Abstract
Purpose
Little is known about how well hospitalized patients can identify errors or injuries in their care. Accordingly, the purpose of this study was to elicit incident reports from hospital inpatients in order to identify and characterize adverse events and near-miss errors.
Subjects
We conducted a prospective cohort study of 228 adult inpatients on a medicine unit of a Boston teaching hospital.
Methods
Investigators reviewed medical records and interviewed patients during the hospitalization and by telephone 10 days after discharge about “problems,”“mistakes,” and “injuries” that occurred. Physician investigators classified patients' reports. We calculated event rates and used multivariable Poisson regression models to examine the factors associated with patient-reported events.
Results
Of 264 eligible patients, 228 (86%) agreed to participate and completed 528 interviews. Seventeen patients (8%) experienced 20 adverse events; 1 was serious. Eight patients (4%) experienced 13 near misses; 5 were serious or life threatening. Eleven (55%) of 20 adverse events and 4 (31%) of 13 near misses were documented in the medical record, but none were found in the hospital incident reporting system. Patients with 3 or more drug allergies were more likely to report errors compared with patients without drug allergies (incidence rate ratio 4.7, 95% CI 1.7, 13.4).
Conclusion
Inpatients can identify adverse events affecting their care. Many patient-identified events are not captured by the hospital incident reporting system or recorded in the medical record. Engaging hospitalized patients as partners in identifying medical errors and injuries is a potentially promising approach for enhancing patient safety.
Keywords: medical error, adverse event, patient participation, incident reporting
Can patients and their families identify errors and injuries that result from medical care? The answer may inform proposals that call for greater participation of patients and families in efforts to improve patient safety.1–5 If patients can identify errors and injuries, then they may be able to intercept the errors before injuries occur or to mitigate the duration or severity of harm.
Patients are potentially acute observers of their own care, and are highly motivated to ensure that correct treatments are correctly delivered.6–9 In consumer surveys, 12% to 42% of U.S. adults report having personally experienced a medical error or seen an error affect the health of a close friend or relative.10–12 In addition, epidemiologic studies of medication-related errors in primary care support the view that adult patients readily identify adverse drug events that are subsequently confirmed by investigators.13
Evidence for patients' ability to identify medical errors and injuries in the hospital is less compelling. Patients in acute care settings may be too ill or confused to participate meaningfully, or may be overwhelmed by the complexity and specialization of modern health care. The medical malpractice experience also argues against a sophisticated understanding of error on the part of hospitalized patients, as most inpatients who file claims have not experienced negligent care, and most cases of negligent care do not result in claims.14,15
In order to understand the role of patient participation in patient safety interventions, we studied adults admitted to a Boston teaching hospital. Our primary goal was to determine whether inpatients and their families could identify adverse events (defined as injuries because of medical care rather than the natural history of the illness) and near misses (defined as “close-call” errors with the potential for injury). Our secondary goals were to characterize patients' reports, to analyze the factors associated with error and injury reporting, and to compare patient reporting to the usual incident-reporting system. We hypothesized that patients would identify adverse events and near misses that affected their care, and that these events would differ from those reported in the hospital incident reporting system.
METHODS
Study Design
We conducted a prospective cohort study of adult inpatients admitted to a 40-bed medical unit at a Boston teaching hospital. The unit included general medical patients, a 10-bed acute-care geriatric program for community-dwelling elders, and overflow patients from oncology, surgery, and Obstetrics–Gynecology units. The hospital's institutional review board approved the study protocol.
Patient Eligibility and Enrollment
Patients admitted to the study unit from January through April 2003 and present on a weekday were potentially eligible to participate.
An investigator (O.P.) approached each patient on the first weekday morning following their admission, explained the purpose of the study, described the protocol, and requested written informed consent from the patient or, if the patient was unable to give consent, the patient's surrogate. Patients unavailable on the first weekday morning were approached on subsequent days. Spanish and Russian interpreters were available as needed for the 2 largest groups of non–English-speaking patients and documents were provided in these languages. Patients with multiple hospitalizations were enrolled at most once.
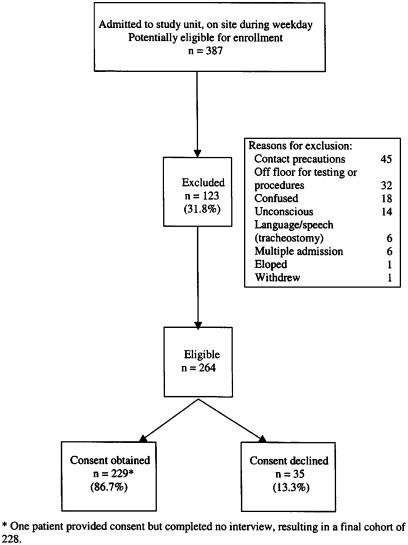
Of 387 potentially eligible patients, 123 (31.8%) could not participate and were excluded (Fig. 1). The only demographic difference between excluded and eligible patients was that fewer excluded patients were nonwhite (12.1% vs 20.2%, P=.04) and fewer had family or friends present at the initial assessment (2.6% vs 15.0%, P<.001). Of the remaining 264 patients, 229 (86.7%) patients or their surrogates provided written informed consent to participate. Consented and declined patients were distinguished only by a greater proportion of non-English speakers among the latter (5.9% vs 15.1%, P=.04). One consented patient was discharged without completing an interview and was excluded from further analyses, yielding a final cohort of 228 patients.
FIGURE 1.
Study eligibility and enrollment.
Patient Interviews
Upon receiving patients' written informed consent, an interviewer (O.P.) conducted brief (5 minute) interviews with patients or their surrogates 2 to 3 times per week throughout the hospitalization. The interviewer used 3 standard interview questions that we developed in studies of clinician respondents:16,17
Do you believe that there have been any problems with your care during this hospitalization?
Do you believe that you were hurt or stayed in the hospital longer than necessary because of problems with your care?
Do you believe that anyone made a mistake that affected your care during this hospitalization?
We encouraged patients to make additional open-ended comments about their care. The interviewer transcribed patients' verbatim responses by hand and recorded the data for subsequent coding and analysis.
We also surveyed patients by telephone 10 days after discharge in order to identify errors and adverse events that occurred at the end of hospitalization and during discharge, because patients may be vulnerable to error and injury during this period.18,19
Medical Record Reviews
An investigator (O.P.) abstracted age, sex, race, ethnicity, insurance type, need for an interpreter, dates of admission and discharge, attending physician, and service from the electronic registration system. The investigator used admission notes and the discharge summary to abstract medication and drug allergy information and medical comorbidities.
Clinician Surveys
We administered written surveys to nurses who worked on the study unit at the end of the study period by anonymous written questionnaires. Respondents were asked whether they were aware of the project, whether it affected their workload or relationship with patients, and whether patient incident reports were useful to them. The response rate was satisfactory, with 19 of 29 (65.5%) surveys completed.
Data Coding
Two of 4 Internists (S.N.W., D.Z.S., J.M.L., and M.D.A.), blinded to patient identification, independently coded each patient-reported event using a classification scheme adapted from previous published work.13,16,17,20 Reviewers classified adverse events, near misses, and medical errors with minimal risk of harm. Adverse events were defined as injuries because of medical care rather than the natural history of the illness. “Preventable” adverse events are injuries because of error. Near misses were defined as errors with the potential for injury, but no harm resulted because the error was intercepted (by the nurse, pharmacist, patient, or someone else) or by good fortune. Preventable adverse events and near misses are of special interest because they represent errors with harm or risk of harm. In contrast, medical errors with minimal risk of harm (e.g., a delayed test or treatment that did not plausibly affect the outcome of care) offer little opportunity for patient safety improvement. Reviewers also classified events such as poor food quality, waits and delays, and poor communication with clinicians as problems with service quality.21 They excluded 9 incidents in which the report was so confusing or incomplete that reviewers could not assess the event.
Reviewers classified the severity of the event as significant (e.g., diarrhea, pain), serious (e.g., large abscess), and life threatening (e.g., anaphylaxis). They classified preventability, involved parties, and process of care deficiencies. Differences were resolved by discussion. Interrater reliability of preconsensus coding was assessed with the kappa statistic to correct for chance association. Interrater agreement was good for reviewers' judgment that the incident had occurred as reported (0.83) and for preventability (0.85). Agreement was excellent for type of incident (0.97), severity (0.92), involved party (0.90), and type of process problem (0.97 to 1.00).
After coding was completed, an investigator (S.N.W.) used patients' narrative reports to guide a review of clinicians' notes, laboratory and radiology reports, medication orders, and the medication administration record for evidence in the medical record to corroborate patient-reported incidents that were confirmed by the physician panel.
Data Analysis
We calculated the number and rate of patient-reported and physician reviewer-confirmed adverse events (preventable and not) and near misses, stratified by severity. We analyzed the involved parties, and process of care deficiencies. We created a multivariable Poisson regression model with forward selection (entry criterion of P<.4) to examine the association of patient-reported adverse events with demographic factors (age, sex, race, ethnicity, need for interpreter), administrative factors (length of stay, insurance type, and service), clinical factors (number of medications, drug allergies, comorbidities), and type of attending physician. We created a second model to examine whether these factors were associated with patient-identified and physician-reviewer–evaluated errors in care, defined as preventable adverse events and near misses.
To test the hypothesis that few patient-reported adverse events are captured by standard reporting techniques, we compared patient-reported adverse events with reports submitted to the hospital incident-reporting system and in the patient's medical record.
RESULTS
Study Cohort
The study cohort included 228 patients. Table 1 presents the demographic, administrative, and clinical attributes of the cohort. A majority of patients had Medicare, reflecting in part the concentration of geriatric patients on the unit. Hospitalists or primary care physicians served as attending of record in most cases. Patients received an average of 7 medications (range 0 to 25), had 2 diagnoses (range 0 to 8), and had an average length of stay of 4 days (range 0 to 36). Sixteen percent had a family member or friend present at the time of consent.
Table 1.
Patient Characteristics (n=228)
| Characteristic | n | % |
|---|---|---|
| Mean age (range), SD | 63.0 (19 to 102), 18.3 | |
| Nonwhite race | 47 | 20.6 |
| Hispanic/Latino | 8 | 3.5 |
| Male gender | 85 | 37.3 |
| Interpreter required | 13 | 5.7 |
| Insurance | ||
| Commercial | 28 | 12.3 |
| Managed care | 55 | 24.1 |
| Medicare | 106 | 46.5 |
| Medicaid | 21 | 9.2 |
| Free care | 6 | 2.6 |
| Other/unknown | 12 | 5.3 |
| Service | ||
| Geriatrics | 93 | 40.8 |
| General medicine | 120 | 52.6 |
| Other/unknown | 15 | 6.1 |
| Attending type | ||
| Hospitalist | 112 | 49.1 |
| PCP | 72 | 31.6 |
| Subspecialist | 44 | 19.3 |
| No. of drug allergies (range), SD | 1.2 (0 to 13), 1.9 | |
| No. of medications | 7.1 (0 to 25), 4.7 | |
| No. of diagnoses | 2.4 (0 to 8), 1.7 | |
| LOS mean (range), SD | 4.4 (0 to 36), 4.9 | |
| Discharge destination | ||
| Home | 180 | 78.9 |
| SNF | 21 | 9.2 |
| Rehabilitation | 15 | 6.6 |
| Died | 4 | 1.8 |
| Hospice | 3 | 1.3 |
| Psychiatric facility | 3 | 1.3 |
| Assisted living | 1 | 0.4 |
| Against medical advice | 1 | 0.4 |
| Family or friend present at consent | 37 | 16.2 |
PCP, primary care physicians; SNF, skilled nursing facility; LOS, length of stay (days)
Patient Incident Reports
Altogether, 228 patients completed 338 inhospital and 190 postdischarge (528 total) interviews (mean 2.3 per person, range 1 to 6). One hundred twelve (49.1%) patients reported at least 1 incident to the interviewer, and a total of 310 distinct incident reports were received (mean 1.4 per person, range 0 to 8). Physician reviewers classified 75 incidents as positive or favorable assessments of clinical care and 173 incidents as problems with service quality. The remaining 62 reports included incidents that the physicians judged to be adverse events, near misses, and medical errors with minimal risk of harm.
Table 2 shows the type and rate of patient-reported incidents by severity and preventability. Seventeen patients (8%) experienced 20 adverse events, for an adverse event rate of 8.8 per 100 admissions. One patient had a serious injury: an abscess at a percutaneous intravenous catheter line site complicated by a deep vein thrombosis that required surgical intervention. Eleven patients (5%) had 13 significant injuries, including 3 cases of swollen, painful arms when intravenous infusions became infiltrated; 3 problems with pain control because of delayed medication administration; and 1 case each of hyperglycemia, hypokalemia, hypotension, dyspnea, diarrhea, hemorrhoidal bleeding that required transfusion, and a spreading hematoma. Eleven of 13 significant adverse events, but no serious adverse event, were judged to be errors, and hence preventable.
Table 2.
Types of Patient-Reported Events
| Type of Event | No. of Patients | % of Patients | No. of Events | Rate/100 Patients |
|---|---|---|---|---|
| Adverse Events | 17 | 7.5 | 20 | 8.8 |
| Life-threatening | 0 | 0.0 | 0 | 0.0 |
| Serious | 1 | 0.4 | 1 | 0.4 |
| Significant | 11 | 4.8 | 13 | 5.7 |
| Minor injury | 5 | 2.2 | 6 | 2.6 |
| Preventable (definite/probable) | 12 | 5.3 | 13 | 5.7 |
| Not preventable | 5 | 2.2 | 7 | 3.1 |
| Serious and preventable | 0 | 0.0 | 0 | 0.0 |
| Significant and preventable | 9 | 3.9 | 11 | 4.8 |
| Near Misses | 8 | 3.5 | 13 | 5.7 |
| Life-threatening | 1 | 0.4 | 1 | 0.4 |
| Serious | 2 | 0.9 | 4 | 1.8 |
| Significant | 1 | 0.4 | 2 | 0.9 |
| Minor injury | 4 | 1.8 | 6 | 2.6 |
| Intercepted | 3 | 1.3 | 7 | 3.1 |
| Not intercepted | 5 | 2.2 | 6 | 2.6 |
| Medical errors with minimal risk of harm | 21 | 9.2 | 29 | 12.7 |
| Total | 46 | 20.2 | 62 | 27.2 |
| Total patients | 228 | 100.0 | 228 | 100.0 |
Similarly, 8 patients (4%) experienced 13 near misses (5.7 per 100 admissions). Three patients had 5 near misses that were judged serious or life threatening. In 3 cases, a physician recommended a medication or test (influenza vaccine, sulfa-containing antibiotic, and IV contrast dye) to which the patient had a known severe allergy. (Subsequently, the hospital implemented an electronic order-entry system with allergy alert checking.) In a fourth case, an antibiotic was delayed by 6 hours. In a fifth case, the patient was referred to the emergency department for evaluation after a fall but sat unsupervised in the waiting room for 2 hours because she had not been registered. Two significant near misses included an error in which the wrong patient's blood pressure was monitored in the case of hypertensive urgency, and a patient who fell in the bathroom and was unattended for 3 hours before being discovered. Seven near misses were intercepted before an injury occurred, in each case by the patient or a family member.
Patients reported most events to the interviewer in person during the hospitalization rather than by telephone after discharge. Patients reported 3 of 20 adverse events, 4 of 13 near misses, and 2 of 29 errors after their discharge. A list of verbatim reports is included in Appendix A.
Involved Parties
Table 3 shows the parties that reviewers judged to have been most closely involved in the patient-reported incident. Clinicians were identified most often, with physicians accounting for 21 (34%) and nurses for 32 (52%) of 62 incidents. In 18 (29%) incidents, the involved party or parties were ill defined or unknown.
Table 3.
Parties Involved in Patient-Reported Events
| Party | Adverse Event (n=20) | Near Miss (n=13) | Medical Error*(n=29) | Total (n=62) |
|---|---|---|---|---|
| Physician | ||||
| Attending | 4 | 3 | 1 | 8 |
| House officer | 5 | 5 | 3 | 13 |
| Subspecialist | 0 | 0 | 0 | 0 |
| Nurse | 8 | 5 | 19 | 32 |
| Emergency room staff | 0 | 2 | 0 | 2 |
| Pharmacist | 2 | 0 | 8 | 10 |
| Phlebotomist | 2 | 1 | 0 | 3 |
| Transportation | 1 | 0 | 0 | 1 |
| Food service | 0 | 1 | 0 | 1 |
| Other | 2 | 0 | 0 | 2 |
| Unknown | 6 | 3 | 9 | 18 |
| Total parties† | 30 | 20 | 40 | 90 |
Medical errors with minimal risk of harm. This category is distinct from near misses, which do pose a risk of injury
Totals exceed 100% because of multiple parties involved
Process of Care Problems
Reviewers identified a variety of process problems associated with patient-reported incidents, such as problems with diagnoses, medications, procedures, clinical services (such as radiology, phlebotomy, and laboratory), and service quality (Table 4). Medication-related process of care problems were implicated in the majority of incidents, including 14 (70%) of 20 adverse events and 47 (76%) of 62 overall.
Table 4.
Process of Care Problems Among Patient-Reported Events
| Type of Process Problem | Adverse Event (n=20) | Near Miss (n=13) | Medical Error (n=29) | Total (n=62) |
|---|---|---|---|---|
| Diagnosis-related problems | ||||
| Diagnostic error | 1 | 0 | 1 | 2 |
| Test/procedure performed on wrong patient | 0 | 1 | 0 | 1 |
| Medication-related problems | ||||
| Overdose or extra dose | 1 | 0 | 0 | 1 |
| Missed dose or wrong time | 5 | 2 | 20 | 27 |
| Failure to order drug | 2 | 0 | 0 | 2 |
| Inappropriate choice of drug | 1 | 0 | 0 | 1 |
| Wrong dose/route | 0 | 1 | 2 | 3 |
| Wrong patient | 2 | 0 | 1 | 3 |
| Known allergy | 0 | 3 | 0 | 3 |
| Failure to recognize contraindication to drug | 0 | 1 | 0 | 1 |
| Inadequate monitoring or follow-up | 2 | 0 | 1 | 3 |
| Failure to observe therapy | 0 | 0 | 2 | 2 |
| Other | 1 | 0 | 0 | 1 |
| Operative- or procedure-related | ||||
| Postprocedure-related problems | 4 | 0 | 0 | 4 |
| Problems with clinical services | ||||
| Failure to perform or delayed performance of a test | 0 | 0 | 2 | 2 |
| Failure to draw blood | 1 | 0 | 0 | 1 |
| Test or procedure performed on wrong patient | 0 | 1 | 0 | 1 |
| Duplicate or unnecessary testing | 0 | 1 | 0 | 1 |
| Other | 1 | 0 | 1 | 2 |
| Service quality problems | ||||
| Waits and delays | 0 | 0 | 1 | 1 |
| Problems with environment and amenities | 0 | 1 | 1 | 2 |
| Poor communication/information for patient | 0 | 0 | 1 | 1 |
| Poor communication among caregivers | 1 | 0 | 0 | 1 |
| Inadequate staffing | 0 | 1 | 0 | 1 |
| Other problems | ||||
| Failure to monitor or follow-up | 1 | 2 | 1 | 4 |
| Equipment malfunction | 1 | 0 | 0 | 1 |
| Total process problems* | 24 | 14 | 34 | 72 |
Totals exceed number of events because multiple process problems were identified for a single event
Factors Associated with Patient-Reported Adverse Events and Errors
To understand the factors associated with patient-reported adverse events, we created a stepwise Poisson regression model of factors that we hypothesized to be associated with patient-reported and reviewer-confirmed adverse events. Patients with more medications were more likely to report adverse events (incidence rate ratio [IRR] 1.1 for each additional medication, 95% confidence interval [CI] 1.0, 1.2), a marginally significant result.
We created a second model that examined the factors associated with errors that had the potential for injury, defined as the number of preventable adverse events and near misses. Patients with 3 or more drug allergies were more likely to report errors compared with patients without drug allergies (IRR 4.7, 95% CI 1.7, 13.4).
Comparison with Hospital Incident Reports
During the study period, the hospital's Department of Healthcare Quality received 25 incident reports from study unit clinicians. Thirteen incidents were slips and falls, 8 were medication-related events, and 4 were problems with nonprocessed laboratory samples, delayed provision of compression bandages, and an elopement. Four events were classified as “level 2” (minor injury); the other 21 were “level 1” (no injury).
Seven study patients were the subject of 8 of these hospital incident reports: 3 falls, 2 medication events, problems with laboratory samples, with bandages, and elopement. Although these 7 study patients reported 4 adverse events, 3 near misses, and 4 errors without risk of injury in our interviews, none of these events had been reported in the hospital incident-reporting system. In other words, there was no overlap between the reports that we elicited and those captured by the hospital's system.
Evidence of Patient-Reported Adverse Events in the Medical Record
Using patients' reports as a guide, we found evidence in the medical record to confirm 11 (55%) of 20 adverse events, 4 (31%) of 13 near misses, and 10 (34%) of 29 medical errors, or 25 (40%) of 62 events overall. Confirmatory data were most often found in the medication administration record, physician and nurse notes, and physician orders.
Patient and Nurse Surveys
A majority (n=190, 83%) of patients completed a postdischarge telephone survey in which we asked about problems, mistakes, and injuries, as well as their assessment of hospital quality and the interview process. Among these respondents, 51 (27%) described the quality of care as “good” and 128 (67%) as “excellent.” Eight of 16 patients who had experienced an adverse event or near miss characterized the care as other than excellent. Most respondents rated their participation in the study favorably: 63 (33%) said the interviews were “satisfactory” and 125 (66%) described them as “rewarding, engaging, interesting, or important.” Two patients characterized the interviews as inconvenient or annoying.
Among 19 nurse survey respondents, only 4 (21.1%) were aware or somewhat aware of the study. All 19 respondents endorsed the statement that medical inpatients can identify problems such as errors and injuries, and that we should continue to ask patients about problems, injuries, and errors that they experience in the hospital.
DISCUSSION
Among 228 patients admitted to the medical unit of a Boston teaching hospital, the patient-reported adverse event rate was nearly 9 per 100 admissions. Serious injuries were uncommon, but two thirds were judged preventable. In addition, 4% of patients experienced near misses. Few patient-reported incidents were identified in the medical record, and none were submitted by clinicians to the hospital's incident-reporting system. Patients were more likely to report preventable adverse events and close calls if they had more drug allergies.
The incidence of patient-reported adverse events in this population is generally similar in magnitude to chart review studies of adverse events among patients in acute care hospitals reported elsewhere. The Medical Practice Study found a rate of 3.7 adverse events per 100 admissions.22 A replication of this study in Colorado and Utah found similar results (2.9%),23 but studies in Great Britain and Australia found higher rates (11.7% and 16.6%).24,25 Although U.S. rates have generally been lower than the 9% rate reported here, these other investigators used a more restrictive definition of adverse events, requiring death, disability, or extended hospitalization.26,27
A significant limitation of these studies is the ascertainment of adverse events based on chart review alone. Many adverse events are not recorded in the medical record, a finding attributed to variable standards for documentation, clinician unawareness or oversight, and concern about liability exposure.28,29 Studies of hospital incident reports elicited from medical house officers showed that as many as half of the reports were not found in the medical record.16,17,30 A study of adverse drug events that compared chart review, computerized detection, and spontaneous reporting found little overlap between the types of events detected.31 An inpatient study that relied on both chart review and clinician queries reported higher adverse event rates than chart review alone.20 In addition, a study in ambulatory care showed that chart review detected fewer than 11% of adverse drug events.13 Overall, these data have 2 implications: the true underlying rate of adverse events is higher than that detected by any single approach, and patient contact represents an important detection approach, at least outside the hospital.
Our study suggests that patient reports represent a valuable source of events inside the hospital as well. In addition, the lack of documentation for many patient-reported adverse events in our study suggests that patient reports represent a reservoir of incidents that were unaccounted for in previous inpatient studies.
We found that patients on multiple medications were more likely to report adverse events. This finding is consistent with studies that document the prominence of adverse drug events among medical inpatients,20,22 and the link between adverse drug events and polypharmacy in the nursing home and primary care settings.13,32 The association between error reporting and multiple drug allergies suggests an increased vulnerability and perhaps vigilance in this group.
Our study has several limitations, including its relatively small sample size, and the fact that it comes from a single site. The unit, which included a small geriatric service, may overrepresent community-dwelling elderly patients compared with an unselected population. We did not enroll patients who were admitted and discharged during the same weekend; this may bias our results if such patients were more or less prone to harm than other inpatients. Ascertainment of adverse events, near misses, and errors relied on the judgment of experienced clinicians, but the data from chart review and patient reports were often limited and in many cases made it difficult to understand or evaluate patients' complaints. In addition, because our interview questions elicited information about “injuries,” we did not collect information about the psychologic that harm the patient may have experienced. Excluding patients on contact precautions may have decreased the observed adverse event rate, as recent data suggest these patients are at a higher risk of adverse events than other patients.33 We did not review all charts for adverse events routinely, so we cannot assess the underlying adverse event rate. Incident classification was retrospective, which did not permit clarification of incidents from patients or clinicians other than the reports elicited by the interviewer. To address these potential problems, reviewers offered generally conservative assessments, excluding events that were difficult to interpret or not credible and produced blinded ratings that were highly reliable.
The results of this study offer some guarded optimism about the prospect for productive partnerships between clinicians and hospitalized patients to create safer health care. Many patients and their families are able to identify errors and injuries during or shortly after the hospitalization, providing a potentially useful source of information that could inform clinical care and guide improvement initiatives. Patients may also be able to identify conditions, such as poor staffing, that increase the risk of harm. This study also adds credibility to “best practice” recommendations proposed by organizations such as the American Hospital Association and National Patient Safety Foundation that call for patient participation in preventing medical errors. Finally, patient reports about the attitudes and behaviors of staff may offer insights into the safety culture of the organization.
Additional studies are needed in order to understand the role that patients can play in promoting safe care. Many of the most basic questions remain unanswered. How can we elicit patient incident reports efficiently and confidentially, and use the information to advance safety? Will this information affect the patient-clinician relationship, or increase the risk of malpractice litigation? How can we work with patients at greatest risk of harm, as those at extremes of age, carrying the greatest burden of comorbid illness, with the most drugs, interventions, and allergies, may be least able to participate in safety prevention? Which interventions are most suitable for patients to play a role?
Many patients are aware of errors and iatrogenic injuries that affect their own care. Engaging patients as partners with clinicians in efforts to identify and prevent medical errors offers a promising strategy to advance patient safety.
Acknowledgments
Dr. Weingart was supported by a K08 Mentored Clinical Investigator Career Development Award from the U.S. Agency for Healthcare Research and Quality (1 K08 HS 11644).
REFERENCES
- 1.Institute for Family-Centered Care. Your Role in Safe Medication Use. Boston, Mass: Massachusetts Coalition for the Prevention of Medical Errors; 1999. Available at http://www.macoalition.org/documents/Best_Practice_Medication_Errors.pdf Accessed December 14, 2003. [Google Scholar]
- 2.American Hospital Association. Successful practices for improving medication safety, 1999. Available at http://www.hospitalconnect.com/aha/key_issues/medication_safety/ahainitiative/medicalsafety20015.html Accessed December 14, 2004.
- 3. National Patient Safety Foundation. You can help improve patient safety. Available at http://www.npsf.org/html/patients.html Accessed January 20, 2003.
- 4. National Patient Safety Foundation. National Agenda for Action: Patients and Families in Patient Safety, 2003. Available at http://www.npsf.org/download/AgendaFamilies.pdf Accessed December 14, 2003.
- 5.Agency for Healthcare Research and Quality. 20 Tips to Help Prevent Medical Errors. Patient Fact Sheet. AHRQ Publication No. 00-PO38. Rockville, Md: Agency for Healthcare Research and Quality; 2000. Available at http://www.ahrq.gov/consumer/20tips.htm Accessed December 14, 2003. [Google Scholar]
- 6.Davies RD, Ware JE. Involving consumers in quality of care assessment. Health Affairs. 1998:33–48. doi: 10.1377/hlthaff.7.1.33. [DOI] [PubMed] [Google Scholar]
- 7.Cleary PD, Edgman-Levitan S, Roberts M, et al. Patients evaluate their hospital care: a national survey. Health Affairs. 1991;11:254–67. doi: 10.1377/hlthaff.10.4.254. [DOI] [PubMed] [Google Scholar]
- 8.Cleary PD, Edgman-Levitan S. Health care quality: incorporating consumer perspectives. JAMA. 1997;278:1608–12. [PubMed] [Google Scholar]
- 9.Cleary PD. A hospitalization from hell: a patient's perspective on quality. Ann Intern Med. 2003;138:33–9. doi: 10.7326/0003-4819-138-1-200301070-00009. [DOI] [PubMed] [Google Scholar]
- 10. Public opinion of patient safety issues: research findings. Report prepared by Louis Harris and Associates for the National Patient Safety Foundation at the AMA. Chicago, Ill: National Patient Safety Foundation, 1997. Available at http://www.npsf.org/download/1997survey.pdf Accessed December 14, 2003.
- 11.How safe is your hospital? Consumer reports readers rate the care they or a relative received. Consumer Rep. 2002;68:12–8. [Google Scholar]
- 12.Blendon RJ, DesRoches CM, Brodie M, et al. Views of practicing physicians and the public on medical errors. N Engl J Med. 2002;347:1933–40. doi: 10.1056/NEJMsa022151. [DOI] [PubMed] [Google Scholar]
- 13.Gandhi TK, Weingart SN, Peterson J, et al. Adverse drug events in ambulatory care. N Engl J Med. 2003;348:1556–64. doi: 10.1056/NEJMsa020703. [DOI] [PubMed] [Google Scholar]
- 14.Brennan TA, Sox CM, Burstin HR. Relation between negligent adverse events and the outcomes of medical-malpractice litigation. N Engl J Med. 1996;335:1963–7. doi: 10.1056/NEJM199612263352606. [DOI] [PubMed] [Google Scholar]
- 15.Studdert DM, Thomas EJ, Burstin HR, Zbar BI, Orav EJ, Brennan TA. Negligent care and malpractice claiming behavior in Utah and Colorado. Med Care. 2000;38:250–60. doi: 10.1097/00005650-200003000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Weingart SN, Ship AN, Aronson MD. Confidential clinician-reported surveillance of adverse events among medical inpatients. J Gen Intern Med. 2000;15:470–7. doi: 10.1046/j.1525-1497.2000.06269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weingart SN, Callanan LD, Ship AN, Aronson MD. A physician-based voluntary reporting system for adverse events and medical errors. J Gen Intern Med. 2001;16:809–14. doi: 10.1111/j.1525-1497.2001.10231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138:161–7. doi: 10.7326/0003-4819-138-3-200302040-00007. [DOI] [PubMed] [Google Scholar]
- 19.Moore C, Wisnivesky J, Williams S, McGinn T. Medical errors related to discontinuity of care from an inpatient to an outpatient setting. J Gen Intern Med. 2003;18:646–51. doi: 10.1046/j.1525-1497.2003.20722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events. JAMA. 1995;274:29–34. [PubMed] [Google Scholar]
- 21.Kenagy JW, Berwick DM, Shore MF. Service quality in health care. JAMA. 1999;281:661–5. doi: 10.1001/jama.281.7.661. [DOI] [PubMed] [Google Scholar]
- 22.Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. N Engl J Med. 1991;324:370–76. doi: 10.1056/NEJM199102073240604. [DOI] [PubMed] [Google Scholar]
- 23.Thomas EJ, Studdert DM, Burstin HR, et al. Incidence and types of adverse events and negligent care in Utah and Colorado. Med Care. 2000;38:261–71. doi: 10.1097/00005650-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Vincent C, Neale G, Woloshynowych M. Adverse events in British hospitals: preliminary retrospective record review. BMJ. 2001;322:517–9. doi: 10.1136/bmj.322.7285.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson RM, Runciman WB, Gibberd RW, Harrison BT, Newby L, Hamilton JD. The Quality in Australian Health Care Study. Med J Aust. 1995;163:458–71. doi: 10.5694/j.1326-5377.1995.tb124691.x. [DOI] [PubMed] [Google Scholar]
- 26.Thomas EJ, Studdert DM, Runciman WB, et al. A comparison of iatrogenic injury studies in Australia and the USA: context, methods, casemix, population, patient and hospital characteristics. Int J Qual Health Care. 2000;12:371–8. doi: 10.1093/intqhc/12.5.371. [DOI] [PubMed] [Google Scholar]
- 27.Runciman WB, Webb RK, Helps SC, et al. A comparison of iatrogenic injury studies in Australia and the USA: reviewer behaviour and quality of care. Int J Qual Health Care. 2000;12:379–88. doi: 10.1093/intqhc/12.5.379. [DOI] [PubMed] [Google Scholar]
- 28.Bates DW. A 40-year-old woman who noticed a medication error. JAMA. 2001;285:3134–40. doi: 10.1001/jama.285.24.3134. [DOI] [PubMed] [Google Scholar]
- 29.Pizzi LT, Goldfarb NI, Nash DB. Other practices related to patient participation. Ch. 50. In: Shojania KG, Duncan BW, McDonald KM, Wachter RM, editors. Making Health Care Safer: A Critical Analysis of Patient Safety Practices. Evidence Report/Technology Assessment, No. 43. Rockville, Md: US Agency for Healthcare Research and Quality; 2001. pp. 575–8. [Google Scholar]
- 30.O'Neil AC, Petersen LA, Cook EF, Bates DW, Lee TH, Brennan TA. Physician reporting compared with medical-record review to identify adverse medical events. Ann Intern Med. 1993;119:370–6. doi: 10.7326/0003-4819-119-5-199309010-00004. [DOI] [PubMed] [Google Scholar]
- 31.Jha AK, Kuperman GJ, Teich JM, et al. Identifying adverse drug events: development of a computer-based monitor and comparison with chart review and stimulated voluntary report. J Am Med Inform Assoc. 1998;5:305–14. doi: 10.1136/jamia.1998.0050305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Field TS, Gurwitz JH, Avorn J, et al. Risk factors for adverse drug events among nursing home residents. Arch Intern Med. 2001;161:1629–34. doi: 10.1001/archinte.161.13.1629. [DOI] [PubMed] [Google Scholar]
- 33.Stelfox HT, Bates DW, Redelmeier DA. Safety of patients isolated for infection control. JAMA. 2003;290:1899–905. doi: 10.1001/jama.290.14.1899. [DOI] [PubMed] [Google Scholar]