Abstract
The kinetics of the electron transfer reaction between reduced [2Fe-2S] ferredoxins and select nitroimidazole antimicrobial agents is reported. The ferredoxins from the protozoan Trichomonas vaginalis and the cyanobacterium Anabaena sp. strain 7120 were studied because they are the proximal electron donors to nitroimidazoles in these two organisms with significantly different nitroimidazole susceptibilities. The rates of electron transfer from Anabaena ferredoxin to all nitroimidazoles were 1 to 2 orders of magnitude lower than for T. vaginalis ferredoxin. Quantitative structure-activity analysis of the kinetic data showed that the size of the alkyl substituent on the N-1 position of the imidazole ring strongly influenced the magnitude of the electron transfer rate constant. This implies that the distance between the iron-sulfur cluster and the nitro group of the imidazole is the critical variable in determining the rate of electron transfer. A correlation between the magnitude of the one-electron transfer rate constant with the susceptibility of the host organism to the cytotoxic effects of nitroimidazoles was also discovered. These results demonstrate that reductive activation is the most crucial step in determining the toxicity of nitroimidazoles.
Metronidazole [1-(2-hydroxyethyl)-2-methyl-5-nitroimidazole (2)] is a member of the nitroheterocycle class of antimicrobial compounds that also includes the nitroimidazole tinidazole and the nitrofuran nitrofurantoin. Metronidazole is a front-line antibiotic in use against a wide range of infectious species. For example, metronidazole is highly effective in treating trichomoniasis and is equally useful against other protozoal infections, including giardiasis and amebiasis (6). Further, metronidazole is the primary drug used to treat postoperative and other infections with anaerobic bacteria, including members of the genera Bacteroides, Fusobacterium, and Clostridium. In addition metronidazole is a component of the therapeutic regimen to eradicate Helicobacter pylori, which is responsible for the majority of cases of peptic ulcer disease and is a risk factor for gastric cancer (4).
The importance of metronidazole in treating various infectious diseases and its unusually broad spectrum of activity have led to investigation of its mechanism of action. Most studies have focused on the activity of nitroimidazoles against the protozoan Trichomonas vaginalis, the causative organism of trichomoniasis in humans. Researchers initially believed that the drug inhibited hydrogenase, an enzyme involved in anaerobic carbohydrate metabolism (6). Further studies showed, however, that the ultimate target of the drug was DNA, and that nitroimidazoles not only inhibited DNA synthesis, but also degraded existing DNA (16). Labeling experiments implicated a radical intermediate as the agent responsible for DNA degradation. Within anaerobic cells nitroimidazoles were found to be reduced to radical anion species with the ability to initiate DNA degradation through abstraction of atoms of the nucleic acid backbone (5). The redox potential of nitroimidazoles is more positive than the internal redox environment of anaerobic cells, but is more negative than the internal redox environment of aerobic cells (5). This provides a driving force for radical anion formation in anaerobic cells and satisfactorily explains the selective toxicity of nitroimidazoles toward anaerobic organisms.
Despite decades of use, metronidazole has been noteworthy for the relatively low level of resistance developed by organisms to its cytotoxic effects. Recently, however, the isolation of resistant strains of H. pylori (19), T. vaginalis (17), Entamoeba histolytica (25), and Giardia lamblia (11) has been reported. Characterization of these strains has revealed altered structures or cellular levels of electron transfer proteins. For example, metronidazole-resistant strains of T. vaginalis, G. lamblia, and E. histolytica have all been shown to harbor reduced levels of small iron-sulfur proteins, termed ferredoxins, relative to sensitive strains (17, 11, 25). Similarly, metronidazole-resistant strains of H. pylori display mutations in the fdrX gene, which codes for an NADPH-dependent nitroreductase (19). These observations implicate decreased reductive activation of metronidazole as a common mechanism for resistance.
To date there is a poor understanding of the structural properties of nitroimidazoles that determine their activity against microorganisms. Studies have shown that neither the nitroimidazole redox potential nor its partition coefficient correlates well with the toxicity of the agent toward T. vaginalis (5, 26). In light of the role of ferredoxin in the reductive activation of metronidazole, the reaction between nitroimidazoles and this iron-sulfur protein became a focus of research. The most-extensive work has centered on the ferredoxin from T. vaginalis. Lindmark and Müller discovered that metronidazole reduction by ferredoxin-depleted extracts of T. vaginalis was greatly increased upon addition of exogenous ferredoxin (10). Moreno et al. observed that the intensity of the signal for the metronidazole radical anion in extracts from the trichomonad Tritrichomonas foetus was substantially amplified by addition of ferredoxin (14).
Detailed structure-activity studies would elucidate the properties of nitroimidazoles that influence reactivity with ferredoxins and, in turn, antimicrobial activity. Prior to the present work, however, no kinetic constants for the reaction of any electron transfer protein with nitroimidazoles have been published. Herein we report the first measurements of the kinetics of reduction of select nitroimidazoles (Table 1) by reduced ferredoxins. We employed [2Fe-2S] ferredoxins from two distinct metronidazole-susceptible organisms, T. vaginalis (7) and the vegetative form of cyanobacterium Anabaena sp. strain 7120 (1). The results of these experiments demonstrate that T. vaginalis ferredoxin is 1 to 2 orders of magnitude more reactive than the cyanobacterial ferredoxin in spite of the smaller driving force for reaction. Further, we report that the in vitro reactivities of the reduced ferredoxins toward nitroimidazoles parallel the susceptibilities of the host organisms to the agents.
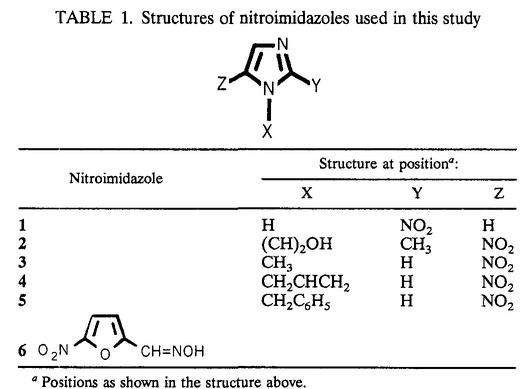
TABLE 1.
Structures of nitroimidazoles used in this study
Positions as shown in the structure above.
MATERIALS AND METHODS
Protein expression.
T. vaginalis ferredoxin was overexpressed in Escherichia coli as described elsewhere (22). Briefly, the E. coli strain HMS174(DE3) was transformed with a recombinant plasmid containing the gene for T. vaginalis ferredoxin. A culture of bacteria harboring the vector was grown in Luria-Bertani medium supplemented with 100 mg of ampicillin/liter followed by isopropyl-β-d-thiogalactopyranoside induction for ferredoxin expression. After cell disruption using a French press, purification of the protein product was achieved through anion-exchange chromatography on a Q-Sepharose column using a linear gradient from 100 to 300 mM Tris-HCl, pH 7.6, followed by fast-performance liquid chromatography gel filtration (Superdex 75). The target protein was greater than 95% pure by polyacrylamide gel electrophoresis and isoelectric focusing. Recombinant vegetative Anabaena sp. strain 7120 ferredoxin was overexpressed and purified according to the previously published procedure (23).
Nitroimidazole preparation.
1-Alkyl-5-nitroimidazoles 3 to 5 were prepared following methods described in the literature (2, 13). Each product was completely characterized by 1H and 13C nuclear magnetic resonance and elemental analysis. The substitution pattern of the products (1-alkyl-5-nitro versus 1-alkyl-4-nitroimidazole) was confirmed by UV spectroscopy, which revealed a characteristic blue shift of the maximum absorbance in 2 M H2SO4 relative to neutral solution for the 5-nitro isomers (Table 2) (4). Reduction potentials were obtained by cyclic voltammetry on a BAS 50W electrochemical system with a platinum electrode using 0.1 M acetonitrile solutions of the nitroimidazoles.
TABLE 2.
Yields and spectroscopic properties of 1-alkyl-5-nitroimidazoles from alkylation of 4-nitroimidazole
| Alkyl group of 1-alkyl-5-nitroimidazole | Alkylating agent | Yield (%) | Max UV absorbance (nm)
|
|
|---|---|---|---|---|
| 2 M H2SO4 | H2O | |||
| CH3 (3) | (CH3)2SO4 | 30 | 266 | 304 |
| CH2CH=CH2 (4) | ICH2CH=CH2 | 48 | 274 | 300 |
| CH2C6H5 (5) | BrCH2C6H5 | 45 | 276 | 298 |
Kinetics assays.
Kinetic measurements were carried out using an OLIS stopped-flow spectrophotometer. All reactions were carried out under pseudo-first-order conditions with oxidant in at least eightfold excess over protein. Ultra-high-purity argon was bubbled through all reactant solutions for 15 to 30 min. Protein solutions were prepared in an anaerobic chamber by adding aliquots of concentrated ferredoxin samples (∼2 mM), degassed by blowing humidified argon over the surface for 15 min, and added to deoxygenated buffer. Sodium dithionite solution (0.1 M) was prepared by adding 5 ml of degassed 10 mM Tris-HCl, pH 8.0, containing 0.15 ml of 3 M NaOH to 87 mg of fresh sodium dithionite. The tube containing solid dithionite was repeatedly evacuated and filled with argon before the buffer solution was added. The reduced protein was generated by adding 2 to 3 equivalents of sodium dithionite to the ferredoxin solution under anaerobic conditions. Solutions of oxidants and ferredoxins were made up at the required pH, and ionic strength was adjusted by addition of 5 M NaCl solution.
Reoxidations of reduced T. vaginalis and Anabaena ferredoxins by nitroheterocycles were monitored at 458 and 422 nm, respectively. At least five kinetic traces were collected at the appropriate wavelengths for each oxidant concentration; first-order rate constants (kobs) were calculated by computer fitting to a single-exponential rate equation using the SI-FIT program (OLIS, Inc.).
RESULTS
The interaction between the nitroimidazoles and the ferredoxins from Anabaena and T. vaginalis was investigated by stopped-flow spectrophotometry by monitoring the one-electron oxidation of the reduced ferredoxins by the nitroimidazoles. 1-Alkyl-5-nitrosubstituted imidazoles (2 to 5) were chosen for detailed investigation because these molecules were previously found to have greater antimicrobial activity than the 2-alkyl-4-nitro or the 2,4-dialkyl-5-nitroimidazole isomers (4). Nitroheterocycles 1, 2, and 6 were obtained from commercial suppliers, while 3 to 5 were prepared according to procedures in the literature (Table 2).
The ferredoxins were obtained through expression of the genes and isolation of the holoproteins in E. coli as described previously (22, 23). The oxidation of the dithionite-reduced [2Fe-2S] ferredoxins by nitroimidazoles was monitored under pseudo-first-order conditions as described by us for the reaction of the ferredoxins with inorganic reagents (24). The reaction traces were accurately modeled by single-exponential fits. The rate of disappearance of the oxidized nitroimidazoles coincided with the rate of the appearance of the oxidized forms of the ferredoxins. Interestingly, neither reduced ferredoxin was reoxidized by the nitrofuran antibiotic nitrofurazone 6.
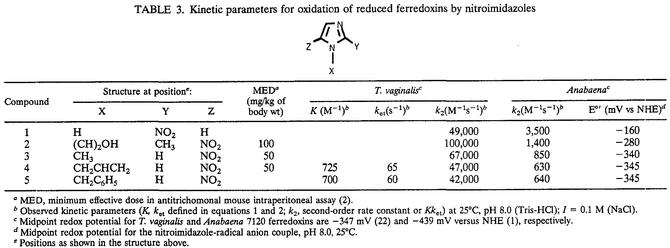
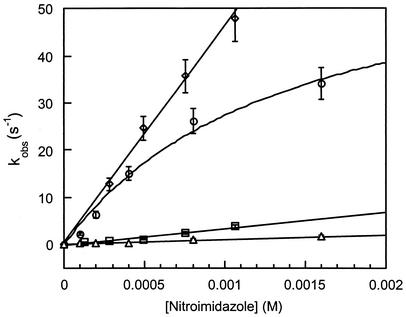
The reduced ferredoxins were readily oxidized by nitroimidazoles to their oxidized forms. Plots of kobs versus the concentration of the oxidant afforded values for the second-order rate constants (k2). (Table 3). The pseudo-first-order rate constants for reaction of Anabaena ferredoxin with nitroimidazoles 1 to 5 and for T. vaginalis ferredoxin with nitroimidazoles 1 to 3 displayed linear dependences on oxidant concentration, consistent with a second-order process (Fig. 1). The rate constants for nitroimidazoles 4 and 5, however, gave nonlinear concentration dependences for reaction with T. vaginalis ferredoxin. The approach to a limiting value for oxidation rate at increased nitroimidazole concentration suggested a mechanism in which bimolecular complex formation preceded electron transfer for these two heterocycles. Assuming the kinetic model shown in equations 1 and 2, in which K is the association constant, and ket is the electron transfer rate constant within the bimolecular complex, the pseudo-first-order rate constants for oxidation of T. vaginalis ferredoxin by nitroimidazoles 4 and 5 obeyed the relationship given in equation 3, where K is k1/k−1 (see reference 24):
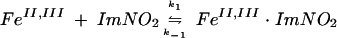 |
(1) |
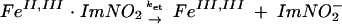 |
(2) |
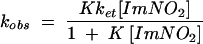 |
(3) |
TABLE 3.
Kinetic parameters for oxidation of reduced ferredoxins by nitroimidazoles
MED, minimum effective dose in antitrichomonal mouse intraperitoneal assay (2).
Observed kinetic parameters (K, ket defined in equations 1 and 2; k2, second-order rate constant or Kket) at 25°C, pH 8.0 (Tris-HCl); I = 0.1 M (NaCl).
Midpoint redox potential for T. vaginalis and Anabaena 7120 ferredoxins are −347 mV (22) and −439 mV versus NHE (1), respectively.
Midpoint redox potential for the nitroimidazole-radical anion couple, pH 8.0, 25°C.
Positions as shown in the structure above.
FIG. 1.
Concentration dependence of the pseudo-first-order rate constant for ferredoxin oxidation (kobs) on nitroimidazole concentration. Data points for oxidation by compound 2 of Anabaena ferredoxin (□) and T. vaginalis ferredoxin (⋄) and by compound 4 of Anabaena (▵) and T. vaginalis ferredoxin (○) were obtained from the average of five experiments. Standard errors of the mean are shown as vertical bars. Fits to concentration dependences were obtained by nonlinear least-squares fits.
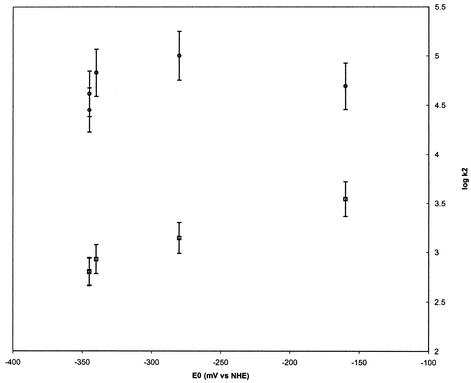
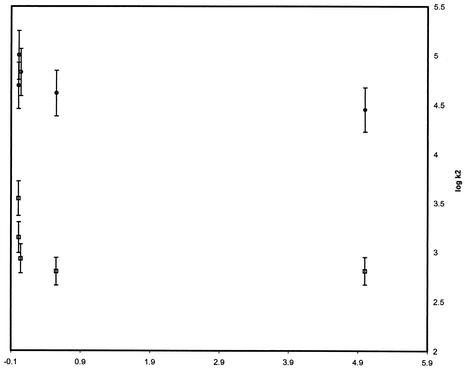
To identify the factors that influence the magnitude of the electron transfer rates, quantitative structure-activity analysis of the kinetics using specific thermodynamic or structural variables was undertaken. In order to discern the dependence of the reaction rate on the thermodynamic driving force, the logarithm of the second-order rate constant was plotted against the reduction potential of the nitroimidazole (Fig. 2). A linear relationship is expected for systems that adhere to Marcus's law, which relates the rate of outer-sphere electron transfer with the thermodynamics of the process (12). A simplified form of the Marcus equation is provided in equation 4, in which ΔG0 is the reaction free energy (directly proportional to the nitroimidazole reduction potential) and λ is the reorganization energy. As seen in Fig. 2, although the nitroimidazole-Anabaena reaction system roughly conformed to the Marcus relationship, the T. vaginalis system did not produce a linear correlation. This finding signified that the latter system adhered particularly poorly to Marcus's law.
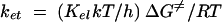 |
(4) |
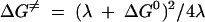 |
FIG. 2.
Marcus plot of logarithm of the second-order rate constant for oxidation of Anabaena (□) or T. vaginalis ferredoxin (○) against the reduction potential of the nitroimidazole. Data points are the average of five experiments. Standard errors of the mean are shown as vertical bars.
To discern which structural factors control electron transfer rates from the ferredoxins to the nitroimidazoles, the logarithms of the rate constants were plotted against the isooctanol-water partition coefficient K for the nitroimidazole (Fig. 3) (4). This parameter is proportional to the hydrophobicity of the species, with more-hydrophobic molecules displaying larger values of K. For both Anabaena and T. vaginalis ferredoxin, electron transfer rates tended to decrease as the value of K increased, although the correlation for neither system was exceptional. This result implies that, although the hydrophobicity of the nitroimidzole influenced the electron transfer rate, it was not the sole factor that determined the kinetics of the reaction process between nitroimidazoles and ferredoxins.
FIG. 3.
Plot of the logarithm of the second-order rate constant for oxidation of Anabaena (□) or T. vaginalis ferredoxin (○) against the partition coefficient K of the nitroimidazole (x axis). Data points are the average of five experiments. Standard errors of the mean are shown as vertical bars.
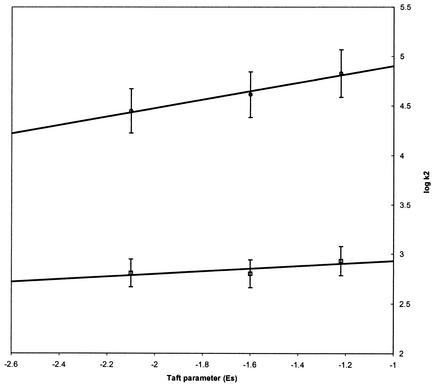
Finally the logarithm of electron transfer rate constants was plotted against the Taft steric parameter Es for the N-alkyl group of the nitroimidazoles 3 to 5 (Fig. 4). This parameter is a measure of the steric bulk of the alkyl group in question, increasing in magnitude with increasing bulkiness of the alkyl moiety (8). In contrast with the partition coefficient correlation, the logarithm of the rate constants for both the Anabaena and T. vaginalis electron transfer reactions displayed good linear correlations with the Es value of the alkyl group. This observation indicated that the steric bulk of the N-alkyl substituent of the nitroimidazole is an important factor that determines the magnitude of the electron transfer reaction between nitroimidazoles and ferredoxins.
FIG. 4.
Plot of the logarithm of the second-order rate constant for oxidation of Anabaena (□) or T. vaginalis ferredoxin (○) against the value of the Taft steric parameter Es of the N-1 substituent of the nitroimidazole. Data points are the average of five experiments. Standard errors of the mean are shown as vertical bars.
DISCUSSION
Ferredoxins have been implicated as the proximal electron donors to metronidazole in vivo, and their altered expression is a common mechanism for resistance to nitroimidazole antimicrobial agents. To characterize the factors that control the reaction between nitroimidazoles and reduced [2Fe-2S] ferredoxins, we measured the electron transfer rates between select 5- and 2-nitroimidazoles and the reduced ferredoxins from Anabaena and T. vaginalis. Several trends were discovered in our analysis of the results.
First, the T. vaginalis ferredoxin was 1 to 2 orders of magnitude more reactive toward all nitroimidazoles than the Anabaena protein. This finding is somewhat surprising in light of the more-negative reduction potential of the Anabaena ferredoxin, which would result in a greater thermodynamic driving force relative to the protozoal ferredoxin. This observation implies that the driving force is not the primary factor that determines reaction rate between [2Fe-2S] ferredoxin and nitroimidazoles. The Marcus plot in Fig. 2 confirms this conclusion. A linear correlation between the logarithm of the rate constant and the electrochemical potential difference is expected for systems of constant reorganization energy and electronic coupling between the redox partners. The fact that the correlation for the T. vaginalis protein was not linear, however, implies that the reorganization energy or the coupling between the redox centers are the defining factors.
Focused analysis of the data leads to the conclusion that the coupling or, in this instance, the distance separating the iron-sulfur cluster and the nitro group of the imidazole is the critical factor that determines the electron transfer rate. Inspection of Fig. 3 reveals a poor correlation between the partition coefficient of the nitroimidazole and its reactivity; this result implies that the reactivity of the nitroimidazole is not particularly influenced by its hydrophobicity. According to Fig. 4, on the other hand, the electron transfer reactivities of the nitroimidazoles with both ferredoxins decreased linearly with the size of the nitrogen substituent, as measured by the Taft steric parameter Es. This finding is consistent with distance being the defining factor in electron transfer, with larger groups hindering approach of the electron accepting nitro group to the electron-donating iron-sulfur center.
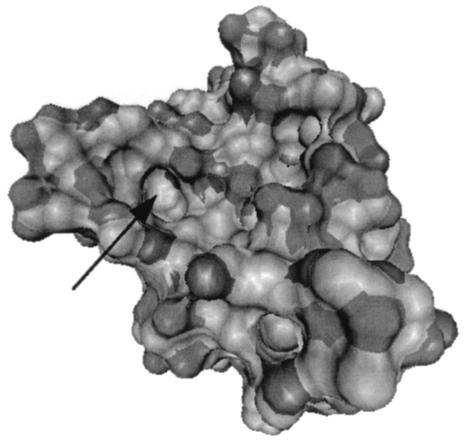
The difference in reactivity between the two ferredoxins is also likely due to distance modulation of the electron transfer rates. Support for this hypothesis comes from analysis of the crystal structures of the two ferredoxins (3, 18). While the [2Fe-2S] clusters of both ferredoxins are bound to side chain sulfhydryl groups on similar peptide loops, the cluster of the T. vaginalis ferredoxin has an exposed bridging sulfur at the bottom of a cavity that is not found in the Anabaena protein (Fig. 5). The cavity measures 3.3 by 6.5 by 4.2 Å and is lined with hydrophobic amino acid side chains. Thus, small molecules, such as nitroimidazoles, may approach the redox center more closely in the trichomonal protein, resulting in faster electron transfer reactions. Another example of prosthetic group exposure affecting electron transfer reactivity was described by Tollin and coworkers (21). They concluded that differences in exposure of heme groups to exogenous molecules was responsible for differences in reactivity of different cytochrome c proteins to reduced flavins.
FIG. 5.
Surface of T. vaginalis ferredoxin showing cavity that exposes the bridging sulfur atom to solvent (arrow). Surface map was calculated using the VMD program by rolling a probe with a 1.4-Å radius over the atoms of the protein.
An intriguing conclusion drawn from our results is that microbial susceptibility to nitroimidazole agents is determined primarily by the reactivity of the cellular electron donor toward the nitroimidazole. For example, reduced T. vaginalis ferredoxin showed no reactivity toward the nitrofuran nifuroxime, and this agent is essentially inactive toward T. vaginalis (14). In addition, the in vitro concentration of metronidazole required to inhibit growth of T. vaginalis is 0.003 to 0.01 mM (15), while that for Anabaena is approximately 1 mM (20); this is consistent with the greater reactivity of T. vaginalis ferredoxin toward nitroimidazoles than Anabaena ferredoxin. It is well known that Anabaena possesses two distinct ferredoxins, the vegetative (18) and heterocyst forms (9), that function independently in photosynthesis and nitrogen fixation, respectively. Interestingly metronidazole displays differential inhibition in vitro of the nitrogen fixation and the photosynthesis activities of Anabaena: while metronidazole strongly inhibited the nitrogenase activity of Anabaena cells, the drug at the same concentration had little effect on photosynthesis (20). It would be interesting to compare electron transfer rates from the vegetative and heterocyst ferredoxins toward nitroimidazoles to see if differences in reactivity are consistent with the differences of in vitro inhibition of the two metabolic processes.
In conclusion, we have shown that nitroimidazoles react rapidly in vitro with reduced [2Fe-2S] ferredoxins and that reactivity of the nitroimidazole is primarily influenced by its ability to approach from the [2Fe-2S] center. Also we demonstrated that the reactivity of nitroimidazoles parallels their cytotoxicity toward T. vaginalis. These results indicate that in vitro kinetic assays may be useful screens for antitrichomonal activity of new nitroheterocycles. New generations of nitroheterocycle agents may be also devised that overcome resistance by selectively attacking different electron donor proteins, perhaps through affinity tags.
Acknowledgments
This publication was supported, in part, by a training fellowship from the Keck Center for Computational Biology (National Science Foundation GRT grant BIR-92-56580). We thank the NIH (grant R15GM61412) and the Robert A. Welch Foundation for generous financial support.
REFERENCES
- 1.Bohme, H., and B. Schrautemeier. 1987. Comparative characterization of ferredoxins from heterocysts and vegetative cells of Anabaena variabilis. Biochim. Biophys. Acta 891:1-7. [Google Scholar]
- 2.Butler, K., H. L. Howes, J. E. Lynch, and D. K. Pirie. 1967. Nitroimidazole derivatives. Relationship between structure and antitrichomonal activity. J. Med. Chem. 10:891-897. [DOI] [PubMed] [Google Scholar]
- 3.Crossnoe, C. R., J. P. Germanas, P. LeMagueres, G. Mustata, and K. L. Krause. 2002. The crystal structure of Trichomonas vaginalis ferredoxin provides insight into metronidazole activation. J. Mol. Biol. 308:503-518. [DOI] [PubMed] [Google Scholar]
- 4.Dunn, B. E., H. Cohen, and M. J. Blaser. 1997. Helicobacter pylori. Clin. Microbiol. Rev. 10:720-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edwards, D. I., J. H. Tochler, L. D. Dale, D. A. Widdick, D. A., and N. S. Virk. 1990. Effects on DNA of bioreducible nitroimidazole and benzotriazine drugs, p. 275-283. In G. E. Adams, A. Breccia, M. Fielden, and P. Wardman, (ed.), Selective activation of drugs by redox processes. Plenum Press, New York, N.Y.
- 6.Edwards, D. I. 1993. Nitroimidazole drugs-action and resistance mechanisms. I. Mechanisms of action. J. Antimicrob. Chemother. 31:9-20. [DOI] [PubMed]
- 7.Gorrell, T. E., N. Yarlett, and M. Muller. 1984. Isolation and characterization of Trichomonas vaginalis ferredoxin. Carlsberg Res. Commun. 49:259-268. [Google Scholar]
- 8.Hansch, C., A. Leo, and D. Hoekman. 1995. Exploring QSAR—hydrophobic, electronic, and steric constants. American Chemical Society, Washington, D.C.
- 9.Jacobson, B. L., Y. K Chae, J. L. Markley, I. Rayment, and H. M. Holden. 1993. Molecular structure of the oxidized, recombinant heterocyst [2Fe-2S] ferredoxin from Anabaena 7120 determined to 1.7 Å resolution. Biochemistry 32:6788-6793. [DOI] [PubMed] [Google Scholar]
- 10.Lindmark, D. G., and M. Muller. 1974. Reduction of metronidazole by homogenates of trichomonad flagellates. J. Protozool. 21:436-440. [DOI] [PubMed] [Google Scholar]
- 11.Liu, S. M., D. M. Brown, P. O'Donoghue, P. Upcroft, and J. A. Upcroft. 2000. Ferredoxin Involvement in metronidazole resistance of Giardia duodenalis. Mol. Biochem. Parasitol. 108:137-140. [DOI] [PubMed] [Google Scholar]
- 12.Marcus, R. A. 1956. On the theory of oxidation-reduction reactions involving electron transfer. J. Chem. Phys. 24:966-978. [Google Scholar]
- 13.Miller, M. W., H. L. Howes, R. V. Kasubick, and A. R. English. 1970. Alkylation of 2-methyl-5-nitroimidazole. Some potent antiprotozoal agents. J. Med. Chem. 13:849-852. [DOI] [PubMed] [Google Scholar]
- 14.Moreno, S. N. J., R. P. Mason, P. A. Muniz, F. S. Cruz, and R. Docampo. 1983. Generation of free radicals from metronidazole and other nitroimidazoles by Tritrichomonas foetus. J. Biol. Chem. 258:4051-4054. [PubMed] [Google Scholar]
- 15.Muller, M., and T. E. Gorrell. 1983. Metabolism and metronidazole uptake in Trichomonas vaginalis isolates with different metronidazole susceptibilities. Antimicrob. Agents Chemother. 24:667-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plant, C. W., and D. I. Edwards. 1976. The effect of tinidazole, metronidazole, and nitrofurazone on nucleic acid synthesis in Clostridium bifermentans. J. Antimicrob. Chemother. 2:203-209. [DOI] [PubMed] [Google Scholar]
- 17.Quon, D. V. K., C. E. D'Oliveira, and P. J. Johnson. 1992. Reduced transcription of the ferredoxin gene in metronidazole-resistant Trichomonas vaginalis. Proc. Natl. Acad. Sci. USA 89:4402-4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rypniewski, W. R., D. R. Breiter, M. M. Benning, G. Wesenberg, B. H. Oh, J. L. Markley, I. Raymet, and H. M. Holden. 1991. Crystallization and structure determination to 2.5 Å resolution of the oxidized [2Fe-2S] ferredoxin isolated from Anabaena 7120. Biochemistry 30:4126-4131. [DOI] [PubMed] [Google Scholar]
- 19.Sisson, G., J.-Y. Jeong, A. Goodwin, L. Bryden, N. Rossler, S. Lim-Morrison, A. Raudonikiene, D. E. Berg, and P. S. Hoffman. 2000. Metronidazole activation is mutagenic and causes DNA fragmentation in Helicobacter pylori and in Escherichia coli containing a cloned H. pylori rdxA+ nitroreductase gene. J. Bacteriol. 182:5091-5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tetley, R. M., and N. I. Bishop. 1979. The differential action of metronidazole on nitrogen fixation, hydrogen metabolism, photosynthesis and respiration in Anabaena and Scendesmus. Biochim. Biophys. Acta 546:43-53. [DOI] [PubMed] [Google Scholar]
- 21.Tollin, G., L. K. Hanson, M. Caffrey, T. E. Meyer, and M. A. Cusanovich. 1986. Redox pathways in electron-transfer proteins: correlations between reactivities, solvent exposure, and unpaired-spin-density distributions. Proc. Natl. Acad. Sci. USA 83:3693-3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vidakovic, M., G. Fraczkiewicz, and J. P. Germanas. 1996. Expression and spectroscopic characterization of the hydrogenosomal [2Fe-2S] ferredoxin from the protozoan Trichomonas vaginalis. J. Biol. Chem. 271:14734-14739. [DOI] [PubMed] [Google Scholar]
- 23.Vidakovic, M., G. Fraczkiewicz, B. C. Dave, R. S. Czernuszewicz, and J. P. Germanas. 1995. The environment of [2Fe-2S] clusters in ferredoxins: the role of residue 45 probed by site-directed mutagenesis and spectroscopic analysis. Biochemistry 34:13906-13911. [DOI] [PubMed] [Google Scholar]
- 24.Vidakovic, M., and J. P. Germanas. 1996. Electrostatic effects in electron transfer reactions of [2Fe-2S] ferredoxins with inorganic reagents. Protein Sci. 5:1793-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wassmann, C., A. Hellberg, E. Tannich, and I. Bruchhaus. 1999. Metronidazole resistance in the protozoan parasite Entamoeba histolytica is associated with increased expression of iron-containing superoxide dismutase and peroxiredoxin and decreased expression of ferredoxin 1 and flavin reductase. J. Biol. Chem. 274:26051-26056. [DOI] [PubMed] [Google Scholar]
- 26.Yarlett, N., T. E. Gorrell, R. Marczak, and M. Muller. 1985. Reduction of nitroimidazole derivatives by hydrogenosomal extracts of Trichomonas vaginalis. Mol. Biochem. Parasitol. 14:29-40. [DOI] [PubMed] [Google Scholar]