholar]
- 68.Luce BR, Manning WG, Siegel JE, Lipscomb J. Estimating costs in cost-effectiveness analysis. In: Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost-effectiveness in Health and Medicine. New York, NY: Oxford University Press; 1996. pp. 176–213. [Google Scholar]
- 69.Sattin RW, Rubbin GL, Webster LA, et al. Family history and the risk of breast cancer. JAMA. 1985;253:1908–1913. [PubMed] [Google Scholar]
- 70.Fox SA, Siu AL, Stein JA. The importance of physician communication on breast cancer screening of older women. Arch Intern Med. 1994;154:2058–2068. [PubMed] [Google Scholar]
- 71.Frazier AL, Colditz G, Fuchs C, Kuntz K. Cost-effectiveness of screening for colorectal cancer in the general population. JAMA. 2000;284:1954–1961. doi: 10.1001/jama.284.15.1954. [DOI] [PubMed] [Google Scholar]
- 72.Guadagnoli E, Weeks JC, Shapiro CL, Gurwitz JH, Borbas C, Soumerai SB. Use of breast-conserving survey for treatment of stage I and stage II breast cancer. J Clin Oncol. 1998;16:101–106. doi: 10.1200/JCO.1998.16.1.101. [DOI] [PubMed] [Google Scholar]
- 73.Giovanazzi-Bannon S, Rademaker A, Lai G, Benson AB III. Treatment tolerance of elderly cancer patients entered onto phase II clinical trials. an Illinois cancer center study J Clin Oncol. 2003;12:2447–2452. doi: 10.1200/JCO.1994.12.11.2447. [DOI] [PubMed] [Google Scholar]
- 74.Boer R, deKoning HJ, van der Maas PJ. A longer breast carcinoma screening interval for women age older than 65 years? Cancer. 1999;86:1506–1510. doi: 10.1002/(sici)1097-0142(19991015)86:8<1506::aid-cncr17>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 75.McCarthy EP, Burns RB, Freund KM, et al. Mammography use, breast cancer stage at diagnosis, and survival among older women. J Am Geriatr Soc. 2000;48:1226–1233. doi: 10.1111/j.1532-5415.2000.tb02595.x. [DOI] [PubMed] [Google Scholar]
- 76.Randolph WM, Goodwin JS, Mahnken JD, Freeman JL. Regular mammography use is associated with elimination of age-related disparities in size and stage of breast cancer at diagnosis. Ann Intern Med. 2002;137:783–790. doi: 10.7326/0003-4819-137-10-200211190-00006. [DOI] [PubMed] [Google Scholar]
- 77.Barratt AL, Les IM, Glasziou PP, Salkeld GP, Houssami N. Benefits, harms and costs of screening mammography in women 70 years and over. a systematic review Med J Aust. 2002;176:266–271. doi: 10.5694/j.1326-5377.2002.tb04405.x. [DOI] [PubMed] [Google Scholar]
- 78.Mandelblatt J, Saha S, Teutsch S, et al. The cost-effectiveness of screening mammography beyond age 65 years. a systematic review for the U S. Preventive Services Task Force. Ann of Intern Med. 2003;139:835–842. doi: 10.7326/0003-4819-139-10-200311180-00011. [DOI] [PubMed] [Google Scholar]
- 79.National Cancer Institute. Screening and testing. Available at: http://www.cancer.gov/cancerinfo/screening/breast. Accessed November 2003. [Google Scholar]
- 80.Lindfors KK, O'Connor J, Acredolo CR, Liston SE. Short-interval follow-up mammography versus immediate core biopsy of benign breast lesions assessment of patient stress. AJR Am J Roentgenol. 1998;171:55–8. doi: 10.2214/ajr.171.1.9648763. [DOI] [PubMed] [Google Scholar]
- 81.Pisano ED, Vokaty K, Earp J, Schell M, Denham A. Screening behavior of women after a false-positive mammogram. Radiology. 1998;208:245–249. doi: 10.1148/radiology.208.1.9646820. [DOI] [PubMed] [Google Scholar]