Abstract
Background
Although vaginitis is a common outpatient problem, only 60% of patients can be diagnosed at the initial office visit of a primary care provider using the office procedures of pH testing, whiff tests, normal saline, and potassium hydroxide preps.
Objective
To determine the most cost-effective diagnostic and treatment approach for the medical management of vaginitis.
Design
Decision and cost-effectiveness analyses.
Participants
Healthy women with symptoms of vaginitis undiagnosed after an initial pelvic exam, wet mount preparations, pH, and the four criteria to diagnose bacterial vaginosis.
Setting
General office practice.
Methods
We evaluated 28 diagnostic strategies comprised of combinations of pH testing, vaginal cultures for yeast and Trichomonas vaginalis, Gram's stain for bacterial vaginosis, and DNA probes for Neisseria gonorrhoeae and Chlamydia. Data sources for the study were confined to English language literature.
Measurement
The outcome measures were symptom-days and costs.
Results
The least expensive strategy was to perform yeast culture, gonorrhoeae and Chlamydia probes at the initial visit, and Gram's stain and Trichomonas culture only when the vaginal pH exceeded 4.9 ($330, 7.30 symptom days). Other strategies cost $8 to $76 more and increased duration of symptoms by up to 1.3 days. In probabilistic sensitivity analysis, this strategy was always the most effective strategy and was also least expensive 58% of the time.
Conclusions
For patients with vaginitis symptoms undiagnosed by pelvic examination, wet mount preparations and related office tests, a comprehensive, pH-guided testing strategy at the initial office visit is less expensive and more effective than ordering tests sequentially.
Keywords: vaginitis, diagnosis, treatment, cost-effectiveness, decision analysis
Vaginitis is one of the 25 most common medical reasons for consulting a physician in the United States,1 resulting in 5 to 10 million office visits per year.2 Although they encounter vaginitis frequently, primary care practitioners have difficulty making an etiologic diagnosis in the office setting.3–9 Specialized centers report diagnosing 80% to 90% of women from the initial visit,10 but primary care providers report only a 50% to 60% chance of reaching a diagnosis despite extensive laboratory testing,4 because the typical practitioner lacks the office resources and expertise found in specialized centers.
The cost of diagnosis in patients presenting with vaginitis symptoms can vary dramatically depending on the amount of testing conducted at the initial visit. Most experts agree that diagnosis should begin with a complete pelvic examination, determining the source of the discharge (cervical or vaginal), gross evaluation of the discharge for consistency, and adherence to the vaginal walls or cervix; followed by wet mount preparations with saline looking for clue cells, white blood cells, and Trichomonas vaginalis; potassium hydroxide (KOH) testing for yeast and the whiff test; and pH testing.11 If these are nondiagnostic, however, should the practitioner: 1) perform extensive testing at the initial visit, or 2) limit initial testing to simple, inexpensive bacteriologic tests to reduce costs and burden to the patient, realizing that more patients will require follow-up visits and testing to reach a definitive diagnosis? We performed decision and cost-effectiveness analyses to compare these strategies for patients who present with symptoms of vaginitis, but who remain undiagnosed after initial office-based evaluation.
METHODS
We constructed a decision tree using a standard computer program (Decision Maker 7.06, Pratt Medical Group, Boston) and data derived from relevant peer-reviewed articles in the English language. We then analyzed the outcomes of 28 different office-based diagnostic strategies for the medical management of vaginitis. We considered a population of healthy women who present with vaginal discharge, pruritus, irritation or odor who could not be diagnosed by initial office evaluation consisting of pH; wet mount (KOH and normal saline [NS]) preparations for Candida species, Trichomonas vaginalis, and mucopurulent discharge; and the four criteria to diagnose bacterial vaginosis (thin, homogeneous discharge; pH>4.5; clue cells and a positive whiff test). We assumed that practitioners would not perform Gram's stain in the office. Pregnant patients and those who used over-the-counter treatment for vaginitis were excluded.
Diagnosis and Treatment
The possible etiologies of vaginitis are yeast, Trichomonas vaginalis, and bacterial vaginosis. Cervicitis caused by Chlamydia trachomatisNeisseria gonorrhoeae can also mimic vaginitis. Herpes infection can present similarly to vaginitis. Other etiologies are less common (Table 1). For simplicity, we assumed that each patient would have only one causative organism.
Table 1.
Probabilities That Specific Etiological Agents Cause Vaginitis
| Etiologic Agents | Base* Case | Low† | High† | References |
|---|---|---|---|---|
| Candida species | .25 | .20 | .33 | 2,10,11 |
| Bacterial vaginosis | .35 | .28 | .50 | 7,10,11 |
| Trichomonas vaginalis | .15 | .10 | .20 | 2,10 |
| Chlamydia trachomatis | .05 | .02 | .07 | 5,7,12,13 |
| Neisseria gonorrhoeae | .02 | 0 | .02 | 5,12,13 |
| Herpes | .02 | .01 | .02 | 7,12 |
| Other | .16 |
Base case is the estimate used in the model, derived from the literature, cited in column 5
“Low”and“High” refer to low and high ranges of the probabilities, derived from the literature, cited in column 5.
We considered initial diagnostic strategies that incorporated the following tests, either alone or in combination: vaginal pH, vaginal cultures for Candida and Trichomonas, Gram's stain for bacterial vaginosis (BV), and DNA probes for N. gonorrhoeae and Chlamydia (GC/Chlamydia probes). We assumed that pH test results would be available during the examination and could be used to guide further testing at the visit with a normal pH excluding BV, trichomonas, and atrophic vaginitis. We estimated that all other test results besides the initial office evaluations would take two days. Specific treatments were based on Centers for disease Control and Prevention (CDC) guidelines (Table 2). We also considered 2 empirical treatment strategies: 1) treatment guided by vaginal pH (treatment with single dose fluconazole for Candida when the pH is less than 4.9, or treatment with 2 g of metronidazole to cover Trichomonas and/or BV when the pH is greater than 4.9) or 2) treatment with both fluconazole and metronidazole.
Table 2.
Treatment Regimens for Vaginitis
| Etiological Agents and Treatments | Course | Side Effects (%) | Average Wholesale Price ($) | Cure Rate (%) |
|---|---|---|---|---|
| Candida species | ||||
| Fluconazole | 150 mg PO × 1 | 10 (5 to 13) | 11.89 | 85 (72 to 93)*14–16 |
| Terconazole,† 0.8% cream | HS × 3 nights | 10 (5 to 18) | 30.96 | 84 (80 to 94)*16,17 |
| Bacterial vaginosis | ||||
| Metronidazole | 500 mg PO BID × 7 days | 10 (10 to 15) | 3.36 | 80 to 92*18 |
| Metronidazole | 2 g PO × 1 | 7 (5 to 10) | 0.48 | 70 to 87*18 |
| Trichomonas vaginalis | ||||
| Metronidazole | 2 g PO × 1 | 7 (5 to 10) | 0.48 | 90 (82 to 93)*10,18 |
| Metronidazole | 500 mg PO BID × 7 days | 10 (10 to 15) | 3.36 | 93 (90 to 95)*10,18 |
| Cervicitis | ||||
| Ceftriaxone | 250 mg IM | 10 (8 to 22) | 8.82 | 98 (95 to 98)*18 |
| Doxycycline | 100 mg PO BID × 7 days | 10 (8 to 15) | 1.68 | 95 (90 to 98)*18 |
| Azithromycin | 2 g PO × 1 | 7 (5 to 10) | 20.98 | 98 (96 to 98)*18 |
References course of treatment and its cure rate
Terconazole was chosen for second treatment of vaginitis as it covers Candida glabrata and Candida tropicalis in addition to Candida albicans.
PO, per os; IM, intramascular; BID, twice daily; HS, nightly.
Further Evaluation
Patients who responded to initial treatment were considered cured. We assumed that patients who failed the initial therapy would receive the prescription for the second therapy by telephone, but those patients who failed empiric therapy or who were undiagnosed after the initial round of tests would return for an office visit and undergo all previously unordered tests. We estimated that symptoms from causes of vaginitis other than those modeled would resolve 20% of the time without treatment before a second visit.
Referral to Specialists
The model presumed that patients not responding to two courses of therapy and those who were undiagnosed despite a complete battery of tests would be referred to an infectious disease or gynecology specialist, who would repeat all tests and treat all diagnosable patients appropriately. For patients with symptoms due to causes other than those modeled, we estimated that specialists could successfully treat half.
Adverse Outcomes
The probability of treatment side effects appears in Table 2. For simplicity, we assumed that side effects would last 2 days and be equal in severity to the vaginitis symptoms.
Outcome Measures
The model summed the costs of all diagnostic tests, office visits, and referrals. Effectiveness was expressed as change in symptom days. We assumed that all vaginitis symptoms would be of equal severity regardless of etiology, would persist until properly treated, and would disappear on the third day of successful treatment.12
Sensitivity Considerations
All quantitative assumptions were subject to one-way sensitivity analysis to discern their relative impact on the cost-effectiveness of different strategies. We also conducted a probabilistic analysis in which we varied all inputs simultaneously to determine confidence intervals for the results. We performed 1,000 Monte Carlo simulations, each time choosing random values from within each variable's 95% confidence interval using logit distributions.
Data and Estimates
Etiologic Agents
Yeast causes 20% to 33% of vaginitis symptoms2,10,11 (Table 1), BV 28% to 50%7,10,11, Trichomonas 10% to 20%2,10,11 and cervicitis 2% to 7%.5,7,13,19 Initial office evaluation correctly diagnoses Candida species 60% of the time 10,11 (Table 3), Trichomonas 70% of the time,2,8,10,11,23,24 GC or Chlamydia 30% of the time,25 and BV 90% of the time.3,8,10,11 Using these data, we calculated the conditional probability of each of these etiologies given a negative initial office evaluation, as well as the probability of each diagnosis depending on vaginal pH (Table 4). For simplicity, we assumed all subsequent tests were conditionally independent and 100% specific.
Table 3.
Test Characteristics of Diagnostics Tests Used in Vaginitis
| Base* Case | Low† | High† | References | |
|---|---|---|---|---|
| Sensitivity of wet mount in | ||||
| Candida species | .60 | .40 | .80 | 10,11,20,21 |
| Bacterial vaginosis | .90 | .80 | .95 | 3,8,10,11,22 |
| Trichomonas vaginalis | .67 | .40 | .80 | 2,8,10,11,20,23 |
| Chlamydia trachomatis | .30 | .18 | .42 | 25 |
| Neisseria gonorrhoeae | .30 | .18 | .42 | 25 |
| Other | 0 | 0 | 0 | Expert assumption |
| Sensitivity of | ||||
| Candida culture | .95 | .95 | .95 | 11 |
| Gram's stain | .95 | .93 | .95 | 8,10,11,22,26 |
| Trichomonas vaginalis culture | .95 | .89 | .95 | 8,10,11 |
| DNA probe (GC) | .90 | .90 | 1 | 27–30 |
| DNA probe (Chlamydia) | .90 | .90 | 1 | 12,19,31,33 |
| Probability of pH>4.9 in | ||||
| Candida species | .29 | .25 | .34 | 7,11 |
| Bacterial vaginosis | 1 | 1 | 1 | 7,11 |
| Trichomonas vaginalis | 1 | 1 | 1 | 7,11 |
| Chlamydia trachomatis | .67 | .60 | .74 | 7 |
| Neisseria gonorrhoeae | .67 | .60 | .74 | Expert assumption |
| Other | .67 | .60 | .74 | 7 |
Base case is the estimate used in the model, derived from the literature, cited in column 5.
“Low” and “High” refer to low and high ranges of the probabilities, derived from the literature, cited in column 5.
Table 4.
Prevalence of Etiological Agents After Negative Wet Prep and pH Testing
| Etiological Agents | Prevalence in General Practice* | Calculated Prevalence After Negative Wet Prep | Calculated Prevalence if pH Is >4.9 | Calculated Prevalence if pH Is <4.9 |
|---|---|---|---|---|
| Candida species | .25 | .21 | .09 | .44 |
| Bacterial vaginosis | .35 | .09 | .13 | .00 |
| Trichomonas vaginalis | .15 | .13 | .19 | .00 |
| Chlamydia trachomatis | .05 | .09 | .09 | .09 |
| Neisseria gonorrhoeae | .02 | .04 | .04 | .03 |
| Other | .18 | .45 | .46 | .44 |
See references in Table 2, column 5.
Treatment Efficacy and Side Effects
We based our estimates of the efficacy and side effects of treatments on data from randomized clinical trials (Table 2). We assumed vaginal creams would cause contact dermatitis in 10% of patients, and that fluconazole would cause gastrointestinal symptoms in the same proportion.14–16 Metronidazole causes secondary yeast infection and gastrointestinal symptoms in 7% to 10% of patients, depending on duration of treatment.18
Costs
All costs were in US dollars for the year 2003 and assumed the societal perspective (Table 5). We included all direct medical costs combining the costs of diagnostic testing, physician visits and prescription medications, as well as indirect costs from lost productivity during physician visits. Costs for diagnostic tests and physician visits were based on the 2003 Medicare Fee Schedule. Drug costs reflect average wholesale prices. Labor costs were based on US average employee compensation for 2003.34
Table 5.
Costs
| Test | Cost ($)* |
|---|---|
| Wet mount preparation | 8.06 |
| Gram Stain | 8.06 |
| Vaginal culture (Candida species) | 15.86 |
| Trichomonas vaginalis culture | 17.86 |
| Neisseria gonorrhoeae DNA probe | 37.86 |
| Chlamydia DNA probe | 37.86 |
| Herpes DNA amplification probe† | 66.27 |
| Human papillomavirus testing† | 66.27 |
| Physician office visit | 50.32 |
| One hour of patient time for physician visit | 24.48 |
| Specialist consultation | 164.34 |
Laboratory and physician costs are from the Medicare Fee Schedule. Indirect costs from lost productivity come from the US Bureau of Labor Statistics
As part of specialist's evaluation.
RESULTS
Prevalence of Disease
Table 1 shows the prevalence of the common causes of vaginitis in patients presenting to practitioners. Using the sensitivity of office evaluation described above for each of the causes, the model calculated the prevalence of each cause in the subset of patients with non-diagnostic office wet mount preparations, both overall and based on vaginal pH (Table 4). Regardless of pH, about 45% of these patients have a diagnosis of “other” which cannot be determined by common office tests.
Diagnostic Strategies
Table 6 lists all 28 diagnostic strategies with the associated average costs and mean symptom days, as determined by the model. The least expensive diagnostic strategy was the most comprehensive: begin with pH testing, yeast cultures and DNA probes for gonorrhoeae and Chlamydia for all patients, but perform Gram's stain and Trichomonas cultures only when vaginal pH exceeded 4.9 ($330, 7.30 symptom days). Other strategies increased average costs by $5 to $81 per patient, and increased duration of symptoms by up to 1.3 days. In general, diagnostic strategies which entailed fewer tests during the initial visit, especially those not testing for yeast, resulted in higher costs because of the greater number of follow-up office visits and the high cost of referral.
Table 6.
Average Cost and Utility of 28 Strategies for Initial Evaluation of Vaginitis Symptoms
| Strategy* | Average Cost ($) | Mean Symptom Days | Incremental Cost†($) | Incremental Symptom Days‡ | Incremental Cost-Effectiveness $/Symptom Day Avoided§ |
|---|---|---|---|---|---|
| YSBTp | 330 | 7.30 | |||
| YBTp | 335 | 7.59 | 5 | 0.29 | Dominated |
| YSBT | 337 | 7.30 | 8 | 0.01 | Dominated |
| YBT | 338 | 7.59 | 9 | 0.29 | Dominated |
| YTp | 351 | 7.87 | 22 | 0.57 | Dominated |
| YT | 353 | 7.87 | 24 | 0.57 | Dominated |
| YBp | 354 | 7.92 | 24 | 0.62 | Dominated |
| YB | 354 | 7.92 | 25 | 0.62 | Dominated |
| YSBp | 358 | 7.68 | 29 | 0.38 | Dominated |
| YSp | 370 | 7.96 | 41 | 0.66 | Dominated |
| Y | 372 | 8.20 | 42 | 0.90 | Dominated |
| YSB | 372 | 7.70 | 42 | 0.40 | Dominated |
| Yp | 373 | 8.20 | 43 | 0.90 | Dominated |
| BT | 374 | 8.19 | 44 | 0.89 | Dominated |
| BTp | 374 | 8.19 | 45 | 0.89 | Dominated |
| YS | 382 | 7.98 | 53 | 0.68 | Dominated |
| SBTp | 385 | 7.97 | 56 | 0.67 | Dominated |
| SBT | 386 | 7.97 | 57 | 0.67 | Dominated |
| T | 392 | 8.47 | 62 | 1.17 | Dominated |
| Tp | 393 | 8.47 | 63 | 1.17 | Dominated |
| B | 395 | 8.53 | 66 | 1.23 | Dominated |
| Bp | 396 | 8.53 | 67 | 1.23 | Dominated |
| SB | 397 | 8.31 | 67 | 1.01 | Dominated |
| STp | 397 | 8.25 | 67 | 0.95 | Dominated |
| ST | 397 | 8.25 | 67 | 0.95 | Dominated |
| SBp | 397 | 8.31 | 67 | 1.01 | Dominated |
| S | 410 | 8.58 | 80 | 1.28 | Dominated |
| Sp | 411 | 8.58 | 81 | 1.28 | Dominated |
Y, vaginal culture for Candida species; S, sexually transmitted disease testing including probes for Neisseria gonorrhoeae and Chlamydia; B, Gram stain for bacterial vaginosis T, vaginal culture for Trichomonas vaginalis; p, vaginal pH testing.
Incremental costs represent the difference between the strategy and the next best nondominated strategy.
Incremental symptom days represents the difference between the strategy and the next best nondominated strategy.
The difference in cost divided by the difference in quality-adjusted life expectancy for each strategy compared with the next best nondominated strategy.
A dominated strategy costs more and is less effective than another available strategy.
Empiric Treatment
Compared to testing strategies, empiric treatment strategies resulted in fewer referrals (40% vs 41% to 46%), but more adverse effects (11% to 19% vs 6%). Diagnostic testing followed by pH-guided empirical therapy while awaiting test results was superior to both empirical treatment and testing alone. Depending on the testing strategy, the savings associated with adding empirical treatment while awaiting test results ranged from $8 to $63 (mean savings $39), and decreased symptom duration by 0.6 and 1.3 days, even after accounting for additional side effects and costs related to empirical treatment. Empirical treatment while awaiting test results was beneficial even if the side effects of the medications were three times as severe as the vaginitis itself.
Sensitivity Analyses
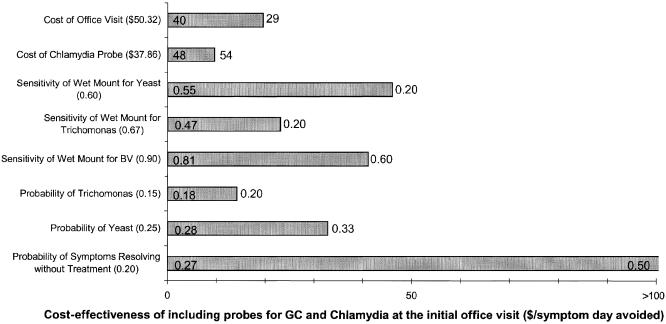
Strategies including empiric treatment and those without were analyzed separately. Results were similar. Despite the small differences between the strategies in cost and effectiveness, the model was robust with respect to the preferred strategies throughout the ranges tested. Under most circumstances, comprehensive testing guided by pH at the initial visit was least expensive. Under certain circumstances, however, ordering GC/Chlamydia probes at the initial visit was expensive (Fig. 1).
FIGURE 1.
One-way sensitivity analysis. Bars show the range of incremental cost-effectiveness of adding Neisseria gonorrhoeae and Chlamydia probes to yeast culture and pH guided testing for bacterial vaginosis and Trichomonas. Baseline values for each variable are shown in parenthesis. Left-hand numbers represent the threshold at which GC/Chlamydia probes become cost-saving. Right-hand numbers represent the upper bound in sensitivity analysis.
Initial GC/Chlamydia testing was least cost-effective when the sensitivities of the initial office evaluations to detect Yeast, BV or Trichomonas were low. As a result, these entities would be relatively more prevalent and GC and Chlamydia less prevalent. One benefit of GC/Chlamydia testing at the initial visit is avoidance of future visits. However, if the cost of an office visit is low, or undiagnosable patients get better without treatment, initial GC/Chlamydia probes are less cost-effective.
Under some circumstances checking pH resulted in a worse outcome. The cost-effectiveness of pH testing relies on the ability of low pH to rule out BV and Trichomonas. BV by definition has a high pH and studies of Trichomonas show that it uniformly causes a high pH. However, if the sensitivity of pH testing for Trichomonas is less than 85%, pH testing should not be done, because false negative tests will lead to unacceptable delay in the diagnosis of Trichomonas.
Results of Probabilistic Sensitivity Analysis
In 1000 Monte Carlo simulations, only two diagnostic strategies were ever cost-effective. Testing for yeast, BV and Trichomonas without GC/Chlamydia probes was the least expensive strategy 42% of the time. Comprehensive testing including GC/Chlamydia probes was always the most effective strategy, and was also the least expensive strategy in 58% of the iterations.
DISCUSSION
Understanding the monetary costs and clinical outcomes of managing common medical problems is increasingly necessary for health care providers and to society.35–37 Unfortunately, the costs and benefits of alternative diagnostic approaches are not always apparent. In medicine, the traditional approach to diagnosis includes a history and physical examination, followed by the development of a differential diagnosis and the performance of serial testing to arrive at the most likely diagnosis. This approach is particularly appealing when tests are potentially harmful, have a high false positive rate, or are expensive.38 The “shotgun” approach, where the clinician orders every possible test during the initial contact, is considered wasteful at best, and potentially harmful, in that it can increase the likelihood of test complications and diagnostic confusion.39 However, in the current healthcare environment, the cost of tests may be small in comparison to the costs of additional office visits, referrals, or emergency department visits resulting from delayed diagnosis.
Symptoms of vaginitis are common in medical practice. The history and physical findings are seldom helpful in diagnosing the etiology of vaginitis symptoms, including the character of the discharge or the presence of an odor.4 While a majority of patients can be diagnosed with simple wet mount preparations and related initial office tests, a substantial number remain undiagnosed. The cost of obtaining a diagnosis for these patients is high, at least $330 (Table 6), more than 6 times the average cost of the office visit ($50.32) itself.
The task of choosing a diagnostic strategy is daunting. The clinician must choose from among five tests, each with a different sensitivity, specificity, and cost, then consider empirical treatment while awaiting test results. The clinician must consider the three common causes of vaginitis, each with a different prevalence, as well as a number of less common causes of these symptoms, such as gonorrhoeae, and Chlamydia. Ordering every potentially useful test at the initial visit may result in the quickest diagnosis, but is it a reasonable use of resources? Our analysis suggests that immediate testing for all diagnostic possibilities—except BV and trichomonas when vaginal pH is normal—is not only reasonable, but the least expensive clinical strategy, considering the cost of follow-up visits and referrals.
Empirical treatment with fluconazole, metronidazole or both in place of testing was not cost-effective, because the majority of patients would not be cured and many would incur unnecessary treatment with attendant side effects, delayed diagnosis and associated costs. By contrast, adding pH-guided empiric therapy to any testing strategy while waiting for culture results both shortened symptom duration and decreased cost. Patients treated empirically by pH had immediate relief, without having to wait for culture results, and if cured, did not have to return for further office visits.
Patients who are at high risk of developing complications from vaginal infections, such as pregnant women or women who are scheduled for an abdominal or vaginal procedure require accurate, diagnostically guided treatment. Empiric treatment would be inappropriate for these women since partial treatment may interfere with interpretation of subsequent tests.
There are a number of limitations to our study. Some results may not be generalizable to all medical practices. For example, the prevalence of disease among patients with negative initial office tests is unknown. For our model, we calculated this probability using the prevalence of each of these etiologies of vaginitis symptoms and the known sensitivity of the initial office tests. In individual practices, however, these results will vary depending upon the prevalence of these etiologies in the specific community and the clinician's skill in interpreting the initial office tests. For example, if a clinician does not easily identify clue cells, undiagnosed patients will have a higher prevalence of BV, and a lower prevalence of GC and Chlamydia, making GC/Chlamydia probes less cost-effective for that clinician. Alternatively, if the patient is at high risk for sexually transmitted diseases, or if the practitioner has difficulty distinguishing between the discharge of cervicitis and that of vaginitis, GC and Chlamydia probes will invariably be cost-saving.
Our study was also limited by available data. Although 45% of the patients in our model could not be diagnosed by simple tests available to the average clinician in office practice, it is unclear how much medical intervention helps these women. We tested these variables in the sensitivity analysis and found that if symptoms resolved in more than one-third of these women without treatment, then testing should be deferred to the follow-up visit, as many women would improve before a follow-up appointment. Because these women constitute such a large percentage of vaginitis patients, more studies are needed to characterize the etiology and prognosis of this condition. For simplicity, we also assumed that each case of vaginitis was caused by a single organism. In reality, concurrent diagnoses in genital tract infections are frequent. This fact only strengthens the argument for broad testing on the initial visit rather than testing for, and then treating, one entity at a time.
There are a number of strengths to our study. A decision analysis allowed us to study this complex problem when doing so in a randomized clinical trial would have been impractical. A second strength is the inclusive nature of our study, which makes the results broadly applicable.
While the cost differences among the diagnostic strategies are modest (no more than $81 per case, on average), the high prevalence of vaginitis makes these differences expensive in aggregate. Choosing one diagnostic strategy over another could result in savings of tens of millions of dollars annually on a national basis. Similarly, 1.3 symptom-days may seem trivial, but when multiplied by one million patients it represents 3,600 patient-years of vaginitis.
CONCLUSION
Vaginitis is common, yet often difficult for primary care practitioners to diagnose effectively in the office setting. Our study suggests that considerable savings and decreased symptoms can be achieved by using vaginal pH to guide testing and treatment at the initial office visit for those patients who are undiagnosed after a complete pelvic examination, evaluation of the discharge, whiff test, pH and wet mount preparations. Under most circumstances, testing for GC and Chlamydia will also improve outcomes and save money.
REFERENCES
- 1.Paavonen J, Stamm WE. Lower genital tract infections in women. Infect Dis Clin North Am. 1987;1:179. [PubMed] [Google Scholar]
- 2.Sparks JM. Vaginitis. J Repro Med. 1991;36:745–52. [PubMed] [Google Scholar]
- 3.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schaaf VM, Perez-Stable EJ, Borchardt K. The limited value of symptoms and signs in the diagnosis of vaginal infections. Arch Intern Med. 1990;150:1929–33. [PubMed] [Google Scholar]
- 5.Berg AO, Heidrich FE, Fihn SD, et al. Establishing the cause of genitourinary symptoms in women in a family practice. JAMA. 1984;251:620–5. [PubMed] [Google Scholar]
- 6.Carr PL, Thabault P, Levenson S, Friedman RH. Vaginitis in a community based practice. Clin Res. 1992;40:554A. [Google Scholar]
- 7.Bleker OP, Folkertsma K, Dirks-Go SIS. Diagnostic procedures in vaginitis. Eur J Obstet Gynec Reprod Biol. 1989;31:179–83. doi: 10.1016/0028-2243(89)90179-2. [DOI] [PubMed] [Google Scholar]
- 8.Eschenbach DA, Hillier SL. Advances in diagnostic testing for vaginitis and cervicitis. J Reprod Med. 1989;34:555–65. [PubMed] [Google Scholar]
- 9.Anderson MR, Klink K, Cohrssen A. Evaluation of vaginal complaints. JAMA. 2004;291:1368–79. doi: 10.1001/jama.291.11.1368. [DOI] [PubMed] [Google Scholar]
- 10.Sobel JD. Vaginitis in adult women. Obs Gyn Clin N Am. 1990;17:851–79. [PubMed] [Google Scholar]
- 11.Sobel JD. Vaginitis. N Engl J Med. 1997;337:1896–1903. doi: 10.1056/NEJM199712253372607. [DOI] [PubMed] [Google Scholar]
- 12.Holmes KK, et al. Lower genital tract infections in women: cystitis/urethritis vulvovaginitis and cervicitis. In: Holmes KK, Sparling PF, Mardh P, et al., editors. Sexually Transmitted Diseases. New York: McGraw-Hill; 1999. pp. 527–45. [Google Scholar]
- 13.Gratton CA, Lim-fong R, Prasad E, Kibsey PC. Comparison of a DNA probe with culture for detecting Chlamydia trachomatis directly from genital specimens. Mol Cell Probes. 1990;4:25–31. doi: 10.1016/0890-8508(90)90036-y. [DOI] [PubMed] [Google Scholar]
- 14.Sobel JD, Brooker D, Stein GE, et al. Single oral dose fluconazole compared with conventional clotrimazole topical therapy of Candida vaginitis. Am J Obstet Gynecol. 1995;172:1263–8. doi: 10.1016/0002-9378(95)91490-0. [DOI] [PubMed] [Google Scholar]
- 15.Oral fluconazole for vaginal candidiasis. Med Lett. 1994;36:81–2. [PubMed] [Google Scholar]
- 16.Reef SE, Levine WC, McNeil MM, et al. Treatment options for vulvovaginal candidiasis, 1993. Clin Infec. Dis. 1995;20(suppl 1):S80–90. doi: 10.1093/clinids/20.supplement_1.s80. [DOI] [PubMed] [Google Scholar]
- 17.Clark C, Cooper CL, Gordon SF, et al. A multicenter comparison of one-dose tioconazole ointment with three–dose terconazole cream in vulvovaginal candidiasis. J Women' Health. 1993;2:189–96. [Google Scholar]
- 18.Centers for Disease Control and Prevention. Guidelines for treatment of sexually transmitted disease. MMWR. 1993;42:171. [Google Scholar]
- 19.Iwen PC, Blair TM, Woods GL. Comparison of the Gen-Probe PACE 2 system, direct fluorescent–antibody, and cell culture for detecting Chlamydia trachomatis in cervical specimens. Am M Clin Pathol. 1991;95:578–82. doi: 10.1093/ajcp/95.4.578. [DOI] [PubMed] [Google Scholar]
- 20.Sexual transmitted disease guidelines, CDC. MMWR. 1993;42:171. [Google Scholar]
- 21.Bertholf MZ, Stafford MJ. Colonization of Candida albicans in vagina, rectum and mouth. J Fam Pract. 1983;95:919–24. [PubMed] [Google Scholar]
- 22.Amsel R, Totten PA, Spiegel CA, et al. Nonspecific vaginitis: diagnostic criteria and microbial and epidemiological associations. Am J Med. 1983;74:14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 23.Krieger JN, Tam MR, Stevens CE, et al. Diagnosis of trichomoniasis: comparison of conventional wet mount preparation with cytological studies, cultures and monoclonal antibody staining of direct specimens. JAMA. 1988;259:1223. doi: 10.1001/jama.259.8.1223. [DOI] [PubMed] [Google Scholar]
- 24.Cotch MF, Pasterek JG, Nugent RP, Yerg DE, Martin DH, Eschenbach DA. Demographics and behavioral predictors of Trichomonas vaginitis infection among pregnant women. Obstet Gynecol. 1991;78:1087–92. [PubMed] [Google Scholar]
- 25.Landers DV, Weisenfeld HC, Heine RP, Krohn MA, Hillier SL. Predictive value of the clinical diagnosis of lower genital tract infection in women. Am J Obstet Gynecol. 2004;190:1004–10. doi: 10.1016/j.ajog.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 26.Spiegel SA, Amsel R, Holmes KK. Diagnosis of bacterial vaginosis by direct gram stain of vaginal fluid. J Clin Microbiol. 1983;18:170–77. doi: 10.1128/jcm.18.1.170-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewis JS, Kranig-Brown D, Trainor DA. DNA probe confirmatory test for Neisseria gonorrhosae. J Clin Microbiol. 1990;28:2349–50. doi: 10.1128/jcm.28.10.2349-2350.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwebke JR, Zajackowski ME. Comparison of DNA probe (Gen-Probc) with culture for the detection of Neisseria gonorrhoeae in an urban STD programme. Genitourin Med. 1996;72:108–10. doi: 10.1136/sti.72.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Granato PA, Franz MR. Evaluation of a prototype DNA probe test for the noncultural diagnosis of gonorrhea. J Clin Microbiol. 1989;27:632–5. doi: 10.1128/jcm.27.4.632-635.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szell A, Tisza T, Horvath A. A comparative study for detection of Chlamydia trachomatisNeisseria gonorrhoeae with DNA probe. Acta Microbiol Immunol Hung. 1994;41:291–3. [PubMed] [Google Scholar]
- 31.Loeffelholz MJ, Lewinski CA, Silver SR, et al. Detection of Chlamydia trachomatis in endocervical specimens by polymerase chain reaction. J Clin Microbiol. 1992;30:2847–51. doi: 10.1128/jcm.30.11.2847-2851.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lees MI, Newman DM, Garland SM. Comparison of a DNA probe assay with culture for the detection of Chlamydia trachomatis. J Med Microbiol. 1991;35:159–61. doi: 10.1099/00222615-35-3-159. [DOI] [PubMed] [Google Scholar]
- 33.Mercer LJ, Robinson DC, Sahm DF, Lawrie MJ, Hajj SN. Comparison of chemiluminescent NA probe to cell culture for the screening of Chlamydia trachomatis in a gynecology clinic population. Obstet Gynecol. 1990;76:114–7. [PubMed] [Google Scholar]
- 34.U.S. Bureau of Labor Statistics. Employer Cost for Employee Compensation. Accessed December 2, 2003 ( http://www.bls.gov/ncs/ect/home.htm#data.
- 35.Bloom BS, Wierz DJ, Pauly MV. Cost and price of comparable branded and generic pharmaceuticals. JAMA. 1986;256:2523–30. [PubMed] [Google Scholar]
- 36. 1993 Red Book. Montvale, NJ: Medical Economics Data Inc; 1993.
- 37.Edelson JT, Weinstein MC, Tosteson ANA, et al. Long-term cost-effectiveness of various initial monotherapies for mild to moderate hypertension. JAMA. 1990;263:407–13. [PubMed] [Google Scholar]
- 38.Pauker SG, Kassirer JP. The threshold approach to clinical decision making. N Engl J Med. 1986;302:1109–17. doi: 10.1056/NEJM198005153022003. [DOI] [PubMed] [Google Scholar]
- 39.Thibault GE. Diagnostic strategy—the shotgun versus the arrow. N Engl J Med. 1995;332:321–5. doi: 10.1056/NEJM199502023320509. [DOI] [PubMed] [Google Scholar]