Abstract
BACKGROUND
Breast-conserving surgery (BCS) has been the recommended treatment for early-stage breast cancer since 1990 yet many women still do not receive this procedure.
OBJECTIVE
To examine the relationship between birthplace and use of BCS in Asian-American and Pacific-Islander (AAPI) women, and to determine whether disparities between white and AAPI women persist over time.
DESIGN
Retrospective cohort study.
SETTING AND PARTICIPANTS
Women with newly diagnosed stage I or II breast cancer from 1992 to 2000 in the Surveillance, Epidemiology, and End Results program.
OUTCOME
Receipt of breast -conserving surgery for initial treatment of stage I or II breast cancer.
MAIN RESULTS
Overall, AAPI women had lower rates of BCS than white women (47% vs 59%; P<.01). Foreign-born AAPI women had lower rates of BCS than U.S.-born AAPI and white women (43% vs 56% vs 59%; P<.01). After adjustment for age, marital status, tumor registry, year of diagnosis, stage at diagnosis, tumor size, histology, grade, and hormone receptor status, foreign-born AAPI women (adjusted OR [aOR], 0.49; 95% CI, 0.32 to 0.76) and U.S.-born AAPI women (aOR, 0.77; 95% CI, 0.62 to 0.95) had lower odds of receiving BCS than white women. Use of BCS increased over time for each racial/ethnic group; however, foreign-born AAPI women had persistently lower rates of BCS than non-Hispanic white women.
CONCLUSIONS
AAPI women, especially those who are foreign born, are less likely to receive BCS than non-Hispanic white women. Of particular concern, differences in BCS use among foreign-born and U.S.-born AAPI women and non-Hispanic white women have persisted over time. These differences may reflect inequities in the treatment of early-stage breast cancer for AAPI women, particularly those born abroad.
Keywords: breast neoplasms, cancer treatment, health disparities, race/ethnicity, immigrant health
In 1990, the National Institutes of Health (NIH) released a consensus statement recommending use of breast-conserving surgery (BCS) with adjuvant radiation instead of mastectomy for the treatment of early-stage (i.e., stage I or II) breast cancer, whenever possible.1 For women diagnosed with early-stage breast cancer, BCS has equivalent survival to mastectomy following initial treatment2 and may afford women better body image and sexual function.3 Despite these guidelines, substantial variations in the use of BCS exist by geographic region, patient characteristics including race/ethnicity, tumor characteristics, hospital characteristics, and provider characteristics.4–9
Previous studies have demonstrated lower rates of BCS among women who are Asian American and Pacific Islander (AAPI).10–14 Morris et al. found that AAPI women with early-stage breast cancer were less likely to receive BCS than non-Hispanic white women.11 Other studies have described lower BCS use than whites among certain AAPI ethnic groups in California, including Vietnamese, Filipino, Chinese, and Japanese women.12,13
These studies did not examine birthplace as a potential mediator of disparities in use of BCS. AAPIs are disproportionately foreign born compared to non-Hispanic white Americans. Moreover, foreign-born individuals are at risk of receiving poorer quality of care due to lower use of preventive services, lack of a regular source of health care, lower rates of insurance coverage, and cultural factors such as low English proficiency and lack of acculturation.15–19 Previous studies have found that AAPIs who are foreign born are less likely to receive cancer screening and hospice care than U.S.-born AAPIs.20,21
In this context, we examined the relationship between use of BCS and birthplace among AAPI and non-Hispanic white women residing in 5 areas included in the Surveillance, Epidemiology, and End Results (SEER) program.22 We hypothesized that foreign birthplace may explain previously described disparities in BCS between white and AAPI women as well as among various AAPI ethnic groups. We further examined trends in BCS from 1992 to 2000. We hypothesized that although use of BCS has increased over time, AAPI women, especially those who are foreign born, remain less likely to receive BCS than white women.
METHODS
Data Source
We used data from the 1992–2000 National Cancer Institute's SEER program.22 SEER consists of 11 population-based tumor registries, which represent approximately 14% of the U.S. population. SEER collects information on diagnosis and initial treatment for all incident cases of cancer diagnosed within geographically defined areas. Registries are located in 5 states (Connecticut, Hawaii, Iowa, Utah, New Mexico) and 6 metropolitan areas (Atlanta, Detroit, San Francisco/Oakland, Seattle/Puget Sound, Los Angeles County, San Jose/Monterey). The latter two registries were added to the SEER program in 1992.
SEER registries identify cases primarily by review of hospital pathology reports and discharge diagnoses. The SEER program maintains rigorous quality control programs, and data are considered highly valid with case ascertainment of 98%. SEER collects information on patient demographics at diagnosis including age, gender, race/ethnicity, marital status, and birthplace, and on cancer characteristics at diagnosis including primary tumor site, stage, size, histology, tumor grade, and estrogen and progesterone receptor status. Treatment is ascertained as the initial treatment course occurring within 4 months of diagnosis.
Study Sample
Non-Hispanic white and AAPI women were eligible if they were diagnosed with a microscopically confirmed first primary invasive breast cancer, stage I or II, between 1992 and 2000 (n=127,458). We limited our sample to women treated with either BCS or mastectomy (n=126,462). Because 96% of AAPI women diagnosed with early-stage breast cancer resided in 5 tumor registries, we restricted our sample to women diagnosed in Hawaii, Los Angeles County, San Francisco/Oakland, San Jose/Monterey, and Seattle/Puget Sound (n=66,995) to reduce geographic variability. We further excluded 967 women with tumor sizes that were unknown or greater than 5 cm who were potentially ineligible for BCS because of unacceptable cosmetic results or who may have required adjuvant therapy prior to surgery, to yield a final sample of 66,028 women.
Outcomes of Interest
Our main outcome was initial surgical treatment for early-stage breast cancer: BCS or mastectomy. We defined BCS as segmental mastectomy, lumpectomy, quadrantectomy, tylectomy, wedge resection, nipple resection, excisional biopsy, or partial mastectomy that was not otherwise specified with or without nodal dissection (n=37,961). We defined mastectomy as subcutaneous, total simple, modified radical, radical, extended radical mastectomy, or mastectomy that was not otherwise specified with or without nodal dissection (n=28,067).
We examined 2 secondary outcomes related to quality of care. The NIH consensus statement recommends axillary node dissection, regardless of surgical procedure, and radiation therapy for women who receive BCS.1
Race/Ethnicity and Birthplace
Our primary factors of interest were race/ethnicity and birthplace. SEER collects information about race/ethnicity largely from extensive chart review. We classified race/ethnicity as non-Hispanic white and AAPI, and birthplace as U.S. born, foreign born, and unknown. Birthplace is ascertained from multiple sources, including medical records, the Department of Motor Vehicles, and death certificates, and has greater than 90% sensitivity and positive predictive value among AAPIs.23 We hypothesized that women born in U.S. territories, such as Guam, would be most similar to foreign-born women, and therefore we classified the 53 AAPI women born in U.S. territories as foreign born. Finally, we combined race/ethnicity and birthplace to classify women as non-Hispanic white (n= 55,666), U.S.-born AAPI (n=3,178), foreign-born AAPI (n=4,418), and AAPI of unknown birthplace (n=2,766).
We did not examine birthplace among non-Hispanic white women for two reasons. First, birthplace is not well documented in medical records for white patients and thus is commonly missing for white patients in SEER.22 Second, the U.S. Census Bureau indicates that only 3.9% of non-Hispanic white individuals residing in the United States are foreign born.24
Because birthplace information was unknown for 27% of AAPI women, we compared their demographic and tumor characteristics with those of U.S.-born AAPI and foreign-born AAPI women (Table 1), and results suggested that AAPI women of unknown birthplace represented a mix of U.S.- and foreign-born AAPIs. Previously, Lin et al. found that overall, AAPIs with unknown birthplace in SEER were more likely to be foreign born than U.S. born, but had higher proportions of U.S.-born AAPIs than the overall AAPI population.23 Therefore, we included AAPI women with unknown birthplace as a separate group rather than impute birthplace information or exclude them from our analyses.
Table 1.
Demographic and Tumor Characteristics of Study Sample by Race/Ethnicity and Birthplace
| AAPI | ||||
|---|---|---|---|---|
| White Americans (N=55,666) n (%) | U.S-born (N=3,178) n (%) | Foreign-born (N=4,418) n (%) | Unknown (N=2,766) n (%) | |
| Demographic characteristics | ||||
| Age at diagnosis,* y | ||||
| <40 | 2,735 (5) | 197 (6) | 482 (11) | 230 (8) |
| 40–49 | 9,653 (17) | 580 (18) | 1,320 (30) | 655 (24) |
| 50–59 | 12,467 (22) | 727 (23) | 1,170 (27) | 690 (25) |
| 60–69 | 12,473 (22) | 820 (26) | 815 (19) | 613 (22) |
| ≥70 | 18,338 (33) | 854 (27) | 631 (14) | 578 (21) |
| Marital status* | ||||
| Married | 31,454 (57) | 1,951 (61) | 3,022 (68) | 1,828 (66) |
| Not married | 24,212 (43) | 1,227 (39) | 1,396 (32) | 938 (34) |
| Tumor registry* | ||||
| Hawaii | 1,442 (3) | 2,395 (75) | 564 (13) | 508 (18) |
| Los Angeles | 19,582 (35) | 429 (14) | 2,098 (48) | 624 (23) |
| SF/Oakland | 12,852 (23) | 191 (6) | 974 (22) | 945 (34) |
| San Jose/Monterey | 6,462 (12) | 73 (2) | 509 (12) | 388 (14) |
| Seattle/Puget Sound | 15,328 (28) | 90 (2) | 273 (6) | 301 (11) |
| Year of diagnosis* | ||||
| 1992 | 5,667 (10) | 310 (10) | 380 (9) | 200 (7) |
| 1993 | 5,645 (10) | 319 (10) | 388 (9) | 186 (7) |
| 1994 | 5,712 (10) | 297 (9) | 408 (9) | 194 (7) |
| 1995 | 5,950 (11) | 338 (11) | 437 (10) | 250 (9) |
| 1996 | 6,093 (11) | 362 (11) | 471 (11) | 294 (11) |
| 1997 | 6,307 (11) | 421 (13) | 546 (12) | 336 (12) |
| 1998 | 6,732 (12) | 452 (14) | 587 (13) | 371 (13) |
| 1999 | 6,875 (12) | 363 (11) | 629 (14) | 443 (16) |
| 2000 | 6,685 (12) | 316 (10) | 572 (13) | 492 (18) |
| Tumor characteristics | ||||
| Stage* | ||||
| I | 32,646 (59) | 1,961 (62) | 2,160 (49) | 1,558 (56) |
| II | 23,020 (41) | 1,217 (38) | 2,258 (51) | 1,208 (44) |
| Lymph nodes* | ||||
| Negative | 38,715 (70) | 2,340 (74) | 2,927 (66) | 1,922 (69) |
| Positive | 16,951 (30) | 838 (26) | 1,491 (34) | 844 (31) |
| Tumor size,* cm | ||||
| 1 or less | 16,068 (29) | 998 (31) | 1,006 (23) | 809 (29) |
| 1–2 | 23,770 (43) | 1,345 (42) | 1,736 (39) | 1,098 (40) |
| 2–3 | 10,306 (19) | 522 (16) | 1,013 (23) | 581 (21) |
| 3–4 | 3,522 (6) | 197 (6) | 421 (10) | 176 (6) |
| 4–5 | 1,614 (3) | 100 (3) | 210 (5) | 83 (3) |
| Grade* | ||||
| Well-differentiated | 10,522 (19) | 513 (16) | 541 (12) | 449 (16) |
| Moderately differentiated | 20,724 (37) | 1,271 (40) | 1,591 (36) | 1,087 (39) |
| Poor/undifferentiated | 16,544 (30) | 937 (30) | 1,718 (39) | 846 (31) |
| Histology* | ||||
| Ductal | 40,895 (74) | 2,661 (84) | 3,598 (81) | 2,241 (81) |
| Lobular | 4,893 (9) | 133 (4) | 162 (4) | 139 (5) |
| Mixed ductal/lobular | 4,492 (8) | 109 (3) | 232 (5) | 119 (4) |
| Estrogen receptor status* | ||||
| Positive | 38,751 (70) | 2,334 (73) | 2,673 (61) | 1,782 (64) |
| Negative | 8,887 (16) | 563 (18) | 922 (21) | 534 (19) |
| Progesterone receptor status* | ||||
| Positive | 32,688 (59) | 2,062 (65) | 2,303 (52) | 1,599 (58) |
| Negative | 13,653 (25) | 773 (24) | 1,165 (26) | 695 (25) |
P<.0001 for differences across race/ethnicity and birthplace groups.
AAPI, Asian American/Pacific Islander.
Finally, recognizing that aggregate data may mask important disparities, we examined AAPI ethnic groups with the largest sample sizes (Japanese [n=2,937], Filipino [n= 2,508], Chinese [n=2,249], Hawaiian [n=791], Korean [n=505], Vietnamese [n=408], and Indian/Pakistani [n= 284]) to determine whether disparities were more prominent in certain ethnicities.
Correlates of Breast-conserving Surgery
We examined demographic and tumor characteristics that have been previously reported in the literature as potential correlates of BCS.4–6 Demographic characteristics at diagnosis included age (<40, 40–49, 50–59, 60–69,≥70), marital status (married, not married), tumor registry (Hawaii, Los Angeles County, San Francisco/Oakland, San Jose/Monterey, Seattle/Puget Sound), and year of diagnosis (1992–2000). Tumor characteristics included American Joint Committee on Cancer stage (I, II), tumor size (in cm), grade (well-differentiated, moderately differentiated, poorly/undifferentiated, unknown), histology (ductal, lobular, mixed ductal and lobular, other), estrogen receptor status (positive, negative, unknown), and progesterone receptor status (positive, negative, unknown).
Statistical Analysis
All analyses were performed using SAS-callable SUDAAN software, version 8.1 (Research Triangle Institute, Research Triangle Park, NC).25 We performed bivariable analyses to compare demographic and tumor characteristics of non-Hispanic white women with AAPI women and repeated these analyses to further compare white women with AAPI women separated by birthplace (U.S.-born, foreign-born, unknown birthplace).
We fit multivariable logistic regression models using generalized estimating equations (GEE) to estimate the unadjusted and adjusted odds ratios for receipt of BCS comparing U.S.-born and foreign-born AAPI women to non-Hispanic white women. To account for potential correlations in treatment patterns among women residing in the same registry, we designated tumor registry as the clustering variable in each model. We adjusted for factors related to BCS in previous studies and those associated with BCS in bivariable analyses (age, marital status, year of diagnosis, tumor stage, tumor size, grade, histology, estrogen receptor status, and progesterone receptor status). Because of the large number of AAPI women without known birthplace, we conducted sensitivity analyses by alternatively categorizing AAPI women of unknown birthplace as U.S.-born AAPIs, then as foreign-born AAPIs. We used similar methods to examine receipt of axillary node dissection and radiation therapy following BCS. Next, we examined use of BCS from 1992 to 2000 to determine whether rates of BCS increased over time and tested for trend. To evaluate whether temporal trends for U.S.-born and foreign-born AAPI women were similar to non-Hispanic white women, we examined the interaction between year of diagnosis and race/ethnicity and birthplace. Finally, we performed bivariable and multivariable analyses to explore differences in receipt of BCS across the AAPI ethnic groups.
RESULTS
Table 1 presents demographic and tumor characteristics of the 66,028 women who were diagnosed with early-stage breast cancer; 10,362 (16%) women were AAPI. Overall, 3,178 (31%) of AAPI women were U.S. born, 4,418 (43%) were foreign born, and 2,766 (27 %) had unknown birthplace. Foreign-born AAPI women were more often diagnosed with stage II disease, positive lymph nodes, larger tumor sizes, and poorly or undifferentiated tumors than either non-Hispanic white or U.S.-born AAPI women.
Table 2 presents use of BCS. Overall, foreign-born AAPI and U.S.-born AAPI women were significantly less likely to receive BCS than non-Hispanic white women (43% and 56% vs 59%, respectively). For most demographic and tumor characteristics, AAPI women, particularly foreign-born women, were less likely to receive BCS than non-Hispanic white women. Of note, foreign-born AAPI women had substantially lower use of BCS even when diagnosed in the earliest stage or with subcentimeter tumors.
Table 2.
Demographic and Tumor Characteristics by Use of Breast-conserving Surgery
| AAPI | ||||
|---|---|---|---|---|
| White Americans % BCS | U.S.-born % BCS | Foreign-born % BCS | Unknown % BCS | |
| Overall | 59 | 56 | 43 | 45 |
| Demographic characteristics | ||||
| Age at diagnosis, y | ||||
| <40 | 55 | 54 | 44 | 46 |
| 40–49 | 60 | 57 | 44 | 48 |
| 50–59 | 63 | 56 | 43 | 47 |
| 60–69 | 60 | 55 | 43 | 42 |
| ≥70 | 57 | 55 | 39 | 40 |
| Marital status (P=.99) | ||||
| Married | 60 | 56 | 43 | 45 |
| Not married | 59 | 56 | 43 | 45 |
| Tumor registry* | ||||
| Hawaii | 60 | 56 | 46 | 48 |
| Los Angeles | 61 | 53 | 39 | 38 |
| SF/Oakland | 61 | 57 | 51 | 49 |
| San Jose/Monterey | 52 | 38 | 35 | 34 |
| Seattle/Puget Sound | 60 | 60 | 52 | 53 |
| Tumor characteristics | ||||
| Stage* | ||||
| I | 69 | 63 | 54 | 53 |
| II | 46 | 43 | 32 | 34 |
| Lymph nodes* | ||||
| Negative | 64 | 59 | 47 | 46 |
| Positive | 50 | 45 | 34 | 42 |
| Tumor size,* cm | ||||
| 1 or less | 70 | 65 | 52 | 54 |
| 1–2 | 64 | 58 | 51 | 49 |
| 2–3 | 49 | 49 | 36 | 35 |
| 3–4 | 33 | 26 | 21 | 25 |
| 4–5 | 22 | 21 | 11 | 16 |
| Grade* | ||||
| Well-differentiated | 71 | 67 | 56 | 57 |
| Moderately differentiated | 61 | 58 | 47 | 46 |
| Poor/undifferentiated | 54 | 48 | 37 | 40 |
| Histology (P=.72) | ||||
| Ductal | 61 | 56 | 43 | 45 |
| Lobular | 49 | 47 | 38 | 40 |
| Mixed duct/lob | 52 | 51 | 39 | 43 |
| Estrogen receptor status* | ||||
| Positive | 61 | 57 | 46 | 48 |
| Negative | 56 | 50 | 40 | 37 |
| Progesterone receptor status* | ||||
| Positive | 62 | 57 | 46 | 49 |
| Negative | 57 | 53 | 41 | 38 |
Pvalue<.0001 by Cochran-Mantel-Haenszel test for homogeneity across strata.
AAPI, Asian American/Pacific Islander; BCS, breast-conserving surgery.
Association of Race/Ethnicity and Birthplace with BCS
Table 3 presents the unadjusted and adjusted odds ratios of receiving BCS. In unadjusted analyses, we found that both U.S.-born and foreign-born AAPI women received BCS less often than non-Hispanic white women. Adjustments for demographic and tumor characteristics did not change the estimated odds ratios appreciably.
Table 3.
Unadjusted and Adjusted Odds of Receiving Breast-conserving Surgery
| Unadjusted Odds Ratio of Receiving BCS (95% CI) | Adjusted Odds Ratio of Receiving BCS (95% CI)* | |
|---|---|---|
| White, non-Hispanic | Reference | Reference |
| AAPI | ||
| Overall | 0.57 (0.40 to 0.82) | 0.53 (0.36 to 0.79) |
| U.S.-born | 0.81 (0.69 to 0.94) | 0.77 (0.62 to 0.95) |
| Foreign-born | 0.51 (0.35 to 0.74) | 0.49 (0.32 to 0.76) |
| Unknown-born | 0.55 (0.40 to 0.76) | 0.47 (0.32 to 0.69) |
Adjusted for age in decades, marital status, year of diagnosis, stage at diagnosis, tumor size, grade, histology, estrogen receptor status, progesterone receptor status, and clustered by tumor registry.
BCS, breast-conserving surgery; AAPI, Asian American/Pacific Islander.
Sensitivity analyses performed by alternatively categorizing AAPIs with unknown birthplace as U.S. born, then as foreign born, only slightly altered results. When AAPIs with unknown birthplace were classified as U.S.-born AAPIs, the unadjusted odds of receiving BCS decreased to 0.65 (95% CI, 0.48 to 0.87) and the adjusted odds decreased to 0.58 (95% CI, 0.41 to 0.82). In contrast, neither the unadjusted nor adjusted odds of receiving BCS among foreign-born AAPIs changed appreciably when we classified AAPIs with unknown birthplace as foreign born.
Association of Race/Ethnicity and Birthplace with Receipt of Axillary Node Dissection and Radiation Therapy Following Breast-conserving Surgery
Overall node dissection rates were lower among non-Hispanic white women (86%) than AAPI women (89% of U.S.-born; 92% of foreign-born; 89% of unknown birthplace) when type of surgery is not considered (P<.05). However, rates of node dissection were higher (95%) and did not vary by race/ethnicity and birthplace among women who had mastectomy. Among women who received BCS, U.S.-born and foreign-born AAPI women experienced higher rates of node dissection than non-Hispanic white women; however, these differences were not significant after adjustment (Table 4).
Table 4.
Receipt of Axillary Node Dissection and Radiotherapy Among Women with Breast-conserving Surgery (n=37,989)
| Receipt of Axillary Node Dissection | Receipt of Radiotherapy following Breast-conserving Surgery | |||||
|---|---|---|---|---|---|---|
| % | Unadjusted Odds Ratio (95% CI) | Adjusted Odds Ratio* (95% CI) | % | Unadjusted Odds Ratio (95% CI) | Adjusted Odds Ratio* (95% CI) | |
| White, non-Hispanic (n=33,097) | 81 | Reference | Reference | 79 | Reference | Reference |
| AAPI | ||||||
| Overall (n=4,892) | 85 | 1.13 (0.83 to 1.54) | 1.01 (0.64 to 1.58) | 80 | 1.31 (1.19 to 1.45) | 0.94 (0.62 to 1.43) |
| U.S.-born (n=1,764) | 85 | 1.31 (1.15 to 1.50) | 1.25 (1.20 to 2.41) | 85 | 1.73 (1.41 to 2.13) | 1.70 (1.20 to 2.41) |
| Foreign-born (n=1,892) | 87 | 1.56 (1.32 to 1.85) | 0.89 (0.74 to 1.69) | 81 | 1.31 (0.93 to 1.85) | 1.12 (0.74 to 1.69) |
| Unknown-born (n=1,236) | 81 | 1.03 (0.87 to 1.22) | 0.68 (0.48 to 0.96) | 73 | 0.69 (0.43 to 1.10) | 0.60 (0.35 to 1.01) |
Adjusted for age in decades, marital status, year of diagnosis, stage at diagnosis, tumor size, grade, histology, estrogen receptor status, progesterone receptor status, and clustered by tumor registry.
AAPI, Asian American/Pacific Islander.
Table 4 also presents receipt of radiation therapy following BCS. U.S.-born Asian women were more likely to receive radiation therapy than non-Hispanic white women even after adjustment for demographic and tumor characteristics.
Trends in Breast-conserving Surgery
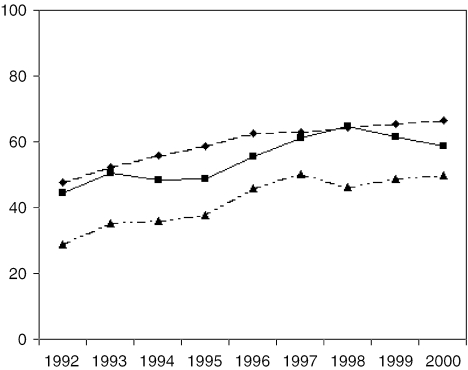
Figure 1 illustrates use of BCS from 1992 to 2000 for non-Hispanic white, U.S.-born AAPI, and foreign-born AAPI women. BCS use increased over time for each group (P≤.05 for trend); however, foreign-born AAPI women received BCS substantially less often than white and U.S.-born AAPI women in any given year. We further examined the interaction between year of diagnosis and each AAPI group compared with whites and found that neither interaction was statistically significant, suggesting that use of BCS remains constantly different for each group.
FIGURE 1.
Unadjusted use of BCS over time. Test of trend examining use of BCS over time, adjusting for age in decades, marital status, year of diagnosis, staging, tumor size, grade, histology, estrogen receptor status, progesterone receptor status, and clustered by tumor registry, was significant (P<.05) for non-Hispanic whites, U.S.-born AAPIs, and foreign-born AAPIs.BCS, breast-conserving surgery; AAPI, Asian American/Pacific Islander.
Asian-American and Pacific-Islander Ethnic Groups, Birthplace, and Treatment
As shown in Table 5, we further explored ethnic groups of AAPI women. We found that certain ethnic groups had very low proportions of foreign-born women, such as Hawaiians, while others, such as Vietnamese, were largely foreign born. Importantly, use of BCS was lower among ethnic groups with larger proportions of foreign-born women.
Table 5.
Demographics and Rates of BCS by AAPI Ethnicity Compared to Non-Hispanic White Women
| Ethnicity | % Foreign-born | % U.S.-born | % BCS | Adjusted Odds of BCS* |
|---|---|---|---|---|
| White (n=55,666) | — | — | 59 | Reference |
| Vietnamese (n=408) | 80 | 2 | 32 | 0.32 (0.14 to 0.71) |
| Korean (n=505) | 73 | 6 | 46 | 0.55 (0.34 to 0.89) |
| Indian/Pakistani (n=284) | 72 | 3 | 51 | 0.76 (0.42 to 1.35) |
| Filipino (n=2,508) | 68 | 9 | 41 | 0.44 (0.31 to 0.64) |
| Chinese (n=2,249) | 48 | 16 | 47 | 0.54 (0.34 to 0.86) |
| Japanese (n=2,937) | 15 | 59 | 52 | 0.61 (0.50 to 0.75) |
| Hawaiian (n=791) | 0.1 | 92 | 56 | 0.78 (0.66 to 0.93) |
Adjusted for age in decades, marital status, year of diagnosis, staging, tumor size, grade, histology, estrogen receptor status, progesterone receptor status, and clustered by tumor registry.
BCS, breast-conserving surgery; AAPI, Asian American/Pacific Islander.
DISCUSSION
AAPI women, particularly those of foreign birth, are less likely to receive BCS for early-stage breast cancer than white women. Importantly, use of BCS increased over time for each group studied, but AAPI women, particularly the foreign-born, continue to have substantially lower rates than non-Hispanic white women. Additionally, AAPI women from ethnic groups with large proportions born abroad were much less likely to receive BCS than white women.
Our findings are consistent with prior work indicating that AAPI women overall are less likely to receive BCS than white women. Morris et al. studied women diagnosed with early-stage breast cancer from the California Cancer Registry between 1988 and 1995 and found that 33% of AAPI women received BCS compared with 43% of white women and that these disparities persisted over time.11 Our study confirmed these findings, and enhanced generalizability by examining women diagnosed in 5 SEER tumor registries from 1992 to 2000. Interestingly, however, our unadjusted odds ratio comparing BCS use of AAPI and white women was similar in magnitude to that reported by Morris et al.
In addition, we examined the role of birthplace in explaining observed racial and ethnic disparities in BCS among AAPI women. We found that while the overall difference in the use of BCS between AAPI and white women was substantial (12%), the absolute difference between U.S.-born AAPI and white women was only 3%. In contrast, the difference between foreign-born AAPI and white women was 16%, suggesting that foreign birthplace may explain a large proportion of observed racial/ethnic disparities. Moreover, our data suggest that if the observed trend continues, foreign-born AAPI women will continue to lag behind both non-Hispanic white and U.S.-born AAPI women and experience substantially lower rates of BCS.
Last, we examined use of BCS among AAPI ethnic groups and found similar results to those reported previously.12,13 Additionally, we demonstrated lower use of BCS in Korean, Hawaiian, and Indian and Pakistani women. After adjustment, Indian and Pakistani women remained less likely to receive BCS than white women, but the odds ratio no longer achieved statistical significance possibly because of inadequate sample size. Overall, disparities were more prominent for ethnic groups comprised largely of foreign-born individuals.
Clinical factors alone are unlikely to explain the observed differences. These disparities persisted despite adjustment for several clinical factors known to influence decisions to perform BCS. Although it is possible that contraindications to BCS such as history of connective tissue disease or undesirable cosmetic result may disproportionately affect AAPI women, they are unlikely to explain the large difference observed. Moreover, it is unlikely that such contraindications could explain the persistently large difference observed between U.S.-born and foreign-born AAPI women.
In contrast, language barriers may contribute to observed differences in BCS use. Foreign-born AAPIs generally have lower English proficiency and thereby may have greater difficulty communicating with their physicians.26 Because physician-patient communication may influence a patient's breast cancer treatment choice and satisfaction with care, lower English proficiency may adversely affect communication and treatment outcomes.27
Another possible explanation for the observed differences is that foreign-born AAPI women may be less likely to choose BCS. Despite conflicting evidence on the impact of mastectomy on the self-image of AAPI women, AAPI women, especially immigrants, may still prefer mastectomy.10,28 AAPI women may place greater emphasis on immediate treatment (mastectomy), which does not require adjunctive radiation therapy. Thus, the choice of mastectomy may be less disruptive to the caretaking roles many women hold in their families10 and may explain why AAPI ethnic groups that are largely foreign born, or generally less acculturated, may choose mastectomy. Because of the paucity of literature on patient preferences in the use of BCS among various racial and ethnic groups, further research is needed to understand the contribution of patient treatment preferences.
Another possible explanation for the observed disparities is that providers caring for AAPI women may be less likely to recommend BCS because of concerns about nonadherence to recommended adjuvant therapy. Although Prehn et al. found that AAPI women in the Greater Bay Area Cancer Registry were less likely to receive appropriate adjuvant therapy compared with white women using 1994 data,13 we found that AAPI women were as likely and sometimes more likely to receive radiation therapy following BCS. It is possible that physicians are more likely to encourage BCS for women who would be adherent to radiation therapy. However, it is also possible that AAPI women may preferentially seek treatment in facilities that are less likely to perform BCS, such as nonurban, nonteaching hospitals.7,9
Our study has important limitations. We were unable to account for socioeconomic status; however, income and education did not explain disparities in BCS between AAPI and white women previously.13 Moreover, we were unable to examine access to care, including insurance status. Foreign-born individuals are less likely to have insurance; however, it is unclear whether insurance would account for the observed disparities, because we only studied women receiving some form of cancer treatment. Importantly, though, overall costs of BCS are less than mastectomy.29 We also lacked information about access to radiation treatment, physician and hospital characteristics, and patient preferences for treatment. Additionally, we lacked complete birthplace information. To address this issue, we included all AAPI women with unknown birthplace in our analyses as a separate group and found that AAPI women of unknown birthplace had similar odds as foreign-born AAPI women. In sensitivity analyses, we found our results remained the same when those with unknown birthplace were reclassified as foreign born. Because Lin et al. found that 74% of AAPIs with unknown birthplace are foreign born, these results further strengthen our findings.23
In summary, AAPI women with newly diagnosed early-stage breast cancer, especially foreign-born AAPI women, have persistently lower rates of BCS than white women. Even though it is troubling that rates of BCS among AAPI women continue to lag behind non-Hispanic white women, it is reassuring that those AAPI women who undergo BCS are as likely as non-Hispanic white women to receive axillary node dissection and to have radiation therapy following surgery. Breast cancer incidence and mortality appears to be rising among AAPIs,30,31 the fastest growing minority group in the United States, underscoring the importance of studying patterns of breast cancer care among AAPI women. Although the use of BCS does not influence mortality, understanding differences in the use of BCS provides insight into possible mechanisms of breast cancer care disparities. More research is required to better understand the role of access to care, physician-patient communication, and patient preferences in the treatment of early-stage breast cancer. In the interim, special efforts should be made to offer this procedure to AAPI women diagnosed with early-stage breast cancer.
Acknowledgments
Dr. Goel was supported by an institutional National Research Service Award (5T32PE11001-15) and by the Ryoichi Sasakawa Fund at the time this research was conducted. Dr. McCarthy is the recipient of a First Independent Research and Transition Award from the National Cancer Institute (R29 CA79052). Dr. Phillips is supported by a Mid-Career Investigator Award from the National Institutes of Health (K24 AT00589-01A1). Dr. Ngo-Metzger is supported by the National Cancer Institute Asian American Network for Cancer Awareness, Research and Training (U01 CA86322).
References
- 1.Treatment of early-stage breast cancer. NIH Consensus Statement Online 1990 June 18–21 (cited 2005 April 11); 8(6); :1–19. [PubMed] [Google Scholar]
- 2.Fisher B, Anderson S, Redmond C, et al. Reanalysis and results after 12 years of follow-up in a randomized clinical trial comparing total mastectomy with lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med. 1995;333:1456–1461. doi: 10.1056/NEJM199511303332203. [DOI] [PubMed] [Google Scholar]
- 3.Kiebert GM, de Haes JC, van de Velde CJ. The impact of breast-conserving treatment and mastectomy on the quality of life of early-stage breast cancer patients. A review J Clin Oncol. 1991;9:1059–1070. doi: 10.1200/JCO.1991.9.6.1059. [DOI] [PubMed] [Google Scholar]
- 4.Farrow DC, Hunt WC, Samet JM. Geographic variation in the treatment of localized breast cancer. N Engl J Med. 1992;326:1097–1101. doi: 10.1056/NEJM199204233261701. [DOI] [PubMed] [Google Scholar]
- 5.Joslyn SA. Racial differences in treatment and survival from early-stage breast carcinoma. Cancer. 2002;95:1759–1766. doi: 10.1002/cncr.10827. [DOI] [PubMed] [Google Scholar]
- 6.Gilligan MA, Kneusel FT, Hoffman RG, et al. Persistent differences in sociodemographic determinants of breast conserving treatment despite overall increased adoption. Med Care. 2002;40:181–189. doi: 10.1097/00005650-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Nattinger AB, Gottlieb MS, Veum J, et al. Geographic variation in the use of breast-conserving treatment for breast cancer. N Engl J Med. 1992;326:1102–1107. doi: 10.1056/NEJM199204233261702. [DOI] [PubMed] [Google Scholar]
- 8.Ayanian JZ, Guadagnoli E. Variations in breast cancer treatment by patient and provider characteristics. Breast Cancer Res Treat. 1996;40:65–74. doi: 10.1007/BF01806003. [DOI] [PubMed] [Google Scholar]
- 9.Johantgen ME, Coffey RM, Harris DR, et al. Treating early-stage breast cancer. hospital characteristics associated with breast-conserving surgery Am J Public Health. 1995;85:1432–1434. doi: 10.2105/ajph.85.10.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kagawa-Singer M, Wellisch DK, Durvasula R. Impact of breast cancer on Asian American and Anglo American women. Cult Med Psychiatry. 1997;21:449–480. doi: 10.1023/a:1005314602587. [DOI] [PubMed] [Google Scholar]
- 11.Morris CR, Cohen R, Schlag R, et al. Increasing trends in the use of breast-conserving surgery in California. Am J Public Health. 2000;90:281–284. doi: 10.2105/ajph.90.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin SS, Phan JC, Lin AY. Breast cancer characteristics of Vietnamese women in the Greater San Francisco Bay Area. West J Med. 2002;176:87–91. [PMC free article] [PubMed] [Google Scholar]
- 13.Prehn AW, Topol B, Stewart S. Differences in treatment patterns for localized breast carcinoma among Asian/Pacific Islander women. Cancer. 2002;95:2268–2275. doi: 10.1002/cncr.10965. [DOI] [PubMed] [Google Scholar]
- 14.Li CI, Malone KE, Daling JR. Differences in breast cancer stage, treatment, and survival by race and ethnicity. Arch Intern Med. 2003;163:49–56. doi: 10.1001/archinte.163.1.49. [DOI] [PubMed] [Google Scholar]
- 15.Frisbie WP, Cho Y, Hummer RA. Immigration and the health of Asian and Pacific Islander adults in the United States. Am J Epidemiol. 2001;153:372–380. doi: 10.1093/aje/153.4.372. [DOI] [PubMed] [Google Scholar]
- 16.Ku L, Matani S. Left out. immigrants' access to health care and insurance Health Aff. 2001;20:247–256. doi: 10.1377/hlthaff.20.1.247. [DOI] [PubMed] [Google Scholar]
- 17.Fox SA, Stein JA. The effect of physician-patient communication on mammography utilization by different ethnic groups. Med Care. 1991;29:1065–1082. doi: 10.1097/00005650-199111000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Woloshin S, Schwartz LM, Katz SJ, Welch HG. Is language a barrier to the use of preventive services? J Gen Intern Med. 1997;12:472–477. doi: 10.1046/j.1525-1497.1997.00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solis JM, Marks G, Garcia M, Shelton D. Acculturation, access to care, and use of preventative services by Hispanics. findings from NHANES 1982–1984 Am J Public Health. 1990;80(suppl.):11–19. doi: 10.2105/ajph.80.suppl.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goel MS, Wee CC, McCarthy EP, et al. Racial and ethnic disparities in cancer screening. the importance of foreign-birth as a barrier to care J Gen Intern Med. 2003;18:1028–1035. doi: 10.1111/j.1525-1497.2003.20807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ngo-Metzger Q, McCarthy EP, Burns RB, et al. Older Asian Americans and Pacific Islanders dying of cancer use hospice less frequently than older white patients. Am J Med. 2003;115:47–53. doi: 10.1016/s0002-9343(03)00258-4. [DOI] [PubMed] [Google Scholar]
- 22.Surveillance, Epidemiology, and End Results (SEER) Program. Public-Use Data (1973–2000), National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2003, based on the November 2002 submission. Available at: [Google Scholar]
- 23.Lin SS, Clarke CA, O'Malley CD, et al. Studying cancer incidence and outcomes in immigrants: methodological concerns. Am J Public Health. 2002;92:1757–9. doi: 10.2105/ajph.92.11.1757-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidley AD. U.S. Census Bureau, Current Population Reports, Series P23–206, Profile of the Foreign-born Population in the United States: 2000. Washington, DC: U.S. Government Printing Office; 2001. [Google Scholar]
- 25. Online Help Manual. SUDAAN Version 8.1. Research Triangle Park, NC: Research Triangle Institute. Available at: http://www.rti.org/sudaan Accessed February 2, 2004.
- 26.U.S. Census Bureau. Age by Language Spoken at Home by Ability to Speak English for the Population 5 Years and Over (PCT38) Census 2000 Summary File 4. Available at: http://www.census.gov Accessed February 3, 2004.
- 27.Liang W, Burnett CB, Rowland JH, et al. Communication between physicians and older women with localized breast cancer: implications for treatment and patient satisfaction. J Clin Oncol. 2002;20:1008–16. doi: 10.1200/JCO.2002.20.4.1008. [DOI] [PubMed] [Google Scholar]
- 28.Fung KW, Lau Y, Fielding R, et al. The impact of mastectomy, breast-conserving treatment and immediate breast reconstruction on the quality of life of Chinese women. ANZ J Surg. 2001;71:202–6. doi: 10.1046/j.1440-1622.2001.02094.x. [DOI] [PubMed] [Google Scholar]
- 29.Barlow WE, Taplin SH, Yoshida CK, et al. Cost comparison of mastectomy versus breast-conserving therapy for early-stage breast cancer. J Natl Cancer Inst. 2001;93:447–455. doi: 10.1093/jnci/93.6.447. [DOI] [PubMed] [Google Scholar]
- 30.Deapen D, Liu L, Perckins C, et al. Rapidly rising breast cancer incidence rates among Asian-American women. Int J Cancer. 2002;99:747–750. doi: 10.1002/ijc.10415. [DOI] [PubMed] [Google Scholar]
- 31.Wingo PA, Ries LAG, Rosenberg HM, et al. Cancer incidence and mortality, 1973–1995. a report card for the U.S Cancer. 1998;82:1197–1207. doi: 10.1002/(sici)1097-0142(19980315)82:6<1197::aid-cncr26>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]