Abstract
Superior mesenteric artery (SMA) syndrome is a rare acquired disorder in which acute angulation of SMA causes compression of the third part of the duodenum between the SMA and the aorta, leading to obstruction. Loss of fatty tissue as a result of a variety of debilitating conditions is believed to be the etiologic factor causing the acute angulation. We report a case of an 86-year-old man with prolonged congestive heart failure and aortic stenosis in which SMA syndrome developed as a result of cardiac cachexia. Because of poor functional status and comorbidities, he was not a suitable candidate for decompressive surgery. Conservative treatment using a gastrostomy tube with jejunal extension led to improvement in nutritional status and resolution of symptoms.
Keywords: Cardiac cachexia, upper gastrointestinal obstruction, SMA syndrome, weight loss, enteral feeding
Superior mesenteric artery (SMA) syndrome (also known as Wilkie's syndrome) is a rare cause of upper gastrointestinal obstruction. This is usually associated with conditions that cause significant weight loss, such as anorexia nervosa, malabsorption, or hypercatabolic states such as burns, major surgery, severe injuries, or malignancies. A commonly overlooked but significant complication of congestive heart failure is cardiac cachexia, which is characterized by severe weight loss and muscle wasting. We describe a patient who developed SMA syndrome as a result of cardiac cachexia. To our knowledge, this association has not been reported previously.
CASE REPORT
An 86-year-old man was hospitalized with abdominal pain, early satiety, epigastric fullness, nausea, vomiting of partially digested food, and 13 kg weight loss over 3 months. Further interview revealed that weight loss had been more chronic and severe, with 29 kg (36% of original body weight) lost in the preceding 3 years. He had a history of hypertension, coronary artery disease, aortic stenosis (valve area 0.9 cm2; gradient 37 mmHg), and severe congestive heart failure with left ventricular ejection fraction (LVEF) of 10% to 15%. His cardiac status was optimized medically.
Physical examination revealed an elderly, cachectic patient. His blood pressure was 90/60 mmHg, heart rate was 110 beats/minute, and respiratory rate 24/minute. Neck veins were collapsed. Lungs were clear with a few basal crackles and scattered rhonchi. A grade 2/6 ejection systolic murmur was heard over the aortic area. Abdominal examination revealed a distended abdomen, mild epigastric tenderness, and hyperactive bowel sounds. There was no palpable organomegaly or clinical signs of ascites. There was trace pitting edema in the lower extremities and global muscle wasting.
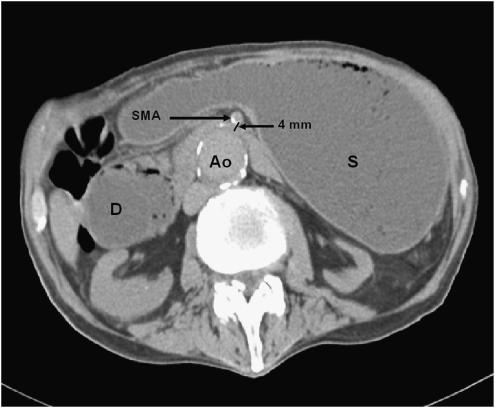
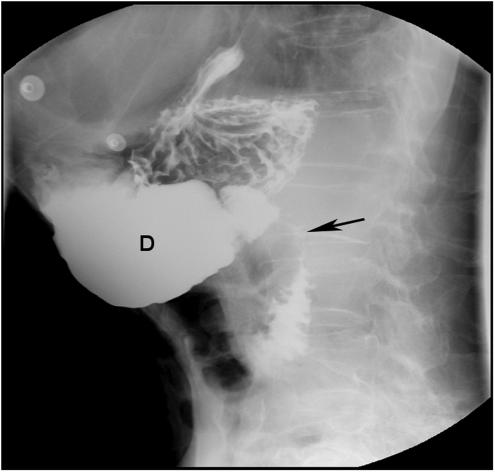
Abdominal radiograph revealed a dilated stomach with a prominent air fluid level. Subsequent abdominal computerized tomography (CT) scan showed a markedly enlarged fluid- and air-filled stomach and dilatation of proximal duodenum that tapered near the SMA (Fig. 1). The small bowel distal to the SMA was decompressed. Endoscopic examination of the upper gastrointestinal tract revealed mild esophagitis, dilated stomach and proximal duodenum, and narrowing of the third part of the duodenum due to a pulsating extrinsic compression. Radiologic evaluation of the upper gastrointestinal (GI) tract showed no intrinsic mucosal abnormalities and confirmed extrinsic compression of the distal duodenum (Fig. 2). The clinical symptoms and signs in conjunction with these studies established the diagnosis of SMA syndrome. Because of poor functional status and comorbidities, the patient was not considered an appropriate candidate for definitive surgical intervention. A decompressive gastrostomy tube with a feeding jejunal extension was placed radiographically, leading to the gradual resolution of his gastrointestinal symptoms, improved nutrition, weight gain, and better overall functional status.
FIGURE 1.
Noncontrast computerized tomography scan of the abdomen showing dilated stomach and proximal duodenum. Compressed third part of the duodenum is seen between the calcified superior mesenteric artery and aorta. D, duodenum; Ao, aorta; S, stomach; SMA, superior mesenteric artery.
FIGURE 2.
Radiological evaluation of the upper gastrointestinal tract showing compression of the third part of the duodenum (←) and dilated proximal duodenum. D, duodenum.
DISCUSSION
SMA syndrome was first described by von Rokitanski in 1861. Later, Wilkie provided a more detailed clinical and pathophysiologic description in a series of 64 patients and suggested treatment approaches.1 The defining feature of this entity is upper gastrointestinal obstruction caused by compression of the third part of the duodenum between the SMA anteriorly and the aorta posteriorly. Normally, fat and lymphatic tissues around the SMA provide protection to the duodenum against compression. Under conditions of severe weight loss, this cushion around the SMA is diminished, causing angulation and reduction in the distance between the aorta and the superior mesenteric artery. Normally, the aortomesenteric angle and aortomesenteric distance is 25° to 60° and 10 to 28 mm, respectively. In SMA syndrome, both parameters are reduced, with values of 6° to 15° and 2 to 8 mm.2 Conditions like increased spinal lordosis, application of a body cast, short ligament of Treitz, or unusually low origin of SMA may also precipitate this syndrome. Rarely, SMA syndrome has been reported after abdominal surgery.
Patients with SMA syndrome may present acutely, with chronic insidious symptomatology, or with an acute exacerbation of chronic symptoms. The acute presentation is usually characterized by signs and symptoms of duodenal obstruction. Chronic cases may present with long-standing vague abdominal symptoms, early satiety and anorexia, or recurrent episodes of abdominal pain, associated with vomiting.
The diagnosis of SMA syndrome is based on clinical symptoms and radiologic evidence of obstruction. Plain radiograph demonstrates a dilated, fluid- and gas-filled stomach. Barium radiography shows dilatation of the first and second part of the duodenum, extrinsic compression of the third part, and a collapsed small bowel distal to the crossing of the SMA. Contrast-enhanced CT scan or magnetic resonance angiography (MRA) enable visualization of vascular compression of the duodenum and measurement of aortomesenteric distance precisely. Both these procedures are noninvasive and are probably equivalent to angiography, which has previously been suggested as the reference standard for establishing the diagnosis.3,4 Endoscopic examination may visualize a pulsatile extrinsic compression suggestive of this condition.4
Traditionally, treatment has consisted of conservative measures such as nasogastric decompression and hyperalimentation followed by oral feeding with frequent small meals. Posturing maneuvers during meals and motility agents may be helpful in some patients. Surgery may be considered if conservative treatment fails. Duodenojejunostomy is effective in the majority of patients.5 A 7-year follow-up study of 16 patients treated with duodenojejunostomy found that outcome was regarded as excellent by 3 patients, good by 6, satisfactory by 5, and poor by 2 patients.6 Although effective, the invasive nature of this treatment is a drawback, especially for debilitated patients. Laparoscopic duodenojejunostomy offers a new minimally invasive therapeutic approach to SMA syndrome.7,8 Laparoscopic surgery involving lysis of the ligament of Treitz with mobilization of the duodenum is another minimally invasive approach.
In our patient, the SMA syndrome was caused by severe malnutrition secondary to advanced, long-standing cardiac failure. Patients with advanced chronic heart failure often develop severe weight loss and generalized wasting, also known as cardiac cachexia. This can lead to a pathophysiologic scenario conducive to the development of SMA syndrome. The resulting chronic anorexia, early satiety, nausea, and vomiting can further exacerbate malnutrition and accelerate the progression of SMA syndrome, as may have happened in our patient, who had chronic gradual weight loss for 3 years before developing more acute symptoms and rapid deterioration over 3 months.
Based on our experience, we feel that it is important for physicians to remain alert to the possible association of SMA syndrome and cardiac cachexia. This association should be considered in patients with congestive heart failure and weight loss, especially if symptoms suggesting proximal bowel obstruction are present. Early recognition may allow appropriate management by interrupting the cycle of weight loss and secondary upper gut obstruction from SMA syndrome. For patients not amenable to more definitive therapy, a gastrostomy tube for decompression with a jejunal extension available for feeding appears to be a reasonable and safe treatment option.
References
- 1.Wilkie D. Chronic duodenal ileus. Br J Surg. 1921:204–14. [Google Scholar]
- 2.Lippl F, Hannig C, Weiss W, Allescher HD, Classen M, Kurjak M. Superior mesenteric artery syndrome: diagnosis and treatment from the gastroenterologist's view. J Gastroenterol. 2002;37:640–3. doi: 10.1007/s005350200101. [DOI] [PubMed] [Google Scholar]
- 3.Bedoya R, Lagman SM, Pennington GP, Kirdnual A. Clinical and radiological aspects of the superior mesenteric artery syndrome. J Fla Med Assoc. 1986;73:686–9. [PubMed] [Google Scholar]
- 4.Gustafsson L, Falk A, Lukes PJ, Gamklou R. Diagnosis and treatment of superior mesenteric artery syndrome. Br J Surg. 1984;71:499–501. doi: 10.1002/bjs.1800710706. [DOI] [PubMed] [Google Scholar]
- 5.Hines JR, Gore RM, Ballantyne GH. Superior mesenteric artery syndrome. Diagnostic criteria and therapeutic approaches. Am J Surg. 1984;148:630–2. doi: 10.1016/0002-9610(84)90339-8. [DOI] [PubMed] [Google Scholar]
- 6.Ylinen P, Kinnunen J, Hockerstedt K. Superior mesenteric artery syndrome. A follow-up study of 16 operated patients. J Clin Gastroenterol. 1989;11:386–91. [PubMed] [Google Scholar]
- 7.Gersin KS, Heniford BT. Laparoscopic duodenojejunostomy for treatment of superior mesenteric artery syndrome. Jsls. 1998;2:281–4. [PMC free article] [PubMed] [Google Scholar]
- 8.Richardson WS, Surowiec WJ. Laparoscopic repair of superior mesenteric artery syndrome. Am J Surg. 2001;181:377–8. doi: 10.1016/s0002-9610(01)00571-2. [DOI] [PubMed] [Google Scholar]