Abstract
A case of 65-year-old male is reported who presented with myalgias, headache, and fever. He subsequently developed myocarditis and was diagnosed to have anaplasmosis on peripheral blood smear. He was treated with doxycycline for 30 days. A coronary angiogram done after recovery showed normal epicardial arteries. The case illustrates the importance of a careful examination of the peripheral smear, with a high index of clinical suspicion, which led to prompt treatment and complete recovery of the patient.
Keywords: Ehrlichiosis, myocarditis, peripheral smear
Ehrlichiosis, first discovered before 1910, has been recognized to cause human infection since 1986.1 It belongs to Rickettsiaceae family. Ehrlichiae are small, obligate intracellular bacteria that grow in cytoplasmic vacuoles to form clusters called morulae. Three distinct species cause human ehrlichiosis. E. chaffeensis predominantly affects the monocytes and is hence termed human monocytic ehrlichiosis (HME) while E. phagocytophilium, and E. ewingii cause human granulocytic ehrlichiosis.2E. phagocytophiliumE. equi are now recognized as the same organism and has been renamed Anaplasma phagocytophilum; the disease is now known as Human Granulocytic Anaplasmosis (HGA). Both HME and HGA share similar clinical and laboratory features and are treated with the same antimicrobials.
Two cases causing myocarditis have been reported with human ehrlichiosis.3,4 On both occasions, diagnoses were made retrospectively. We report a case of human ehrlichiosis involving the heart where the diagnosis was made on careful examination of peripheral smear which led to prompt treatment and complete recovery of the patient.
CASE REPORT
A previously healthy 65-year-old male presented to the emergency room with 4 days of generalized myalgias, fever, chills, diaphoresis, headaches, nonproductive cough with progressive dyspnea on minimal exertion, nausea, vomiting, and diarrhea. He reported no tick bite or rash. He worked in real estate and approximately 2 weeks prior to his illness, he was out in a prairie in central Minnesota, an area known for Lyme's disease and Human Ehrlichiosis.5
On admission, he had a fever of 103.7°F with relative bradycardia of 75/minutes and blood pressure of 92/50 mm Hg. His respiratory rate was 24/minutes with oxygen saturations of 98% on room air. He appeared pale but anicteric. There was no jugular venous distension. Chest, cardiac, and abdominal examination was unremarkable. There was no lower extremity edema. Skin examination did not reveal rashes or petechiae. Neurologically, he was alert and oriented without meningeal signs or focal neurologic deficits.
Laboratory data revealed a low WBC count of 3,300/mm3, hemoglobin of 11.5 g/dL, and platelets of 31,000/mm3. He had mild hyponatremia (sodium of 129 meq/L) with blood urea nitrogen of 57 mg/dL, creatinine of 3.4 mg/dL without acidosis. Prothrombin time and partial thromboplastin times were within normal limits. Liver function tests showed normal bilirubin with elevated serum aspartate aminotransaminase of 318 IU/L, serum alanine aminotransaminase of 143 IU/L, and alkaline phosphatase of 198 IU/L. Amylase and lipase were normal. Chest X-ray and urinalysis were normal.
The patient was assessed as being dehydrated and treated with fluid boluses. The next morning, he was noted to be in congestive heart failure which was confirmed on chest X-ray. Electrocardiogram showed hyperacute T waves and 0.5 mm ST segment elevation in the lateral leads. Troponin I was 130 ng/mL (0 to 0.5 ng/mL) while CK was 1,483 IU/L (30 to 107 U/L) with a CK-MB of 105 IU/L (<12 IU/L). Echocardiogram was done subsequently which showed a reduced ejection fraction of 35% with global hypokinesis and only trace mitral regurgitation. There was no evidence of pericardial effusion.
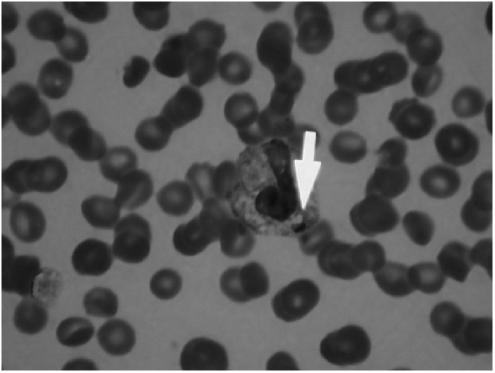
Patient was transferred to the intensive care unit for hemodynamic support with vasopressors. In the interim, his peripheral smear showed intracytoplasmic inclusions consistent with HGA (see Fig. 1). There was no evidence of microangiopathic hemolytic anemia. Lyme's titers were negative.
FIGURE 1.
Peripheral blood smear showing characteristic intracytoplasmic inclusions of human granulocytic anaplasmosis.
The patient was treated with intravenous doxycycline. He rapidly improved and continued to do so over the next few days. Echocardiogram 3 days later showed improvement in ejection fraction to 45%. Five days after hospitalization, he was discharged home on oral doxycycline 100 mg twice a day to complete a 30 days treatment course. His platelet counts returned to normal and creatinine improved to 1.9 mg/dL on discharge.
Because of his age, ECG changes and new diagnosis of heart failure with enzymatic evidence of myocardial necrosis, he was scheduled for an elective coronary angiogram/ventriculogram, a few weeks after hospital discharge. This revealed normal epicardial coronary arteries with normal left ventricular size and function. His clinical course has remained uneventful for over 3 years since.
DISCUSSION
HGA is a zoonotic infection spread to human by tick bite. HGA has been documented in Wisconsin, Minnesota, Connecticut, New York, Massachusetts, California, Florida, and western Europe. Most cases occur in the spring and summer months.
Typically, patients present with fever, myalgias, and malaise mimicking influenze-like illness.6 A small percentage has gastro-intestinal complaints. Severe complications occur at extremes of age including adult respiratory distress syndrome (ARDS) and toxic shock-like syndrome.6 Typical laboratory findings include leucopenia, thrombocytopenia, and elevated liver enzymes. A thorough peripheral smear examination can identify 20% to 75% of infections.7 Intracytoplasmic inclusions (morulae) are characteristic and diagnostic.6 Polymerase chain reaction on blood collected before initiation of antibiotics leads to a better diagnostic yield. Serodiagnosis is based on 4-fold increase in Ig M antibodies.8
The differential diagnosis for HGA is varied. Rocky Mountain spotted fever can sometimes be confused with HGA. However, absence of rash and leucopenia makes ehrlichia more likely. Ehrlichiosis and anaplasmosis may also be confused with a wide array of common viral illnesses (such as mononucleosis), thrombotic thrombocytopenic purpura, hematologic malignancy, cholangitis, the early phases of hepatitis A infection, and community acquired pneumonia especially in the immunosuppressed population.
A substantial proportion of patients with HGA develop serologic reactions considered diagnostic of Lyme disease in the absence of clear findings consistent with that diagnosis.9 Hence, HGA should always be considered in the differential diagnosis of atypical or early presentations of Lyme disease. Presence of high grade fever, cough, leucopenia, thrombocytopenia, and abnormal liver function tests in a patient who has erythema migrans and serologic tests positive for Lyme disease should prompt the clinician for a superimposed human ehrlichiosis or babesiosis.
Myocardial involvement has been frequently documented with rocky mountain spotted fever.10 Typically patients present with heart failure, myocardial infarction, cardiac arrhythmias, or conduction disturbances. Most of the cases have been fatal. However, myocarditis with Ehrlichiosis and Anaplasmosis is a rare finding with only 2 previously reported cases, 1 of which was fatal. A case of human ehrlichiosis causing left ventricular dilatation and dysfunction without electrocardiographic or chemical evidence of myocarditis has been described by Vanek et al.11 The pathogenic mechanism of cardiac involvement is incompletely known. While immunohistochemical staining demonstrated HGA in the cytoplasm of inflammatory cells in autopsy specimens of the perivascular myocardial tissue, it is uncertain whether HGA directly induces myocardial damage or if it induces transient immunosuppression and concomitant inflammatory cell dysfunction that causes nonspecific damage to myocytes.
Although our patient denied any history of tick bite, indirect questioning made us suspicious of a possible zoonotic infection. Interestingly, Central Minnesota where this patient visited, was reported to have the highest number of cases of Human Ehrlichiosis in Minnesota in 2000.5
We believe that our patient had myocarditis related to Anaplasmosis as manifested by peripheral smear examination, profoundly elevated cardiac enzymes, clinical evidence of congestive heart failure with a moderately decreased ejection fraction, normal coronary angiogram, and recovery of cardiac function after prompt treatment. As with any infectious agent, anaplasmosis can be complicated by development of ARDS and/or sepsis. However, the severity of myocardial enzyme leak with depression in cardiac function made myocarditis the more likely underlying cause. Serologies for human ehrlichiosis were not done on our patient as intracytoplasmic morulae are considered diagnostic. Although, acute coronary syndrome was a strong differential and under ideal circumstances should be immediately intervened, we could not start our patient on aspirin or anticoagulants secondary to thrombocytopenia, β-blockers owing to acute heart failure and angiotensin converting enzyme inhibitor secondary to renal failure. For the same reason, no coronary angiogram could be done at that time.
Although Lyme serology was negative, this did not rule out lyme carditis as positive titers take 6 to 8 weeks.12 Although the recommended treatment for human ehrlichiosis is at least 7 days or for 3–5 days after defervescence, we treated our patient with a 30 days course of oral doxycycline as we could not definitively rule out lyme carditis.
In summary, myocarditis with human ehrlichiosis can be fatal. Careful examination of the peripheral smear along with a high index of suspicion based on clinical symptoms and laboratory values is the key to prompt diagnosis and complete recovery.
Acknowledgments
We are grateful to Dr. Amin Rahmatullah for editing the manuscript.
References
- 1.Chen SM, Dumler JS, Bakken JS, Walker DH. Identification of a granulocytotrophic ehrlichia species as the etiologic agent of human disease. J Clin Microbiol. 1994;32:589. doi: 10.1128/jcm.32.3.589-595.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakken JS, Dumler JS. Human granulocytic ehrlichiosis. Clin Infect Dis. 2000;31:554. doi: 10.1086/313948. [DOI] [PubMed] [Google Scholar]
- 3.Jahangir A, Kolbert C, Edwards W, Mitchell P, Dumler JS, Persing DH. Fatal pancarditis associated with human granulocytic Ehrlichiosis in a 44-year-old man. Clin Infect Dis. 1998;27:1424–7. doi: 10.1086/515014. [DOI] [PubMed] [Google Scholar]
- 4.Williams JD, Snow RM, Arciniegas JG. Myocardial involvement in a patient with human ehrlichiosis. Am J Med. 1995;98:414–5. doi: 10.1016/S0002-9343(99)80324-6. [DOI] [PubMed] [Google Scholar]
- 5. Annual Summary of Communicable Diseases Reported to the Minnesota Department of Health, 2000; June 2001; 29:17–36.
- 6.Bakken JS, Krueth J, Wilson-Noldskog C, et al. Clinical and laboratory characteristics of human granulocytic ehrlichiosis. JAMA. 1996;275:199–205. [PubMed] [Google Scholar]
- 7.Bakken JS, Aguero-Rosenfeld ME, Tilden RL, et al. Serial measurements of hematologic counts during the active phase of human granulocytic ehrlichiosis. Clin Infect Dis. 2001;32:862–70. doi: 10.1086/319350. [DOI] [PubMed] [Google Scholar]
- 8.Walder G, Tiwald G, Dierich MP, Wurzner R. Serological evidence for human granulocytic ehrlichiosis in Western Austria. Eur J Clin Microbiol Infect Dis. 2003;22:543–7. doi: 10.1007/s10096-003-0986-3. [DOI] [PubMed] [Google Scholar]
- 9.Nadelman RB, Horowitz HW, Hsieh TC, et al. Simultaneous human granulocytic ehrlichiosis and Lyme borreliosis. N Engl J Med. 1997;337:27–30. doi: 10.1056/NEJM199707033370105. [DOI] [PubMed] [Google Scholar]
- 10.Walker DH, Paletta CE, Cain BG. Pathogenesis of myocarditis in Rocky Mountain spotted fever. Arch Pathol Lab Med. 1980;104:171–4. [PubMed] [Google Scholar]
- 11.Vanek NN, Kazi S, Cepero NM, Tang S, Rex JH. Human ehrlichiosis causing left ventricular dilatation and dysfunction. Clin Infect Dis. 1996;22:386–7. doi: 10.1093/clinids/22.2.386. [DOI] [PubMed] [Google Scholar]
- 12.Sigal LH. The polymease chain reaction assay for Borrelia burgdorferi in the diagnosis of Lyme disease (editorial) Ann Intern Med. 1994;120:520–1. doi: 10.7326/0003-4819-120-6-199403150-00013. [DOI] [PubMed] [Google Scholar]