Abstract
Objective
Medical school and residency are formative years in establishing patterns of prescribing. We aimed to review the literature regarding the extent of pharmaceutical industry contact with trainees, attitudes about these interactions, and effects on trainee prescribing behavior, with an emphasis on points of potential intervention and policy formation.
Design
We searched MEDLINE from 1966 until May 2004 for English language articles. All original articles were included if the abstract reported content relevant to medical training and the pharmaceutical industry. Editorials, guidelines, and policy recommendations were excluded.
Measurements and Main Results
Contact with pharmaceutical representatives was common among residents. The majority of trainees felt that the interactions were appropriate. A minority felt that their own prescribing could be influenced by contact or gifts, but were more likely to believe that others' prescribing could be influenced. Resident prescribing was associated with pharmaceutical representative visits and the availability of samples. A variety of policy and educational interventions appear to influence resident attitudes toward interactions with industry, although data on the long-term effects of these interventions are limited. Overall, residents reported insufficient training in this area.
Conclusions
The pharmaceutical industry has a significant presence during residency training, has gained the overall acceptance of trainees, and appears to influence prescribing behavior. Training programs can benefit from policies and curricula that teach residents about industry influence and ways in which to critically evaluate information that they are given. Recommendations for local and national approaches are discussed.
Keywords: medical students, interns and residents, medical education, drug industry
Interactions between physicians and the pharmaceutical industry have been coming under increasing scrutiny both within the medical field and among the public.1,2 Pharmaceutical companies spend, on average, twice as much on marketing to physicians and the public as they do on research and development.3 In 1999, 87% of the $13.9 billion spent on drug promotions was aimed at physicians,1 with an estimated expenditure of $8,000 to $13,000 spent per year per physician.1 Total expenditures for drug promotion rose to $16 billion in 2000.4 The medical literature has demonstrated that promotional activities can effectively influence the behaviors of practicing physicians, such as prescribing decisions and requests to add medications to a formulary.5 Despite many layers of evidence that marketing is effective, physicians may frequently be unaware of being influenced by marketing when making prescribing decisions.6–8
The effects of gifts from industry to physicians in general have been summarized elsewhere, but no published reports have reviewed the literature on the pharmaceutical industry's influence over medical trainees.5,9 The circumstances and effects of pharmaceutical industry promotions on medical trainees may differ substantially from those of practicing physicians. Because medical students, residents, and fellows are forming early preferences and practice patterns, they may be particularly vulnerable to the effects of industry promotions. Moreover, the educational, financial, social, and institutional contexts in which these interactions occur are often markedly different from those of practicing physicians.
Understanding trainees' attitudes and behavior in response to pharmaceutical promotions is essential to inform policy and to guide the growing numbers of programs in medical schools and post-graduate programs that aim to educate trainees about marketing and prescribing. Our aim is to review the literature regarding the extent of pharmaceutical industry interactions with trainees, the presence of guidelines for appropriate interactions with trainees, attitudes about these interactions, and effects on trainee prescribing behavior, with an emphasis on points of potential intervention and policy formation.
METHODS
We searched MEDLINE from 1966 until May 2004 for English language articles using the key words “pharmaceutical industry,”“drug industry,”“pharmaceutical manufacturers,”“pharmaceutical companies,”“pharmaceutical representatives,” or “pharmaceutical firms,” combined with the subject headings “education, medical,”“education, medical, graduate,”“education, medical, undergraduate,”“internship and residency,” or the key word “housestaff.” All original English language articles were included if the abstract reported content relevant to any aspect of medical training and the pharmaceutical industry. Studies focused on training program faculty were also included, because faculty serve in a teaching and role-modeling capacity for trainees, and transmission of educational values occurs through them. Articles without abstracts were examined directly for relevance. Editorials were reviewed for content and were included if original investigations not published elsewhere were described. Guidelines and policy recommendations were excluded. All bibliographies of included articles were cross-referenced for other relevant studies, which were then included, as were recent articles brought to our attention.
In order to summarize the common themes in the studies, questions from surveys were grouped by the consensus of the authors when the themes they addressed were equivalent, even if worded differently between studies. These questions are represented in the tables by compact question statements intended to preserve the meaning of the theme. In certain cases, we categorized items in studies using implicit criteria (e.g., deeming a type of gift “educational” or “noneducational”) to allow comparison with other studies. Survey results regarding trainee knowledge, attitudes, and behavior were extracted by one of us (DZ) in the form of percent agreement with a statement or Likert-scale responses (1=strongly agree, 2=agree, 3=neutral, 4=disagree, 5=strongly disagree). To maintain consistency in our presentation of data, results of negatively worded questions or those using an inverted Likert scale (i.e., 1=strongly disagree, 5=strongly agree) were reversed when grouped with other questions that addressed the same theme. Instances where this was done are noted in the tables. No papers for which we display Likert-scale data used a smaller or larger number of items.
In places where we discuss data from students and residents, we have used the term “trainees”; otherwise, we have used the more specific term “students” or “residents” (no studies evaluated fellows). All data for training program faculty, program directors, and chief residents are reported separately in the tables.
RESULTS
The initial search retrieved 155 articles. Twenty additional articles were found upon review of the bibliographies of these articles and through notification of recent publications. Fifty-eight articles were excluded because their subject matter did not deal with the pharmaceutical industry and trainees. Sixty-nine articles were excluded because they were editorials or commentary or news items, and 5 were excluded because they were guidelines or policy recommendations. The 44 articles that were ultimately included are summarized in Table 1.
Table 1.
Summary of Articles Included in Review
| Study | Population | N* | Methods | Intervention | Main Results |
|---|---|---|---|---|---|
| Educational interventions | |||||
| Watkins and Kimberly10 (2004) | IM† residents and faculty | 85/88, 86/109 | Needs assessment, survey pre and post | Integrated 4-unit curriculum using small and large group seminars | All residents reported increased awareness of patients' perceptions, how to identify conflict of interest, and feeling more prepared to interact with pharmaceutical representatives |
| Wilkes and Hoffman11 (2001) | Medical students | 120/136 | Survey, pre and post | Mock pharmaceutical representative presentation with discussion, literature search tools | Intervention increased belief that sponsored trips cause bias and advertisements are not educational. Many students who initially felt issues were not problematic became uncertain of their ethical position |
| Kelcher et al.12 (1998) | FP residents | 12/15 | Survey | Educational seminar and 8 actual pharmaceutical representative presentations | 92% of residents felt better prepared to interact with pharmaceutical representatives after the seminar |
| Hopper et al.13 (1997) | IM faculty, IM residents | 14/18, 28/31 | Survey, pre and post | 40-min lecture/discussion | Postintervention residents more likely to agree that pharmaceutical representatives can be unethical, marketing can be inappropriate, and prescribing can be influenced |
| Shear et al.14 (1996) | FP residents, medical students | N/A | Descriptive | 1-h video and discussion of physician/detailer interaction | No formal outcomes |
| Anastasio et al.15 (1996) | FP residents | 29/30 | Survey, pre and post | Educational seminar with role play and feedback | Increased confidence in meeting 10 goals of interaction with pharmaceutical representatives |
| Shaughnessy et al.16 (1995) | FP residents | 12/12 | Validated survey, pre and post | Lecture/discussion and evaluation of actual pharmaceutical representative presentation | More likely to believe that pharmaceutical representatives influence prescribing, less likely to believe they support important conferences and speakers |
| Vinson et al.17 (1993) | Medical students | 134/214 | Survey | 50-min lecture/discussion | “Marketing practice acceptability” score showed students less accepting of pharmaceutical representatives postintervention |
| Palmisano and Edelstein18 (1980) | Medical students, nurse practitioner students | 100/100, 95/100 | Survey, pre only | 90-min seminar to introduce students to drug industry strategies | 85% of medical students think it is improper for public official to take gift; 46% think it is improper for medical students to do same |
| Daniel and Leedham19 1966 | Medical, pharmacy, and dental students | 197/227, 98/101, 25/25 | Survey | Small group evaluation of drug advertising as part of pharmacology course | Students more skeptical of drug company claims |
| Garb20 1960 | Medical students | N/A | Descriptive | Evaluation of drug company ads, pharmaceutical representative presentations | 11 of 26 companies found to be reliable |
| Knowledge, attitudes, practices, and other research | |||||
| Keim et al.21 (2004) | EM program directors | 106/125 | Survey | Attitudes and practices toward industry interactions | Majority accepted industry support, while >90% agreed that industry support is an attempt to change prescribing |
| Brett et al.22 (2003) | IM residents and faculty | 39/42, 37/51 | Survey | Which pharmaceutical industry gifts are ethically problematic? | Most activities not believed to create ethical problems |
| Monaghan et al.23 (2003) | Medical, pharmacy, and nurse practitioner students | 59/108, 53/94, 17/17 | Survey | Knowledge and attitudes toward the pharmaceutical industry | Medical students were unsure of the usefulness of interactions and felt that most types of gifts were appropriate |
| Boltri et al.24 (2002) | FP residents and faculty | 24, 8 (clinic totals) | Sample use tracking | Prescriptions for patients with hypertension, pre and postrestriction of samples | Prescribing of first-line antihypertensive medication by residents increased from 39% to 72% after sample availability was restricted |
| Chakrabarti et al.25 (2002) | Psychiatry chief residents and program directors | 12/16, 15/16 | Survey | Attitudes, awareness of guidelines, and perceptions of compliance with guidelines | 75% unaware or noted absence of policy on industry influence, and 11% described influence of industry on their program as “restricted” |
| Wolfsthal et al.26 (2002) | IM program directors | 287/394 | Program Survey | Factors that correlate with American Board of Internal Medicine (ABIM) program pass rate | Financial support for residency programs from industry is a negative predictor of ABIM pass rates |
| McCormick et al.27 (2001) | IM residency graduates | 205/299 | Survey | Association between industry policy in residency and future behavior | Presence of policy negatively associated with perceived benefit of pharmaceutical representative information, OR 0.37 (95% CI 0.14 to 0.96) |
| Schwartz et al.28 (2001) | Patients admitted by psychiatry residents | N/A | Chart review | Prescriptions for psychiatric patients newly enrolled in a resident clinic | Choice of initial medication associated with recent pharmaceutical representative visits for that medication |
| Sigworth29 (2001) | IM residents | 164/181 | Interview and survey | Branded items carried in white coat pockets | 97% carried at least 1 branded item, median 4 items |
| Steinman et al.30 (2001) | IM residents | 105/117 | Survey | Resident attitudes and practices towards industry gifts | Residents found most gifts appropriate and felt their own prescribing was not influenced, while others' can be |
| Ferguson31 (1999) | IM residency graduates | 346/865 | Survey | Current interactions with pharmaceutical representatives, presence of policy in residency | Presence of policy in residency did not predict likelihood of interaction with pharmaceutical representatives or acceptance of samples |
| Razack et al.32 (1999) | Pediatric residency program | N/A | Descriptive | Program experience with policy development | An example of attempt to address conflicts of interest |
| Brewer33 (1998) | FP residents | N/A | Prescription inventory | Prescriptions at 3 residencies with differing sample policies | Greater use of generics seen at programs with limited or no medication samples. No cost differences |
| Gibbons et al.34 (1998) | Residents and practicing physicians, patients | 268/394, 196/200 | Survey | Physician and patient attitudes | Patients found gifts more influential than physicians, but patients more likely than doctors to find several kinds of gifts appropriate |
| Mahood et al.35 (1997) | FP program directors | 16/16 | Survey | Presence of policy or curriculum, extent of industry interaction | 4 of 16 FP programs had policies, 13 taught critical appraisal of industry claims, 4 taught industry marketing techniques |
| Sandberg et al.36 (1997) | Medical students | 205/205 | Survey | Recall of drug company name after receipt of textbooks as gifts | 90% received at least 1 free book, 25% recalled name of company |
| Shaughnessy and Bucci37 (1997) | FP residents and program directors | 248/800, 232/436 | Survey | Knowledge, attitudes, and behavior related to sample medication use | Samples were valued and used often; training regarding sample use was not viewed as adequate |
| Sergeant et al.38 (1996) | FP residents | 226/262 | Survey | Attitudes, knowledge, self-reported behavior | 82% approved of industry interaction, 58% found industry literature useful, and 34% believed pharmaceutical representatives influenced prescribing |
| Spingarn et al.39 (1996) | IM residents | 75 | Survey, retrospective cohort | Knowledge of Lyme disease treatment and information recall after grand rounds by pharmaceutical representative | Attendees (n=22) more likely to name company drug for complicated Lyme disease, less likely to name appropriate drug for mild disease |
| Stryer and Bero40 (1996) | IM residency, HMO, private practice | N/A | Assessment of promotional items | Compliance with FDA regulations | 42% of items failed to comply with at least 1 regulation; items favor the distributing company |
| Hodges41 (1995) | Psychiatry residents and students | 74/106 | Validated survey | Attitudes, extent of interaction with pharmaceutical representatives | 77% felt pharmaceutical representatives support important conferences; 57% felt promotional items do not affect prescribing |
| Ziegler et al.42 (1995) | IM residents | 27/N/A | Survey, pharmacist transcription | Accuracy of drug information stated during pharmaceutical representative presentations | 12 of 106 (11%) statements were incorrect; 26% of residents recalled hearing a false statement |
| Johnstone and Valenzuela43 (1995) | Anesthesia residency program | N/A | Descriptive | Departmental experience with pharmaceutical industry | Industry influence was pervasive and often unethical; restrictions on pharmaceutical representative activity were instituted |
| Reeder et al.44 (1993) | EM chief residents | 72/87 | Survey | Extent of pharmaceutical representative involvement, attitudes | 80% felt programs benefit from industry presence, and 20% felt their own prescribing habits were affected |
| Keim et al.45 (1993) | EM residents and program directors | 1385/1836, 80/81 | Survey | Attitudes and self-reported behaviors | 60% of residents felt gifts were appropriate, 74% felt pharmaceutical representatives cross ethical boundaries, and 49% felt they affect prescribing |
| Brotzman and Mark46 (1993) | FP residents | 265/378 | Survey | Attitudes stratified by type of policy (“unrestricted” vs “restricted” residency) | Unrestricted program residents more likely to view interaction as beneficial, view detailing as helpful, and view gifts as appropriate |
| Lichstein et al.47 (1992) | IM program directors | 272/444 | Survey | Extent of industry involvement, attitudes | 88% allowed industry sponsored conferences, 67% felt benefits outweighed risks, and 35% had formal policy |
| Banks and Mainous48 (1992) | Medical school faculty | 248/462 | Survey | Attitudes of medical school faculty toward American Medical Association guidelines | Majority felt samples and gifts do not influence prescribing, and 66% felt personal pharmaceutical representative relationship does influence prescribing |
| Brotzman and Mark49 (1992) | FP programs | 328/386 | Survey | Presence of policies regarding industry interactions | 58% had policy; 41% had prohibitions |
| Bucci and Frey50 (1992) | FP program directors | 325/383 | Survey | Level of pharmaceutical curriculum development | Presence of pharmacy faculty associated with presence of curricula to evaluate industry marketing materials and presence of guidelines |
| Morelli and Koenigsburg 51 (1992) | Drug samples in FP residency | N/A | Samples inventory and tracking | Characteristics of the distribution of samples | 54% of samples given to patients, 46% family/self/other, 39% economic rationale, and 53% therapeutic rationale; simultaneous written prescription matched sample brand 100% of time |
| McKinney et al.52 (1990) | IM faculty and residents | 277/335, 190/240 | Validated survey | Attitudes | 23% faculty/15% residents felt physicians cannot ever be compromised; both denied influence of contact on their own prescribing behavior |
| Lurie et al. 53 (1990) | IM faculty and residents | 240/309, 131/175 | Survey | Reported number of pharmaceutical representative contacts, self-reported behavior | 25% faculty/32% residents changed practice based on a discussion with a representative |
Presented as number of respondents/total number of subjects available.
IM, Internal Medicine; EM, emergency medicine; FP, family practice; N/A, not available or not applicable.
CHARACTERISTICS OF STUDIES
The identified studies were published between 1960 and 2004, with 3 studies published prior to 1990, 11 studies between 1990 and 1994, 18 between 1995 and 1999, and 12 between 2000 and the beginning of 2004. Eleven studies reported educational interventions, 9 of which also reported attitude surveys.10–20 Twenty-four studies reported the results of surveys.21–23,25,27,30,31,34–39,41,42,44–50,52,53 The remaining 9 studies described other aspects of the interface between trainees and the pharmaceutical industry (Table 1).24,26,28,29,32,33,40,43,51
The vast majority of studies used written surveys and descriptive statistics. Of the total of 31 studies that surveyed attitudes, 3 used modifications of a scale originally validated by McKinney, while the remaining 28 utilized unvalidated instruments.16,41,52 Sample size across studies ranged from 12 to 1,917, with a median of 214. Response rates ranged from 31% to 100%.
TYPE AND EXTENT OF INDUSTRY CONTACT
Pharmaceutical representatives interact frequently with trainees and provide small gifts during sponsored meals, conferences, and scientific meetings. In 1 study of medical students, 90% reported having received at least 1 book from a drug company.36 Residents in Internal Medicine and psychiatry have reported attending between 1.5 and 8 industry-sponsored lunches or rounds per month,27,41,42,46,52 emergency medicine residents have been reported to interact with pharmaceutical representatives 1 to 3 times per week,44 and in a more recent study, medical students reported a mean of 10.6 contacts per month.23 A study of Internal Medicine program directors revealed that 89% allowed industry sponsorship of conferences, 84% allowed residents to meet with pharmaceutical representatives, and 85% accepted industry food for conferences, although 71% of programs had other sources of funding for food.47 Pharmaceutical representatives were allowed to give presentations in roughly half of Internal Medicine and emergency medicine programs.21,47 Among emergency medicine chief residents, 93% reported involvement of pharmaceutical representatives in their programs,44 while in another study of emergency medicine programs, 18% at least occasionally allowed unrestricted interactions between representatives and residents at work, 32% allowed sponsored speakers to present topics to the residency, 41% allowed representatives to teach residents directly, and 40% accepted cash support for social activities.21
TRAINING ABOUT INTERACTIONS WITH INDUSTRY
A number of studies have assessed medical student and resident training for interactions with pharmaceutical representatives and their awareness of guidelines that address these interactions. In 3 of 4 studies, a majority of trainees reported insufficient training about how to interact with pharmaceutical representatives,16,23,41,52 and about half desired more teaching on the subject.38 In another study, the majority of residents also stated that training was insufficient in the area of sample medication use.37 In a survey of Internal Medicine residency programs, Lichstein found that 26% of programs offered instruction on how to assess pharmaceutical representative marketing claims.47 Resident, chief resident, and program director awareness of and familiarity with guidelines and position statements ranged from 1% to 70% between different studies,10,25,34,44 and in another study, 23% of residents reported having read them.38
ATTITUDES
Attitudes toward interactions with and information received from pharmaceutical representatives varied in different study settings (Table 2). Several of the studies surveyed both trainees and faculty in the same medical centers, and others surveyed faculty program directors; where applicable, data from both groups are presented to highlight similarities and differences.
Table 2.
Attitudes Toward Interactions with Pharmaceutical Representatives
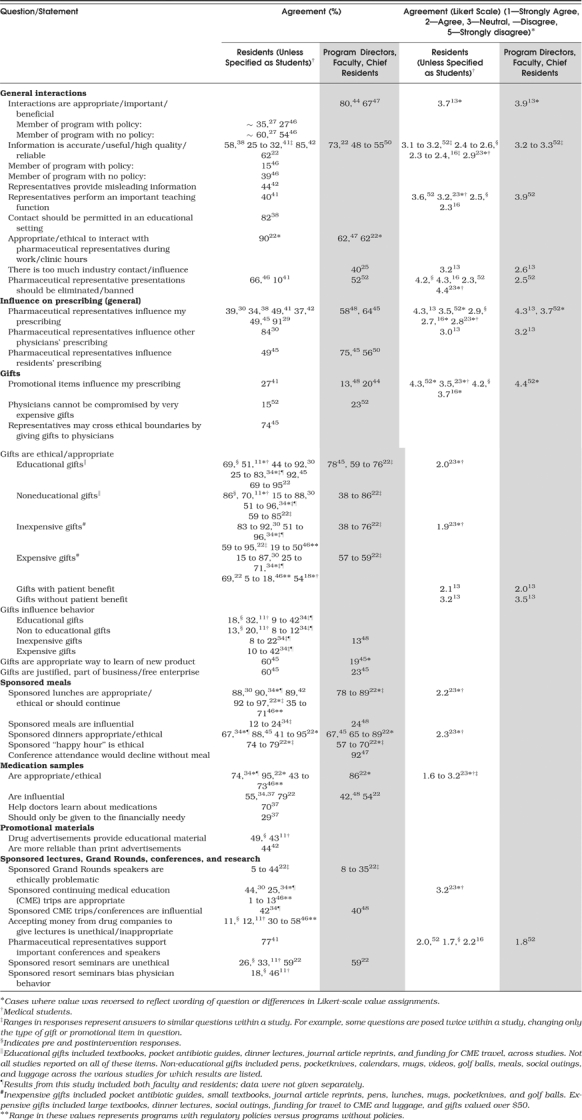 |
Substantial numbers of residents and faculty found pharmaceutical representative interactions to be “appropriate,”“important,”“beneficial,” or “ethical” and felt that they should be permitted. However, at least 2 studies suggest that residents from programs with regulatory policies took a more skeptical approach to interactions than residents from programs without policies.27,46
Individual residents believed themselves to be more immune to industry influence than their colleagues. In 5 studies, fewer than half of the residents believed that interactions with pharmaceutical representatives could influence their own prescribing,29,30,38,41,42 while in 1 study, 84% believed that representatives could influence the prescribing of other physicians30; similar results were also found in studies using Likert scales.13,52 In 1 study, faculty were more likely than residents to believe that residents' prescribing can be influenced in general.45
The theme of personal immunity to industry influence was also reflected in questions about receiving gifts. Across a number of studies, residents stated that most types of gifts (including sponsored meals) were appropriate, and a minority felt that gifts influenced behavior.11,30,34,45 Even for the most expensive of gifts (worth $10,000 and above), 1 study found that 23% of faculty and 15% of residents felt that physicians would not be influenced.52 Other data on differences between residents and supervising faculty are conflicting. In a recent study, only 3 of 18 industry promotion scenarios received significantly different ethical ratings by the 2 groups.22 In contrast, a study in an emergency medicine program revealed that residents were far more likely than faculty to believe that gifts were an appropriate way to learn about new products.45
Finally, most residents and faculty considered medication samples to be appropriate, with one half to three quarters of the respondents feeling that they influence prescribing,22,34,37 and studies document a wide range of opinions on the ethics and influence of sponsored lectures and conferences.
BEHAVIOR
Most of the studies of trainee behavior resulting from interactions with industry did not measure objective behavioral outcomes such as prescribing, attendance at sponsored events, or use of promotional gifts. Instead, most surveys asked respondents to report on their own past behaviors or expected future behaviors (Table 3).
Table 3.
Behavior in Residents' Interactions with Pharmaceutical Representatives
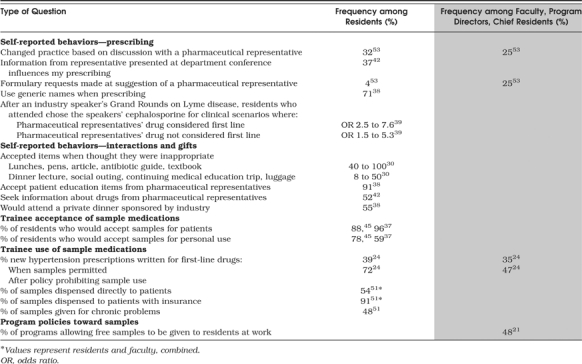 |
One study that did use objective outcomes used chart review to evaluate resident prescribing in a psychiatry residency and found a statistically significant association between individual company sales visits and initiation of the company's medication by residents within 12 weeks of the sales visit.28 In 2 studies using self-report surveys, about one third of residents reported changing their practices based on discussions with pharmaceutical representatives and information learned from industry presentations at teaching conferences.42,53 In the latter case, although 11% of statements by representatives were inaccurate, only 1 in 4 residents recalled ever hearing a false statement.42 A study which surveyed residents after a Grand Rounds presentation on Lyme disease was given by a pharmaceutical representative found that attendees were more likely than non-attendees to name the speaker's drug as first line both when it was indicated and when it was not.39
Many residents also report accepting gifts and patient education items from industry representatives, even when residents think these gifts are inappropriate (Table 3).30
The use of samples is another area in which objective outcomes have been measured. In 1 study of new prescriptions for hypertension among family practice residents and faculty, prescriptions written for guideline-recommended agents nearly doubled among residents (from 39% to 72%) after a policy prohibiting sample use was instituted.24 Other studies have shown that the vast majority of residents accept samples to use for patients.37,45 When samples were given for a chronic condition and a written prescription accompanied the sample, it matched the sample 100% of the time.51
POLICIES AND EDUCATIONAL INTERVENTIONS
Several studies have examined the presence of policies for the interaction of pharmaceutical representatives with residents as well as for the use of sample medications. In 1992 and 1993 in the United States, 35% of Internal Medicine programs stated that they had a formal policy or guidelines regarding interactions with pharmaceutical representatives, while 30% to 58% of family practice programs and 61% of emergency medicine program directors reported the same.45,47,49,50 Twenty-five percent of Canadian psychiatry and family practice residencies reported the presence of a formal policy.25,35 However, in 2 studies, many residents (73%–77%) and chief residents (54%) were not aware of the presence or absence of policies or established guidelines.38,44
In a random sample of family practice programs, residents from programs without policies were more likely than residents from other programs to view interactions with pharmaceutical representatives as beneficial (54% vs 27%, P=.003), to view detailing as helpful (41% vs 10%, =0.001), and to feel that the gifts were appropriate (50% vs 19% for inexpensive gifts, P=.001, 18% vs 5% for expensive gifts, P=.005).46 Another sample of family practice residency programs found that programs with pharmacists on the faculty were more likely than other programs to have printed guidelines for interactions with pharmaceutical representatives, and more likely to include methods of evaluating promotional materials in their curriculum.50 In a survey of psychiatry chief residents and program directors, respondents from departments and programs without guidelines perceived an increased degree of industry influence on their residents' psychiatry training.25
The long-term effects of the presence of policies in residency have been evaluated in 2 studies.27,31 In these studies, physicians who had graduated from programs with or without a restrictive policy were equally likely to interact with pharmaceutical representatives27,31 or to accept samples.31 However, 1 of these studies found that interactions were less “intense” among graduates exposed to a policy during residency (i.e., in 1 of 2 comparisons, they scored lower on a combined “contact score” that included participation in out-of-hospital social events and acceptance of consulting fees, among other interactions).27 In addition, this study also found that graduates of the program with a policy were significantly less likely to find pharmaceutical representative information helpful in guiding their practice.27
Among studies of educational interventions for which pre and postsurveys were conducted, receiving the intervention was associated with a stronger belief that discussions with pharmaceutical representatives and promotional items can impact prescribing,16 stronger beliefs that pharmaceutical representatives may use unethical practices and that other physicians' prescribing patterns can be negatively influenced by gifts,13 beliefs that resort seminars bias physician behavior and that it is unethical to accept funding to attend these seminars,11 less agreement that drug advertisements provide educational material,11 less approval of pharmaceutical gifts,17 and an increase in resident confidence in managing aspects of the interaction with pharmaceutical representatives.15 There was no significant change in residents' view of their own prescribing practices after the program in one study,13 but another study found that after the educational intervention residents were more likely to believe that promotional items can impact their prescribing.16
Certain program policies were directed at the use of sample medications. Sixty-six percent of family practice programs reported having a policy regarding sample medications, although only 15% of those incorporated recommendations of the Society of Teachers of Family Medicine.37 In that study, 41% of residents from programs with a sample policy were aware that a policy existed, whereas 42% of residents not governed by a sample policy thought that one was in place.37
CONCLUSIONS
In our review of studies on interactions between the pharmaceutical industry and physicians in training, several themes emerge. The frequency of resident interaction with the pharmaceutical industry was high, and the majority of residents believed that industry interactions were appropriate. Despite evidence suggesting that pharmaceutical representative interactions can influence prescribing, a majority of residents felt that their own prescribing could not be influenced by interactions or gifts, while allowing that others' prescribing could be influenced. Many programs lacked policies to regulate or inform interactions with industry representatives, and trainees reported insufficient training in how to approach these interactions and other forms of industry promotion, despite a high frequency of contact.
Trainees' self-assured attitudes toward influence and their lack of training in how to interpret pharmaceutical marketing techniques, combined with limited but suggestive evidence that education and policies can change attitudes and practices, suggests a central role for education and policy. The 9 educational interventions reviewed provide a limited set of models by which curricula may be implemented, including lecture-, video-, and seminar-based curricula, as well as evaluations of pharmaceutical representative presentations, both real and staged. The studies reviewed here suggest that these interventions can be effective at raising trainee awareness of influence and increasing their skepticism towards information presented by industry. However, the time to the follow-up survey was short in each of these studies, so the long-term effects on attitudes remain uncertain, as do the effects of these interventions on skills and behavior. While the current data are not sufficient to recommend the inclusion of one particular teaching intervention in residency curricula, larger scale randomized evaluations of educational curricula are warranted.
While these curricula represent an important first step, a more vigorous set of interventions may be necessary to effectively counter the effects of pharmaceutical marketing. In particular, such interventions should focus on problem areas in knowledge, attitudes, and practices that have been documented in the literature. For example, curricular- and practice-based interventions should emphasize training students and residents to critically assess marketing practices and information received from industry, challenging prevailing local beliefs about acceptable levels of interaction with industry, and debunking the common belief among trainees that they are impervious to influence. This might take the form of an ongoing series of seminars to address educational deficiencies in these areas. Simultaneously, interventions that target faculty and chief residents to encourage appropriate role modeling in their daily interactions with trainees are likely to play a critical role in establishing new cultural norms for trainees and promoting alignment of the overt with the “hidden” curriculum. It is important that these interventions incorporate proven methods to change physician behavior, namely, they should be sustained, interactive, and multifaceted, and should use local opinion leaders (e.g. chief residents and respected faculty members) and/or “academic detailers.”54–56
In addition, the widespread absence of policies and high frequency of interactions with industry representatives suggest an important role for regulation. The implementation or modification of residency program policies is likely to be helpful, as some studies suggest that the presence of policies is associated with more skeptical attitudes toward industry and fewer future interactions with representatives. Such policies, when clear and explicit, can not only modify current behavior but can also help establish norms that may remain with trainees into subsequent stages of their careers. Moreover, these policies and norms should guide not only the interactions between trainees and industry representatives but also the interactions that the industry enjoys with program leaders and the training programs they represent. Senior program faculty and chief residents should set an example by moderating their own contact with industry representatives, as well as by minimizing or eliminating industry funding for and involvement with the educational and social components of their programs.
Given the breadth and importance of this issue, a nationwide approach is merited. Curricular recommendations should be established by the Accreditation Council for Graduate Medical Education (ACGME) and the American Association of Medical Colleges (AAMC) as part of their larger efforts to standardize and improve medical education. For example, the ACGME's Practice-based Learning (PBL) core competency addresses residents' skills in evaluating scientific evidence and critically appraising the medical literature in making patient-related decisions.57 Because physicians may adopt practice patterns and attitudes from pharmaceutical marketing, even when they believe they rely on scientific sources,6 evaluating the validity of pharmaceutical marketing information is an important part of mastering this competency. Similarly, educating trainees about the influence and ethics of marketing can contribute to meeting the standards of the ACGME's Professionalism competency,58 as well as providing skill-based training on preferred methods of interacting with industry representatives.
Educational curricula will only be effective if trainees view them as necessary, making it important to understand trainee attitudes so as to tailor curricula to their interests and needs. In addition, it is important to understand the attitudes and practices of supervising faculty, given their central role in developing and implementing curricula and their status as role models for professional behavior. Therefore, while national recommendations may be warranted, local factors are also critical to acknowledge and incorporate into training curricula, as opportunities for change are highly contextual within cultures of medical practice.32 The experience of McMaster University is illustrative, wherein faculty, residents, and pharmaceutical representatives were all involved in the process of creating guidelines, with the result being the successful implementation of policies ending drug-sponsored lunches, industry presence at educational events, and funding of events requiring the inclusion of materials by the sponsor.59 Finally, further high-quality research is needed to evaluate the long-term effectiveness of interventions and thus guide future efforts in this area.
While the literature informs our understanding of this topic, several limitations of the studies we reviewed merit attention. Many of the studies are small, and of limited power. Most of the survey instruments used were not validated; the validated attitude scale introduced by McKinney et al. has been used by other authors, but with few subjects. Also, few studies have used objective measures of actual behavior change in trainees. However, the self-report and knowledge surveys among trainees reviewed here suggest that interactions with pharmaceutical representatives influence prescribing, which is also suggested by studies of behavioral outcomes in practicing physicians as well as the inherent goals of advertising and promotion in a profit-based marketplace.3,7,8 Another limitation is that the potentially charged nature of this subject may have led to bias in assessments of knowledge, attitudes, and behavior. However, the wide variety of studies that produce a generally consistent message about knowledge, attitudes, and behavior suggests that these effects are both real and generalizable.
In summary, the pharmaceutical industry has a significant presence during medical training and has gained the overall acceptance of trainees. Residents acknowledge the potential for industry influence in others, but generally not in themselves, despite evidence that they themselves are influenced as well. Given this state of affairs, it is time for a major shift in the culture of medical training. Serious and sustained interventions have the potential to substantially modify these attitudes and behavior, and to improve the skills of trainees in dealing with industry marketing and information. Such efforts can foster a climate of best practices that is less likely to be compromised by the promotional efforts of individual pharmaceutical companies.
Acknowledgments
We would like to thank Dr. Warren Browner, Dr. Ralph Gonzales, Dr. Seth Landefeld, Dr. Mack Lipkin, Jr., and Dr. Steven Reidbord for their encouragement and critical reviews of the manuscript.
Grant support: Dr. Zipkin has received no funding for the work on this manuscript. Dr. Steinman is supported by a VA Research Career Development Award, and part of this work was supported by a fellowship grant from the VA National Quality Scholars Program.
References
- 1.Stolberg SG, Gerth J. High-tech stealth being used to sway doctor prescriptions. The New York Times. November 16, 2000; National Desk. [PubMed] [Google Scholar]
- 2.Zuger A. When your doctor goes to the beach, you may get burned. The New York Times. February 24, 2004; F:5. [Google Scholar]
- 3.Profiting from Pain: Where Prescription Drug Dollars Go. Washington, DC: Families USA; 2002. Publication No. 02-105. [Google Scholar]
- 4.Prescription Drugs and Mass Media Advertising, 2000. Washington, DC: National Institute for Health Care Management; November 2001. [Google Scholar]
- 5.Wazana A. Physicians and the pharmaceutical industry: is a gift ever just a gift? JAMA. 2000;283:373–80. doi: 10.1001/jama.283.3.373. [DOI] [PubMed] [Google Scholar]
- 6.Avorn J, Chen M, Hartley R. Scientific versus commercial sources of influence on the prescribing behavior of physicians. Am J Med. 1982;73:4–8. doi: 10.1016/0002-9343(82)90911-1. [DOI] [PubMed] [Google Scholar]
- 7.Orlowski JP, Wateska L. The effects of pharmaceutical firm enticements on physician prescribing patterns: there's no such thing as a free lunch. Chest. 1992;102:270–3. doi: 10.1378/chest.102.1.270. [DOI] [PubMed] [Google Scholar]
- 8.Chren MM, Landefeld CS, Murray TH. Doctors, drug companies, and gifts. JAMA. 1989;262:3448–51. [PubMed] [Google Scholar]
- 9.Lexchin J. Interactions between physicians and the pharmaceutical industry: what does the literature say? Can Med Assoc J. 1993;149:1401–7. [PMC free article] [PubMed] [Google Scholar]
- 10.Watkins RS, Kimberly J., Jr What residents don't know about physician–pharmaceutical industry interactions. Acad Med. 2004;79:432–7. doi: 10.1097/00001888-200405000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Wilkes MS, Hoffman JR. An innovative approach to educating medical students about pharmaceutical promotion. Acad Med. 2001;76:1271–7. doi: 10.1097/00001888-200112000-00026. Dec. [DOI] [PubMed] [Google Scholar]
- 12.Kelcher S, Brownoff R, Meadows LM. Structured approach to pharmaceutical representatives. Family medicine residency program. Can Fam Physician. 1998;44:1053–6. 1059–60. [PMC free article] [PubMed] [Google Scholar]
- 13.Hopper JA, Speece MW, Musial JL. Effects of an educational intervention on residents' knowledge and attitudes toward interactions with pharmaceutical representatives. J Gen Intern Med. 1997;12:639–42. doi: 10.1046/j.1525-1497.1997.07126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shear NH, Black F, Lexchin J. Examining the physician–detailer interaction. Can J Clin Pharmacol. 1996;3:175–9. [Google Scholar]
- 15.Anastasio GD, Little JM., Jr Pharmaceutical marketing: implications for medical residency training. Pharmacotherapy. 1996;16:103–7. [PubMed] [Google Scholar]
- 16.Shaughnessy AF, Slawson DC, Bennett JH. Teaching information mastery: evaluating information provided by pharmaceutical representatives. Fam Med. 1995;27:581–5. [PubMed] [Google Scholar]
- 17.Vinson DC, McCandless B, Hosokawa MC. Medical students' attitudes toward pharmaceutical marketing: possibilities for change. Fam Med. 1993;25:31–3. [PubMed] [Google Scholar]
- 18.Palmisano P, Edelstein J. Teaching drug promotion abuses to health profession students. J Med Educ. 1980;55:453–5. doi: 10.1097/00001888-198005000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Daniel EE, Leedham L. Effect on student attitudes of a program of critical evaluation of claims for drugs. J Med Educ. 1966;41:49–60. doi: 10.1097/00001888-196601000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Garb S. Teaching medical students to evaluate drug advertising. J Med Educ. 1960;35:729–39. [PubMed] [Google Scholar]
- 21.Keim SM, Mays MZ, Grant D. Interactions between emergency medicine programs and the pharmaceutical industry. Acad Emerg Med. 2004;11:19–26. doi: 10.1197/j.aem.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 22.Brett AS, Burr W, Moloo J. Are gifts from pharmaceutical companies ethically problematic? A survey of physicians. Arch Intern Med. 2003;163:2213–8. doi: 10.1001/archinte.163.18.2213. [DOI] [PubMed] [Google Scholar]
- 23.Monaghan MS, Galt KA, Turner PD, et al. Student understanding of the relationship between the health professions and the pharmaceutical industry. Teach Learn Med. 2003;15:14–20. doi: 10.1207/S15328015TLM1501_04. [DOI] [PubMed] [Google Scholar]
- 24.Boltri JM, Gordon ER, Vogel RL. Effect of antihypertensive samples on physician prescribing patterns. Fam Med. 2002;34:729–31. [PubMed] [Google Scholar]
- 25.Chakrabarti A, Fleisher WP, Staley D. Interactions of staff and residents with pharmaceutical industry: a survey of psychiatric training program policies. Ann R Coll Physicians Surg Can. 2002;(35 (suppl)):541–6. [PubMed] [Google Scholar]
- 26.Wolfsthal SD, Beasley BW, Kopelman R, Stickley W, Gabryel T, Kahn MJ. Benchmarks of support in internal medicine residency training programs. Acad Med. 2002;77:50–6. doi: 10.1097/00001888-200201000-00013. [DOI] [PubMed] [Google Scholar]
- 27.McCormick BB, Tomlinson G, Brill-Edwards P, Detsky AS. Effect of restricting contact between pharmaceutical company representatives and internal medicine residents on posttraining attitudes and behavior. JAMA. 2001;286:1994–9. doi: 10.1001/jama.286.16.1994. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz TL, Kuhles DJ, II, Wade M, Masand PS. Newly admitted psychiatric patient prescriptions and pharmaceutical sales visits. Ann Clin Psychiatry. 2001;13:159–62. doi: 10.1023/a:1012237709036. [DOI] [PubMed] [Google Scholar]
- 29.Sigworth SK, Nettleman MD, Cohen GM. Pharmaceutical branding of resident physicians. JAMA. 2001;286:1024–5. doi: 10.1001/jama.286.9.1024-a. [DOI] [PubMed] [Google Scholar]
- 30.Steinman MA, Shlipak MG, McPhee SJ. Of principles and pens: attitudes and practices of medicine housestaff toward pharmaceutical industry promotions. Am J Med. 2001;110:551–7. doi: 10.1016/s0002-9343(01)00660-x. [DOI] [PubMed] [Google Scholar]
- 31.Ferguson RP, Rhim E, Belizaire W, Egede L, Carter K, Lansdale T. Encounters with pharmaceutical sales representatives among practicing internists. Am J Med. 1999;107:149–52. doi: 10.1016/s0002-9343(99)00192-8. [DOI] [PubMed] [Google Scholar]
- 32.Razack S, Arbour L, Hutcheon R. Proposed model for interaction between residents and residency training programs, and pharmaceutical industry. Ann R Coll Physicians Surg Can. 1999;32:93–6. [PubMed] [Google Scholar]
- 33.Brewer D. The effect of drug sampling policies on residents' prescribing. Fam Med. 1998;30:482–6. [PubMed] [Google Scholar]
- 34.Gibbons RV, Landry FJ, Blouch DL, et al. A comparison of physicians' and patients' attitudes toward pharmaceutical industry gifts. J Gen Intern Med. 1998;13:151–4. doi: 10.1046/j.1525-1497.1998.00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahood S, Zagozeski C, Bradel T, Lawrence K. Pharmaceutical policies in Canadian family medicine training. Survey of residency programs. Can Fam Physician. 1997;43:1947–51. [PMC free article] [PubMed] [Google Scholar]
- 36.Sandberg WS, Carlos R, Sandberg EH, Roizen MF. The effect of educational gifts from pharmaceutical firms on medical students' recall of company names or products. Acad Med. 1997;72:916–8. [PubMed] [Google Scholar]
- 37.Shaughnessy AF, Bucci KK. Drug samples and family practice residents. Ann Pharmacother. 1997;31:1296–300. doi: 10.1177/106002809703101103. [DOI] [PubMed] [Google Scholar]
- 38.Sergeant MD, Hodgetts PG, Godwin M, Walker DM, McHenry P. Interactions with the pharmaceutical industry: a survey of family medicine residents in Ontario. Can Med Assoc J. 1996;155:1243–8. [PMC free article] [PubMed] [Google Scholar]
- 39.Spingarn RW, Berlin JA, Strom BL. When pharmaceutical manufacturers' employees present grand rounds, what do residents remember? Acad Med. 1996;71:86–8. doi: 10.1097/00001888-199601000-00022. [DOI] [PubMed] [Google Scholar]
- 40.Stryer D, Bero LA. Characteristics of materials distributed by drug companies. An evaluation of appropriateness. J Gen Intern Med. 1996;11:575–83. doi: 10.1007/BF02599024. [DOI] [PubMed] [Google Scholar]
- 41.Hodges B. Interactions with the pharmaceutical industry: experiences and attitudes of psychiatry residents, interns and clerks. Can Med Assoc J. 1995;153:553–9. [PMC free article] [PubMed] [Google Scholar]
- 42.Ziegler MG, Lew P, Singer BC. The accuracy of drug information from pharmaceutical sales representatives. JAMA. 1995;273:1296–8. [PubMed] [Google Scholar]
- 43.Johnstone RE, Valenzuela RC, Sullivan D. Managing pharmaceutical sales activities in an academic anesthesiology department. J Clin Anesth. 1995;7:544–8. doi: 10.1016/0952-8180(95)00101-m. [DOI] [PubMed] [Google Scholar]
- 44.Reeder M, Dougherty J, White LJ. Pharmaceutical representatives and emergency medicine residents: a national survey. Ann Emerg Med. 1993;22:1593–6. doi: 10.1016/s0196-0644(05)81266-1. [DOI] [PubMed] [Google Scholar]
- 45.Keim SM, Sanders AB, Witzke DB, Dyne P, Fulginiti JW. Beliefs and practices of emergency medicine faculty and residents regarding professional interactions with the biomedical industry. Ann Emerg Med. 1993;22:1576–81. doi: 10.1016/s0196-0644(05)81262-4. [DOI] [PubMed] [Google Scholar]
- 46.Brotzman GL, Mark DH. The effect on resident attitudes of regulatory policies regarding pharmaceutical representative activities. J Gen Intern Med. 1993;8:130–4. doi: 10.1007/BF02599757. [DOI] [PubMed] [Google Scholar]
- 47.Lichstein PR, Turner RC, O'Brien K. Impact of pharmaceutical company representatives on internal medicine residency programs. A survey of residency program directors. Arch Intern Med. 1992;152:1009–13. [PubMed] [Google Scholar]
- 48.Banks JW, III, Mainous AG., III Attitudes of medical school faculty toward gifts from the pharmaceutical industry. Acad Med. 1992;67:610–2. doi: 10.1097/00001888-199209000-00017. [DOI] [PubMed] [Google Scholar]
- 49.Brotzman GL, Mark DH. Policies regulating the activities of pharmaceutical representatives in residency programs. J Fam Pract. 1992;34:54–7. [PubMed] [Google Scholar]
- 50.Bucci KK, Frey KA. Involvement of pharmacy faculty in the development of policies for pharmaceutical sales representatives. J Fam Pract. 1992;34:49–52. [PubMed] [Google Scholar]
- 51.Morelli D, Koenigsberg MR. Sample medication dispensing in a residency practice. J Fam Pract. 1992;34:42–8. [PubMed] [Google Scholar]
- 52.McKinney WP, Schiedermayer DL, Lurie N, Simpson DE, Goodman JL, Rich EC. Attitudes of internal medicine faculty and residents toward professional interaction with pharmaceutical sales representatives. JAMA. 1990;264:1693–7. [PubMed] [Google Scholar]
- 53.Lurie N, Rich EC, Simpson DE, et al. Pharmaceutical representatives in academic medical centers: interaction with faculty and housestaff. J Gen Intern Med. 1990;5:240–3. doi: 10.1007/BF02600542. [DOI] [PubMed] [Google Scholar]
- 54.Bero LA, Grilli R, Grimshaw JM, Harvey E, Oxman AD, Thomson MA. Closing the gap between research and practice: an overview of systematic reviews of interventions to promote the implementation of research findings. The Cochrane Effective Practice and Organization of Care Review Group. BMJ. 1998;317:465–8. doi: 10.1136/bmj.317.7156.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davis DA, Taylor-Vaisey A. Translating guidelines into practice. A systematic review of theoretic concepts, practical experience and research evidence in the adoption of clinical practice guidelines. Can Med Assoc J. 1997;157:408–16. [PMC free article] [PubMed] [Google Scholar]
- 56.Oxman AD, Thomson MA, Davis DA, Haynes RB. No magic bullets: a systematic review of 102 trials of interventions to improve professional practice. Can Med Assoc J. 1995;153:1423–31. [PMC free article] [PubMed] [Google Scholar]
- 57.Hayden SR, Dufel S, Shih R. Definitions and competencies for practice-based learning and improvement. Acad Emerg Med. 2002;9:1242–8. doi: 10.1111/j.1553-2712.2002.tb01584.x. [DOI] [PubMed] [Google Scholar]
- 58.Larkin GL, Binder L, Houry D, Adams J. Defining and evaluating professionalism: a core competency for graduate emergency medicine education. Acad Emerg Med. 2002;9:1249–56. doi: 10.1111/j.1553-2712.2002.tb01585.x. [DOI] [PubMed] [Google Scholar]
- 59.Development of residency program guidelines for interaction with the pharmaceutical industry. Education Council, Residency Training Programme in Internal Medicine, Department of Medicine, McMaster University, Hamilton. Ont. Can Med Assoc J. 1993;149:405–8. [PMC free article] [PubMed] [Google Scholar]


