Abstract
Background
In New Zealand, more than 5% of people aged 50 years and older have undiagnosed diabetes; most of them attend family practitioners (FPs) at least once a year.
Objectives
To test the effectiveness of patients or computers as reminders to screen for diabetes in patients attending FPs.
Design
A randomized-controlled trial compared screening rates in 4 intervention arms: patient reminders, computer reminders, both reminders, and usual care. The trial lasted 2 months. The patient reminder was a diabetes risk self-assessment sheet filled in by patients and given to the FP during the consultation. The computer reminder was an icon that flashed only for patients considered eligible for screening.
Participants
One hundred and seven FPs.
Measurements
The primary outcome was whether each eligible patient, who attended during the trial, was or was not tested for blood glucose. Analysis was by intention to treat and allowed for clustering by FP.
Results
Patient reminders (odds ratio [OR] 1.72, 95% confidence interval [CI] 1.21, 2.43), computer reminders (OR 2.55, 1.68, 3.88), and both reminders (OR 1.69, 1.11, 2.59) were all effective compared with usual care. Computer reminders were more effective than patient reminders (OR 1.49, 1.07, 2.07). Patients were more likely to be screened if they visited the FP repeatedly, if patients were non-European, if they were “regular” patients of the practice, and if their FP had a higher screening rate prior to the study.
Conclusions
Patient and computer reminders were effective methods to increase screening for diabetes. However, the effects were not additive.
Keywords: diabetes, screening, family practice, computer reminder, patient intervention
Treating diabetes and its associated disorders can prevent or delay microvascular and macrovascular damage. In New Zealand, at least 5% of people aged 50 years or older, within each major ethnic group, have undiagnosed diabetes.1 Some 80% to 90% of these people visit a family practitioner (FP) at least once a year.2 As most people with undiagnosed diabetes have no symptoms, their diabetes can be found only by screening. The best opportunity to screen is when people attend an FP for another reason. However, it is not clear what method is most effective to trigger diabetes screening when the patient attends for some other reason.
It is well established that computer reminders can be effective in increasing FP preventive care such as immunizations and cervical smears.3,4 Nevertheless, not all interventions have been successful, and effect sizes vary markedly so that effectiveness needs to be confirmed in specific settings.
It also seems that having a patient remind an FP may be effective, although the evidence is mostly less direct, comes from a range of study types, and the expected size of effect is not always clear. First, patients have been reported as an important barrier to evidence-based medicine or guideline implementation.5,6 Second, patient expectations can “drive” FPs,7 or, more specifically, FPs are primarily driven by what they think the patient expects.8 Third, a limited number of studies have shown a positive effect “using” patients to influence FPs.9–11 Finally, further indirect evidence comes from the boom in direct-to-consumer marketing that is currently allowed only in the United States and New Zealand.12,13 This study compared the effectiveness of computer reminders and a diabetes risk self-assessment form completed by patients, who gave the form to FPs as a way of having patients remind the FP to screen.
This trial was conceived as implementing a guideline in preventive care, the guideline being recommendations on diabetes screening previously published by Kenealy et al.1 Factors that may relate to increased preventive care include a slow consultation rate, doctors being younger and female, and continuity of care with patients regularly attending the same doctor.14–17 In New Zealand, there is concern about the increasing rate of diabetes, about its greater prevalence in non-European peoples,18 and the cost of attending doctors has been raised as a possible barrier to attending FPs.
METHODS
Planned Study Population, Inclusion, and Exclusion Criteria
Family practitioners were eligible for the trial if they:
used MedTech 32 patient management computer software (Health Technology Ltd., Auckland, New Zealand)—used by over half of New Zealand GPs at the time of the study,
recorded their medical consultation notes on the computer within their consultations—about 65% of FPs according to data collected from phoning all family practices in Auckland in preparation for this study,
had received laboratory glucose results electronically for at least 1 year—about 92% of Auckland FPs,
saw at least 10 individual patients aged 50 years or older per month—as both power calculations and analyses that allow for clustering may be misled by extremes of cluster size, and
worked in the Auckland region—approximately one third of FPs in New Zealand.
Patients were considered eligible for diabetes screening if they were aged 50 years or older, were not coded in the computer as having diabetes, and had no glucose test result in the computer in the previous 3 years.
Interventions and Timing
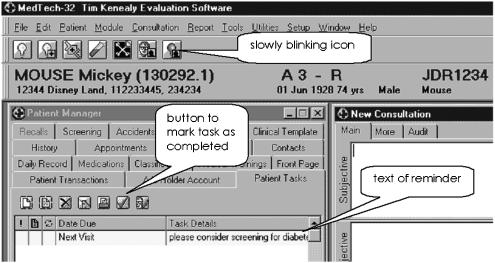
The computer showed a slowly flashing icon on the task bar when the doctor opened the file of an eligible patient (Fig. 1). When the FP clicked on this icon, a brief message appeared suggesting screening for diabetes. The icon flashed each time the patient record was opened until the FP marked the task as “complete.”
FIGURE 1.
Screen shot from MedTech 32 showing an active reminder at the right end of the toolbar and the text of the patient task near the bottom line of the screen shot
The patient reminder was a diabetes risk self-assessment form, adapted from the American Diabetes Association.19,20 The forms asked patients their age and ethnicity, whether their weight was over that listed for their height on an accompanying chart (set at body mass index 28), whether they had a mother, father, sister, or brother with diabetes, whether they were a woman who had a baby weighing more than 4 kg at birth, and whether they did little or no exercise on most days. Practice receptionists were asked to give the form to all patients age 50 years or older attending study FPs. They asked patients to complete what they could before seeing the doctor and to give the form to the doctor.
Author T.K. visited all FPs to confirm recruitment for the study, to explain the study aims and design, to provide uniform education on diabetes screening, and to instruct FPs on using both the computer reminder and the patient reminder form. The visits lasted from 5 to 15 min/FP, some one to one and some in small groups within a practice. All FPs were given a copy of a recent article on diabetes screening in New Zealand1 and a laminated card summarizing the same information. At the end of each meeting, 1 FP opened an envelope revealing the intervention group assigned to all FPs participating in that practice. As these visit meetings were completed prior to randomly assigning FPs to intervention groups, the effect of the visit on screening rates was presumed to be equivalent across the groups. The effect of these visits on screening rates was measured by comparing the screening rate in the “usual care” group with the prior screening rate across all the groups.
The active interventions lasted for 2 months, which power calculations suggested was sufficient to establish the effectiveness of the interventions.
Primary Outcome Measures
The primary study question was whether or not a patient who was eligible for diabetes screening and who visited an FP during the study was screened for diabetes. A visit was defined by the presence of an invoice during the study period. A patient was considered “screened” if they had a laboratory glucose test result in the computer during the trial.
Other Measures
FP data included gender, year of graduation, half-days worked per week, proportion of total patients aged 50 years or older, proportion of eligible patients screened for diabetes in the 2 months prior to the trial, whether they ever screened for diabetes using only HbA1c (i.e., without a glucose test), and use of MedTech 32 reminders prior to the trial. The total number of patients seen by each doctor was divided by the number of half-days they worked in the trial as a measure of workload and average length of appointment. Practice size was represented by the total number of doctors in the practice. The usual FP charge for a patient receiving no government subsidies was recorded as a proxy for socioeconomic status of patients and practice location. Patient data included ethnicity, age, number of visits during the trial, and whether the patient was “regular” or “casual” with the practice.
The patient reminder forms asked for a statement from the FP about whether the patient was screened for diabetes following the consultation; if so, whether they would have screened the patient in the absence of the form, and if no, why not.
Sample Size Calculations and Statistical Analysis
Pilot data from 37 FPs in 9 practices were used for power calculations (power 80% and significance 5%). Simulations with boot-strap selection of practices assumed a mean improvement with either intervention of 15% with a normal distribution of effect and a standard deviation of 5. It was also assumed that the effects of the interventions were additive with a combined additional improvement of between 0% and 15%. Comparisons of computer reminder versus none, patient reminder versus none, both reminders versus none, and either reminder versus both suggested that at least 13 practices were needed in each group. To allow for practices dropping out, 15 practices were sought for each group, a total of 60 practices.
The statistical analyses were carried out in Stata version 8. Logistic regressions allowed for clustering of individual patients within groups (FP), as selection and intervention were conceived as based on individual doctors rather than practice. Alternative calculations based on clustering by practice were also performed, but made no substantial difference to the results. The χ2 test was used for comparison of proportions, and the t test was used for comparison of means. All analyses were performed on an intention-to-treat basis, statistical significance is cited at P<.05, with 1 exception explained in the text, and all tests were 2-tailed. The Auckland Ethics Committees approved this study.
Unit of Randomization, Allocation Schedule
Randomization was undertaken in 2 stages. The first was to sample from FPs who probably fulfilled the inclusion criteria based on a telephone survey of practices. The second was to allocate to an intervention arm those who were confirmed as eligible after a visit by author T.K. and who consented to take part, at the same time stratifying for practice size to protect against grossly uneven distribution of practice size across the intervention arms. Even so, 5 FPs who were initially entered into the trial were withdrawn from analysis as they proved not to fulfill the selection criteria of seeing 10 or more individual patients aged 50 years or older per month, i.e., if this had been known earlier, they would not have been included in the trial.
For the first randomization, an independent person used Excel to assign a random number between 0 and 1 to each of the 398 FPs. A prior decision was made to invite FPs assigned random numbers 0 to 0.5.
For the second randomization, practices were stratified according to number of doctors (solo, 2 to 4 doctors, 5 or more doctors), to protect the intervention groups from gross discrepancies in practice size. An independent person used Excel to generate random numbers in blocks of 8 and place the names of intervention groups in sealed and consecutively numbered envelopes. Each block of 8 assigned 2 practices to the computer reminder, 2 to the patient intervention, and so on. The envelopes were numbered, were opened in sequence, and the number, date, and time of opening were recorded.
RESULTS
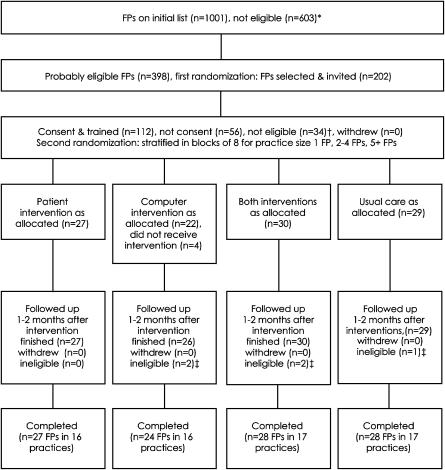
The number of FPs at each stage of the trial is shown in Fig. 2. One hundred and seven FPs in 66 practices completed the trial. Table 1 compares their baseline data across the intervention groups. During the trial, 19,187 individual patients aged 50+ years visited an FP at least once. Of these, 5,628 were eligible for screening for diabetes. The percentage screened in each group were: computer 31.8%, patient 23.9%, both 23.7%, and usual 15.5%. Computer reminders were more effective than patient reminders (odds ratio [OR] 1.49, 95% confidence interval [CI] 1.07, 2.07). The usual group screening rate was close to that for all groups combined in the 2 months prior to the trial (15.7%, P .80).
FIGURE 2.
Flow diagram of family practitioner (FP) numbers in trial. *Not using MedTech 32 (474), not having electronic laboratory results for ≥1 year (75), not recording clinical notes on computer (32), not in Auckland (5), not an FP (9), 1 entry each for doctors working in 2 practices (7), and author T.K. (1). †Left practice, leaving practice, or on maternity leave (13), not willing or able—within the timeframe of this trial—to upgrade to the version of MedTech 32 needed for the computer intervention and data collection (15), not an FP (4), and saw <10 individual patients age ≥50 per month (2). ‡Two FPs in the computer group, 2 in the patient group, and 1 in the usual group saw <10 individual patients age ≥50 per month
Table 1.
Comparative Data for FPs, Practices, and Patients by Intervention Group
| Both | Computer | Patient | Usual | |
|---|---|---|---|---|
| FPs (n female, n male) | 11, 17 | 12, 12 | 13, 14 | 13, 15 |
| Practices (n) | 17 | 16 | 16 | 17 |
| FPs per practice (median, range) | 3, 7 | 4, 20 | 3.5, 6 | 4, 9 |
| Years since FP graduated (median, range) | 15, 28 | 19, 30 | 18, 30 | 19, 28 |
| Half-days worked per week (median, range) | 10, 6 | 8.5, 8 | 8, 7 | 5.5, 8* |
| Fee for patient without government subsidy (median, range) | 40, 38 | 42, 40 | 45, 42 | 45, 18 |
| Patients seen | ||||
| Total | 24,614 | 22,829 | 23,960 | 23,136 |
| Total age 50+ | 7,010 | 7,083 | 7,615 | 8,964 |
| Individuals age 50+ | 4,295 | 4,509 | 4,727 | 5,656 |
| Ethnicity of individuals age 50+ | ||||
| N recorded | 3,517 | 3,413 | 3,443 | 4,311 |
| % Recorded | 81.8 | 75.7 | 72.8 | 76.2 |
| % Not European | 26.8 | 31.6 | 19.5 | 14.5 |
Median test for differences in medians between groups (dropping any value equal to the median): P= .10.
FP, family practitioner.
A Model to Predict Screening of Eligible Patients
Family practitioner, practice, and patient items that were common to all intervention groups were considered for a model predicting screening across all patients in the trial, shown in Table 2. This model was manually refined to include only those items that, in combination, each retained a significance P<.05 apart from non-European ethnicity, which was retained because of its plausibility in the New Zealand context; see Table 3.
Table 2.
Odds Ratio for Eligible Patients Being Screened, All Variables Initially Considered Likely to Predict Screening*
| Odds Ratio | Standard Error | z | P>|z| | 95% Confidence Interval | |
|---|---|---|---|---|---|
| Both reminders | 1.86 | 0.39 | 2.94 | .003 | 1.23 to 2.82 |
| Computer reminders | 2.66 | 0.72 | 3.63 | <.001 | 1.57 to 4.53 |
| Patient reminders | 1.95 | 0.37 | 3.53 | <.001 | 1.35 to 2.80 |
| FP female | 1.12 | 0.17 | 0.72 | .47 | 0.83 to 1.50 |
| FP number in practice | 0.97 | 0.02 | −1.35 | .18 | 0.94 to 1.01 |
| FP year graduation | 1.01 | 0.01 | 1.35 | .18 | 0.99 to 1.03 |
| FP tenths worked | 0.98 | 0.03 | −0.54 | .59 | 0.92 to 1.05 |
| FP mean number patients per day | 1.00 | 0.001 | −0.59 | .56 | 1.00 to 1.00 |
| FP proportion patients age 50+ | 27.3 | 1.83 | 1.53 | .13 | 0.75 to 10.14 |
| FP prior screen rate | 4.38 | 2.46 | 2.63 | .01 | 1.46 to 13.18 |
| FP fee to patient | 1.00 | 0.01 | −0.01 | .99 | 0.98 to 1.02 |
| Patient age | 1.00 | 0.004 | −0.82 | .41 | 0.99 to 1.00 |
| Patient “regular” | 2.04 | 0.27 | 5.44 | <.001 | 1.58 to 2.64 |
| Patient number of visits | 1.23 | 0.05 | 5.44 | <.001 | 1.14 to 1.32 |
| Patient non-European ethnicity | 1.45 | 0.20 | 2.70 | .007 | 1.11 to 1.89 |
Logistic regression with clustering by FP, n=4,081.
The number of patients included in this regression is reduced because of 1,547 patients missing ethnicity data.
FP, family practitioner.
Table 3.
Odds Ratio (OR) for Eligible Patients Being Screened, Showing Contribution of Key Variables
| OR | 95% Confidence Interval | P | |
|---|---|---|---|
| Computer reminders | 2.31 | 1.40 to 3.82 | .001 |
| Patient reminders | 1.90 | 1.34 to 2.71 | <.001 |
| Both reminders | 1.73 | 1.16 to 2.59 | .008 |
| FP prior screen rate | 5.23 | 1.63 to 16.86 | .005 |
| Patient number of visits | 1.22 | 1.14 to 1.31 | <.001 |
| Patient “regular” | 2.09 | 1.58 to 2.77 | <.001 |
| Patient non-European ethnicity | 1.29 | 0.99 to 1.67 | .06 |
Logistic regression adjusted for clustering on FP, n=5,628.
Computer, patient, and both reminders indicate the OR of the patient being screened if they attended an FP in each intervention group, compared with their being in the usual care group. FP prior screen rate indicates an OR of 5.23 for being screened if the patient attended an FP whose screening rate prior to the trial was 100% compared with an FP who screened 0% prior to the trial. Patient number of visits indicates an OR of 1.22 per additional visit during the trial. Patient “regular” indicates an OR of 2.09 for patients who were “regular” patients of the practice compared with those who were “casual” or “temporary visitors” to the practice.
FP, family practitioner.
The reminders increased glucose testing for both patients who were defined as eligible for screening and, to a lesser extent, those who were not. For example, comparing the computer group with the usual group, the OR for eligible patients being screened was 2.55 (P<.001) and for those who were not eligible was 1.31 (P .04).
Patient Sheet Analysis
There were 1,883 patient reminder sheets returned from 28 practices. The number of sheets per practice ranged from 0 to 262. It seemed possible that patient reminders—when delivered—were highly effective, but that the measured effect was minimized in the previous intention-to-treat analyses. Logistic regression comparing practices that returned patient sheets from more or less than 25% (approximately the mean) of their eligible patients showed no statistically significant difference, but practice numbers were relatively small.
Examining the Low Screening Rate with Both Reminders
There was no statistically significant difference between the proportion of sheets delivered to patients in the both reminders group (mean 0.30, SE 0.12) and in the patient reminders group (mean 0.23, SE 0.10), P .65.
The data were examined to see whether the both reminders group, in comparison with either the patient or computer reminders groups, had smaller numbers of patients who visited more often, were regular, or were non-European patients, or whether the FPs had lower rates of screening prior to the trial. For the first 3 variables, the figures given are percentages of patients in each group with significance testing using clustered logistic regression for odds of being screened. Prior screening rates were tested as proportions between groups of FPs. Compared with the computer group, the both reminders group had a smaller proportion of regular patients (77% vs 79%, OR 2.46, SE 0.50, CI 1.65, 3.66), no statistically significant difference in the proportion of non-European patients (27% vs 36%, OR 1.11, SE 0.19, CI 0.80, 1.54), or in FP prior screening rate (20% SE 7% vs 21% SE 8%, P .95) but a higher mean number of visits per patient during the trial (1.54 vs 1.45, OR 1.22, SE 0.05, CI 1.11, 1.32). Compared with the patient group, the both reminders group had a smaller proportion of regular patients (77% vs 85%, OR 3.14, SE 0.58, CI 2.18, 4.52), no statistically significant difference in proportion of non-European patients (27% vs 18%, OR 1.10, SE 0.18, CI 0.80, 1.51) or FP prior screening rate (20% SE 7% vs 15% SE 7%, P .65), but higher mean number of patient visits during the trial (1.54 vs 1.53, OR 1.23, SE 0.05, CI 1.14, 1.33).
DISCUSSION
Principal Findings
The principal finding of this study was that a simple and unobtrusive computer reminder approximately doubled the rate of appropriate opportunistic screening for diabetes in a routine family practice setting in New Zealand. This is the first study to show that a computer reminder will increase the rate of diabetes screening in family practice, and the first to show that a patient reminder is effective. However, the computer reminders were more effective than patient reminders, and the screening rate was nearly identical for the patient and both groups. The computer reminders appeared more acceptable to FPs and practice staff than the patient reminders. The reminders increased both the rate of screening and the relative rate of screening those who were eligible compared with those who were not eligible.
A logistic regression model of the probability of eligible patients being screened included 1 “trial” variable (intervention group), 1 FP variable (“prior screen,” which was the FP screening rate in the 2 months prior to the trial), and 2 “patient” variables (“regular” patient vs not, and non-European ethnicity). The number of visits by an individual patient within the trial period can reflect FP working style or patient medical problems.
Strengths and Weaknesses
There are several strengths of this study. The sample size exceeded recruitment goals. The participation rate was acceptably high at 112 participants from 168 who proved eligible for the study (67%). The design was robust as was the intention-to-treat analysis, which allowed for clustering.
The trial compared 2 quite different interventions, each expected to increase diabetes screening in “real-life” primary care. We envisage that the first task of a screening program is to deliver the intended test to the relevant population. At the time of this study, it was not readily possible to extract glucose values from the FP computers. The trial did not test the appropriateness of the criteria used to determine who was eligible for screening, or what decisions FPs made as a result of any glucose test.
Routinely collected data may have given imprecise counts of a patient visit, an eligible patient, and a screened patient. In each case any error is likely to decrease the apparent screening rate but the effect is not likely to differ between the intervention arms. We also recognize that testing a patient for glucose, even though not “eligible” by the definition used here, may nevertheless be appropriate medical care.
The 2-month duration of the intervention is relatively short. The success of the computer reminder will encourage longer-term studies. On the other hand, it would not be worth extending a trial of the patient reminder unless the process was substantially modified.
The degree of participation by FPs and practice staff was very variable in the 2 groups using the patient reminders. Many practices had difficulties because of a series of part-time receptionists dealing with a range of doctors, only some of whom were in the study, being visited by a range of patients, only some of whom “needed” forms. On the other hand, the only negative comments about the computer reminders were from those who found the flashing icon to be too inconspicuous.
Interpretation
The most surprising finding was that the effect of both reminders was lower than computer reminders alone, and close to that of patient reminders alone. In the most comparable study we found, Turner et al.9 tested computer-generated paper reminders and patient reminders as a means to increase 4 categories of preventive care. It is interesting to note that they, too, found that combining both computer and patient reminders was slightly less effective in improving physician-dependent care than were computer reminders alone.
There are several possible explanations in this study, including a negative interaction of patient reminders on computer reminders, and vice versa. Perhaps a patient reminder, when it occurred, was so obvious that doctors were distracted from recalling that there was a separate, additional, and easily missed reminder on the computer. This could be summarized as “if I am not being reminded by the patient, then I am not being reminded at all.” It seems unlikely that computer reminders had a negative effect on patient reminders; if this were the case, one would expect the patient reminder rate to be considerably higher than the rate with both reminders.
Another possible explanation would be that fewer patient sheets were delivered in the group with both reminders than in the patient reminder group. However, the converse was true, i.e., the delivery rate was higher in the group with both reminders, albeit the difference was not statistically significantly different.
Finally, the data were examined in secondary analyses for chance misdistribution of significant variables between the groups. Compared with both the computer and patient reminders groups, the both reminders group had some statistically significant differences in variables, some of which would be expected to increase the screening rate while some would be expected to decrease it. No pattern was found to explain the relatively low rate of screening in this group.
Future Research
While not required for this study, collecting glucose data and determining what decisions FPs made as a result of screening would enable a future study to measure the effectiveness of a screening program more accurately. It still seems to us that a patient reminder could be more effective than in this study. However, one would need to engage the receptionists more strongly than was achieved in this trial, perhaps aided by a computer reminder to them!
Acknowledgments
The authors thank Alistair Stewart for statistical advice. Funding from the Health Research Council of New Zealand (Training Fellowship for T.K.) and Auckland Faculty of the Royal New Zealand College of General Practitioners (study costs) is gratefully acknowledged.
References
- 1.Kenealy T, Braatvedt G, Scragg R. Screening for type 2 diabetes in non-pregnant adults in New Zealand: practical recommendations. N Z Med J. 2002;115:194–6. [PubMed] [Google Scholar]
- 2.Ministry of Health. Taking the Pulse: The 1996/97 New Zealand Health Survey. Wellington: Ministry of Health; 1999. [Google Scholar]
- 3.Johnston M, Langton K, Haynes B, Mathieu A. Effects of computer-based clinical decision support systems on clinician performance and patient outcome; a critical appraisal of research. Ann Intern Med. 1994;120:135–42. doi: 10.7326/0003-4819-120-2-199401150-00007. [DOI] [PubMed] [Google Scholar]
- 4.Shea A, DuMouchel W, Bahamonde L. A meta-analysis of 16 randomised controlled trials to evaluate computer-based clinical reminder systems for preventive care in the ambulatory setting. J Am Med Inform Assoc. 1996;3:399–409. doi: 10.1136/jamia.1996.97084513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larme A, Pugh J. Attitudes of primary care providers toward diabetes: barriers to guideline implementation. Diabetes Care. 1998;21:1391–6. doi: 10.2337/diacare.21.9.1391. [DOI] [PubMed] [Google Scholar]
- 6.Nutting P, Rost K, Dickinson M, et al. Barriers to initiating depression treatment in primary care practice. J Gen Intern Med. 2002;17:103–11. doi: 10.1046/j.1525-1497.2002.10128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maly R, Abrahamse A, Hirsch S, et al. What influences physician practice behaviour? An interview study of physicians who received consultative geriatric assessment recommendations. Arch Fam Med. 1996;5:448–54. doi: 10.1001/archfami.5.8.448. [DOI] [PubMed] [Google Scholar]
- 8.Cockburn J, Pit S. Prescribing behaviour in clinical practice: patients' expectations and doctors' perceptions of patients' expectations—a questionnaire study. BMJ. 1997;315:520–3. doi: 10.1136/bmj.315.7107.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turner B, Day S, Borenstein B. A controlled trial to improve delivery of preventive care: physician or patient reminders? J Gen Intern Med. 1989;4:403–9. doi: 10.1007/BF02599691. [DOI] [PubMed] [Google Scholar]
- 10.Greenfield S, Kaplan S, Ware J, et al. Patients' participation in medical care: effects on blood sugar control and quality of life in diabetes. J Gen Intern Med. 1988;3:448–57. doi: 10.1007/BF02595921. [DOI] [PubMed] [Google Scholar]
- 11.Yabroff K, Mandelblatt J. Interventions targeted toward patients to increase mammography use. Cancer Epidemiol Biomarkers Prev. 1998;8:749–57. [PubMed] [Google Scholar]
- 12.Woloshin S, Schwartz L, Tremmel J, Welch H. Direct-to-consumer advertisements for prescription drugs: what are Americans being sold? Lancet. 2001;358:1141–6. doi: 10.1016/S0140-6736(01)06254-7. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman J, Wilkes M. Direct to consumer advertising of prescription drugs (editorial) BMJ. 1999;318:1301–2. doi: 10.1136/bmj.318.7194.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Streja D, Rabkin S. Factors associated with implementation of preventive care measures in patients with diabetes mellitus. Arch Intern Med. 1999;159:294–302. doi: 10.1001/archinte.159.3.294. [DOI] [PubMed] [Google Scholar]
- 15.Osborn E, Bird J, McPhee S, et al. Cancer screening by primary care physicians. Can we explain the differences? J Fam Pract. 1991;32:465–71. [PubMed] [Google Scholar]
- 16.Cabana M, Rand C, Powe N, et al. Why don't physicians follow clinical practice guidelines? JAMA. 1999;282:1458–65. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 17.Kenealy T, Arroll B, Kenealy H, et al. General practice changes in south Auckland from 1990 to 1999: a threat to continuity of care? N Z Fam Phys. 2002;29:387–90. [Google Scholar]
- 18.Health Funding Authority. Diabetes 2000. Wellington: Health Funding Authority; 2000. [Google Scholar]
- 19.Herman W, Smith P, Thompson T, et al. A new and simple questionnaire to identify people at increased risk for undiagnosed diabetes. Diabetes Care. 1995;18:382–7. doi: 10.2337/diacare.18.3.382. [DOI] [PubMed] [Google Scholar]
- 20.American Diabetes Association. Screening for type 2 diabetes. Diabetes Care. 2000;23(suppl 1):S20–3. [PubMed] [Google Scholar]