Abstract
Introduction
Many smokers reduce their cigarette consumption during failed attempts to quit. We report the impact of changes in consumption on smoking-related respiratory symptom severity (SRRSS).
Methods
Between February 2002 and May 2004 we recruited 383 smokers from 5 methadone maintenance programs for a randomized trial of nicotine replacement plus behavioral treatment versus nicotine replacement alone for smoking cessation. Cigarette use in the 28 days prior to the interview, and severity of SRRSS using a 7-item respiratory index, were assessed at baseline and at 3-month follow-up.
Outcome
Baseline minus 3-month assessment difference in SRRSS score.
Results
Follow-up of 319 participants (83.3%), mean age 40.4 years, 51.4% male, who smoked 26.4 cigarettes per day, demonstrated a mean reduction of 16.7 cigarettes per day. A reduction in cigarette use was positively and significantly (b=0.29, t=5.16, P<.001) associated with a reduction in smoking-related symptom severity after adjusting for age, gender, race, years of regular smoking, baseline nicotine dependence, and history of treatment for asthma or emphysema. A 1 standard deviation reduction in average daily smoking (about 14.1 cigarettes) was associated with a 0.28 standard deviation decrease in smoking-related symptom severity.
Conclusion
Reduction in symptom severity increases as absolute reduction in daily smoking increases. This is the first study to demonstrate an association between subjective short-term health changes and reduction in smoking.
Keywords: cigarettes, symptoms, methadone, nicotine, quality of life
A variety of treatments to help smokers quit have been tested but have demonstrated limited efficacy.1 With many smokers either unable or unwilling to quit, the proportion of “treatment resistant” smokers may have increased as the overall smoking rate in the United States has decreased over the past decades.2 In response to this possibility, the concept of harm reduction through reducing cigarettes smoked per day has evolved both as an outcome measure in clinical trials and as a treatment strategy.3,4 A risk reduction model proposes that for many smokers reducing consumption is the first step to cessation. Those who are unable to quit are often highly dependent and have the highest tobacco-related health risk.5
Even in clinical trials that aim at cessation, reduction is a more common outcome than cessation, particularly among heavy smokers. In The Lung Health Study, a 3,923-person 5-year smoking cessation trial, for instance, nearly two-thirds had not continuously quit at the end of the first study year.6 However, most smokers in this study who tried to stop and failed subsequently reduced their smoking, and many were able to maintain reductions for long periods of time. Marked decreases in the number of daily cigarettes smoked have been consistently maintained across a variety of studies, and ongoing nicotine replacement therapy (NRT) has been shown to be safe as an aid in reduction.7,8
The impact of reducing the number of cigarettes smoked daily is uncertain. Demonstration of a reduced incidence of cancer or smoking-related cardiovascular or pulmonary diseases would require very large cohorts to be followed over many years; even then, the results could be confounded by other etiological factors. But exposure reduction may translate into clinically relevant health benefits over a much shorter time frame. For example, smoking-related respiratory symptoms (SRRS) may be 1 of the factors that lead smokers to quit attempts. It is possible that reduction in cigarette use may produce symptom relief and smokers may stop short of cessation when they experience symptom reduction, diminishing the perceived harms of smoking enough to disrupt a cessation attempt. Alternatively, respiratory symptom relief could reinforce efforts to quit. Yet there are no published studies specifically addressing smoking-related symptoms in the literature of cigarette reduction or cessation. Examining self-reports of respiratory symptom changes represents an important step in understanding smoking cessation and reduction.
Methadone maintenance treatment programs (MMTP) for opiate-dependent persons frequently have 80% to 90% prevalence of smokers, most often heavy smokers.9 Furthermore, among persons stable in methadone maintenance, tobacco use is strongly related to subsequent mortality, controlling for a wide array of health-risk behaviors.10 Considering these factors, this study explores the impact of changes in cigarette consumption on SRRS among methadone-maintained smokers enrolled in a smoking cessation trial.
METHODS
Procedure
Case Identification and Recruitment
Between February 2002 and May 2004, methadone-maintained smokers were recruited at 5 MMTP clinics in the greater Providence, RI, area for a randomized clinical trial to test, in combination with the nicotine patch, the incremental efficacy of an individually tailored behavioral treatment. Individuals who expressed an interest in the study met briefly with a research assistant (RA) who explained the study, determined interest, and assessed eligibility. To be eligible for the study, participants were: (1) age 18 years or older, (2) current, regular smokers of at least 10 cigarettes per day for the past 3 months, (3) English speaking, and (4) enrolled in MMTP for at least 3 months. However, participants did not have to agree to try to quit smoking or to use the nicotine patch.
Assessment
After the RA reviewed the inclusion/exclusion criteria, eligible participants signed an informed consent statement approved by the Lifespan Institutional Review Board. The participants then completed a questionnaire assessing sociodemographic information, aspects of smoking history (e.g., years, smoking quit attempts, etc.), level of motivation to quit smoking, mood, and social support items. The RA also assessed the participants' carbon monoxide concentration via a breath sample. The time to complete the introduction, consent, questionnaire, and breath sample averaged 45 minutes.
After a participant completed the questionnaire, the RA introduced the participant to the study interventionist. At this point, randomization and group assignment occurred. Study interventionists then performed the minimal treatment for those assigned to the control group or the maximal treatment for those assigned to the treatment condition.
Interventions
Minimal Treatment
Persons assigned to this group received self-help materials and direct advice from the interventionist to quit smoking. The advice to quit smoking message (≤3 minutes) followed NCI's 4 A's model (Ask, Advise, Assist, Arrange) for smoking cessation counseling.11 For smokers ready to set a quit date within 30 days, a follow-up visit was scheduled with the study interventionist on the participant's quit day to provide the nicotine patch and directions for its use.
Maximal Treatment
Participants in this condition received 2 visits: (a) an initial motivational interviewing session with the study interventionist that included information from the participant's breath sample, along with the written feedback report on the participant's level of motivation, level of smoking, prior quit attempts, environmental factors, and perceived vulnerability to smoking-related illnesses; (b) for smokers ready to set a quit date within 30 days, a follow-up visit was scheduled with the study interventionist on the participant's quit day to provide the nicotine patch and directions for its use. The motivational interviewing technique is intended to minimize participant resistance and increase response-efficacy and self-efficacy by providing a set of alternative response strategies.12
In both study arms, for those participants attending their quit day appointment, the interventionist dispensed the nicotine patch and described its proper use (i.e., placement, use of 1 patch per day, importance of not smoking while using the patch, and tapering of patches). Potential side effects were also described and participants were urged to call should they experience significant discomfort. The patch, Nicoderm® (Marion Merrill Dow, Kansas City, MO), was given such that the prescription had to be renewed once for an 8-week course of therapy or twice for a 12-week course of therapy. The 8-week course of therapy began with 4 weeks at full strength (21 mg), followed by tapering to the 14 mg patch for 2 weeks, and then reducing to 7 mg for the remaining 2 weeks. For those participants smoking more than 2 packs per day, a 12-week course of therapy was initiated: 4 weeks full strength (42 mg), followed by tapering to 35 mg for 2 weeks, 28 mg for 2 weeks, 21 mg for 2 weeks, 14 mg for 1 week, and then reducing to 7 mg for the remaining weeks.
Variables
Severity of SRRSS was assessed at both baseline and follow-up using a 7-item summated rating index adapted from the ATS/NHLBI respiratory symptoms scale.13,14 Items included coughing, phlegm, wheezing, sinus congestion, fatigue, pain/tightness in chest, and shortness of breath during exercise such as walking or going up the stairs. Each item was evaluated on a 4-point scale (none=0, mild=1, moderate=2, severe=3) following the question, “Have you had any of the following symptoms persistently in the last 3 months?” Internal consistency was 0.82 at baseline and 0.83 at the 3-month follow-up. The primary outcome variable is the baseline to 3-month difference score with higher scores indicating greater reduction of symptom severity. The observed distribution of difference scores was approximately normal.
Using time-line follow-back methods we assessed the number of cigarettes participants smoked on the 28 days prior to baseline and on the 28 days prior to the 3-month assessment.15 We calculated the baseline to 3-month difference in average daily smoking; higher scores indicate greater reductions in average daily cigarette consumption. It should be noted that measures of change in average daily smoking are partially constrained by average daily smoking at baseline. Specifically, heavier smokers have the potential for larger absolute reductions in cigarette use than lighter smokers. Therefore, in addition to the absolute change in average daily smoking, we present data giving the relative reduction in average daily smoking, expressed as a percentage. Additionally, we present data regarding the change in symptom severity at selected cut points along both the absolute and relative smoking change continua. These include absolute reductions of 1 or more, 5 or more, 10 or more, 20 or more, 30 or more, and 40 or more cigarettes per day and relative reductions of 25% or more, 50% or more, 75% or more, and complete abstinence. Participants who quit smoking at follow-up were included as smoking 0 cigarettes.
Additional covariates included age, race, gender, the Fagerström test for nicotine dependence,16 the number of years since the participant began to smoke regularly, and self-reported history of treatment for asthma or emphysema/chronic obstructive pulmonary disease (COPD). We also assessed motivation to quit smoking using a single item at baseline and at 3 months, “How motivated are you to try to quit smoking within the next month?” with response categories from “definitely” to “definitely not” using a 6-point scale.
Analytical Methods
We report means and percentages to describe the characteristics of the sample. Standard statistical tests (t- and Pearson χ2-statistics) and product-moment correlation coefficients were used for bivariate analyses. Locally weighted scatterplot smoothing (lowess) was used to explore bivariate associations and assess linearity. Because regression diagnostics indicated the presence of outliers, we used iteratively re-weighted least-squares regression analysis to estimate the adjusted effects of selected covariates on change in smoking-related respiratory symptom severity (SRRSS). All continuous variables were standardized to 0 mean and unit variance prior to estimating the regression equation. All analyses were conducted using Stata.17
RESULTS
Three-month follow-up data were available for 319 (83.3%) of the 383 participants who completed baseline assessments. Those lost to follow-up did not differ significantly with respect to age (t381=−1.20, P=.231), gender (χ2=2.40, P=.121), race (χ2=0.62, P=.432), average daily smoking at baseline (P=.77 and .441), severity of nicotine dependence at baseline (t381=0.24, P=.814), or treatment assignment (χ2=1.48, P=.223). The following results are based on the 319 participants for whom follow-up data were available.
Participants averaged 40.3 years of age, 51.4% were male, and 79.0% were Caucasian (Table 1). On average participants had smoked cigarettes regularly for 24.2 years and smoked 26.4 cigarettes per day during 28 days preceding baseline data collection. About 29.2% had a history of treatment for asthma and 6.9% for emphysema/COPD. The mean Fagerström test of Nicotine Dependence was almost 6.7 and the mean score on the SRRSS index was 10.04 on a scale with a range of 0 to 21.
Table 1.
Sample Characteristics (n=319)
| Years of age | 40.34 (±8.27) |
| % male | 51.4 |
| % Caucasian | 79.0 |
| % history of asthma | 29.2 |
| % history of COPD | 6.9 |
| Years regular smoking | 24.21 (±9.53) |
| Fagerstrom FTND | 6.68 (±2.23) |
| Mean cigarettes per day | 26.40 (±12.12) |
| Symptom index | 10.04 (±4.71) |
COPD, chronic obstructive pulmonary disease; FTND, Fagerström test for nicotine dependence.
Two hundred eighty-nine participants (90.6%) had 1 or more quit attempts during the 3-month follow-up. Average daily smoking decreased significantly (t319=21.03, P<.001) between baseline and follow-up, at which time participants averaged 9.73 (±10.59) cigarettes per day. The mean reduction was 16.7 cigarettes per day. Thirty-two (10.0%) participants reported either no reduction or increased smoking at follow-up, 19 (6.0%) reduced average daily smoking by 5 or fewer cigarettes per day, 60 (18.8%) reduced smoking by 5 to 10 cigarettes per day, 109 (33.9%) by 10 to 20 cigarettes per day, 88 (27.9%) by 20 to 40 cigarettes per day, and 12 (3.8%) by 40 or more cigarettes per day.
On average, participants used NRT on 41.4 (±24.4) days during the follow-up period. Among the 48 participants assigned to the 12-week course of therapy who provided follow-up data, 19% completed therapy, defined as using NRT >95% of days, with a mean use of 53.4 days. Among 271 participants assigned to the 8-week course of therapy who provided follow-up data, 41% completed therapy, with a mean use of 39.5 days. Number of days on which participants used NRT did not differ significantly by treatment condition (t319=0.24, P<.813). However, reduction in average daily smoking was strongly associated with NRT; participants averaged 14.8 fewer cigarettes on days when a 7 mg or stronger patch was used. Absolute reductions in average daily smoking tended to be largest among those reporting heavier smoking at baseline. Indeed, the correlation between change in daily smoking and average daily smoking at baseline was 0.69 (P<.001).
At baseline, the linear association between average daily smoking and symptom severity was statistically significant (r=.14, P<.01). The relatively weak magnitude of association may in part reflect the absence of nonsmokers and preponderance of relatively heavy smokers, which produced a restricted range in this cohort. All participants smoked at least 10 cigarettes per day; 61 (21.3%) smoked less than 20 cigarettes per day on average, 59 (18.5%) said they smoked a pack a day, 150 (47.0%) smoked between 1 and 2 packs of cigarettes per day, and 41 (12.9%) smoked 2 or more packs of cigarettes per day. Exploratory analysis using nonparametric regression indicated the association between SRRSS and average daily smoking prior to baseline was not linear. SRRSS increased with increased daily smoking to a threshold of about 30 cigarettes per day. As smoking increased beyond 30 cigarettes per day, reported symptom severity did not increase. Among those who smoked less than 30 cigarettes per day, the linear association between SRRSS and average daily smoking was 0.21 (P<.001); by comparison, the product-moment correlation was only 0.07 (P=.387) among those who smoked 30+ cigarettes per day.
The correlation between average daily smoking and SRRSS, both assessed at the 3-month follow-up, was statistically significant (r=.19, P<.001). Nonparametric regression again suggested that the association was not linear; as average daily smoking exceeded about 30 cigarettes per day, there was little additional increase in SRRSS.
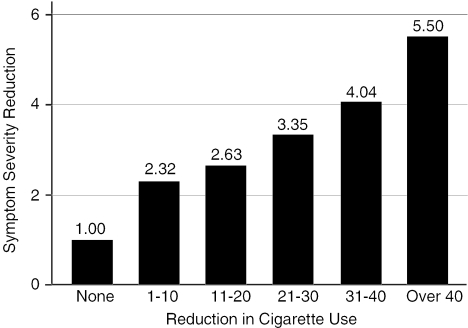
The correlation between reduction in average daily smoking and reduction in symptom severity was 0.26 (P<.001). The correlation between percent reduction in smoking and symptom severity was 0.20 (P<.001). Smoking-related health symptom severity decreased significantly between baseline and follow-up (t319=10.56, P<.001); mean SRRSS at follow-up was 7.29 (±4.81). Figure 1 gives the mean reduction in SRRSS by smoking reduction (categorized). Even those who reported no reduction in average daily smoking reported slightly lower symptom severity at 3 months than at baseline; however, average reduction in symptom severity tended to increase as the absolute reduction in daily smoking increased, and the largest improvements in symptom severity are observed among those with the largest absolute reductions in average daily smoking. Furthermore, these data indicate that even relatively small reductions in daily smoking are associated with significant reductions in symptom severity (see Table 2 which gives the difference in mean change in SRRSS by indicators of smoking reduction dichotomized at a range of absolute and relative cut points). Mean symptom-severity scores improved significantly more (t=2.26, P=.025) among participants who reported any reduction in daily smoking (2.94) than for those who did not reduce cigarette consumption (1.00) over the study period. Statistically significant differences in symptom-severity reduction are observed across a range of absolute and relative smoking reduction cut points (Table 2).
FIGURE 1.
Respiratory symptoms and smoking reduction
Table 2.
Mean Change in Smoking-Related Health Symptoms (SRHSs): Dichotomous Indicators of Absolute and Relative Smoking Reduction
| Cigarette Change | Mean SRHSs Change | t (P=) | d |
|---|---|---|---|
| Any reduction | |||
| Yes (n=287) | 2.94 | 2.26 (.025) | 0.42 |
| No (n=32) | 1.00 | ||
| 5+ reduction | |||
| Yes (n=275) | 3.05 | 2.90 (.004) | 0.46 |
| No (n=44) | 0.88 | ||
| 10+ reduction | |||
| Yes (n=221) | 3.12 | 2.14 (.033) | 0.26 |
| No (n=98) | 1.92 | ||
| 20+ reduction | |||
| Yes (n=118) | 3.75 | 2.97 (.003) | 0.34 |
| No (n=201) | 2.16 | ||
| 30+ reduction | |||
| Yes (n=41) | 4.36 | 2.44 (.015) | 0.40 |
| No (n=278) | 2.49 | ||
| 40+ reduction | |||
| Yes (n=12) | 5.50 | 2.10 (.036) | 0.61 |
| No (n=307) | 2.64 | ||
| 25% reduction | |||
| Yes (n=266) | 3.12 | 3.28 (.001) | 0.49 |
| No (n=53) | 0.82 | ||
| 50% reduction | |||
| Yes (n=222) | 3.17 | 2.47 (.014) | 0.30 |
| No (n=97) | 1.78 | ||
| 75% reduction | |||
| Yes (n=148) | 3.72 | 3.52 (.001) | 0.39 |
| No (n=171) | 1.91 | ||
| Abstinent | |||
| Yes (n=49) | 4.27 | 2.50 (.013) | 0.39 |
| No (n=270) | 2.47 | ||
To adjust for possible confounds, we estimated the regression model presented in Table 3. Change in symptom severity and all continuous predictors were standardized prior to estimating the model. A reduction in cigarette use was positively and significantly (b=0.29, t=5.16, P<.001) associated with a reduction in smoking-related symptom severity after adjusting for age, gender, race, years of regular smoking, and baseline nicotine dependence. A 1 standard deviation reduction in average daily smoking (about 14.1 cigarettes) was associated with a 0.28 standard deviation decrease in smoking-related symptom severity.
Table 3.
Regression of Change in Symptom Severity on Cigarette Change and Selected Covariates (n=319)
| Covariate | b | t (P=) |
|---|---|---|
| Age | −0.14 | −1.34 (.182) |
| Gender | 0.04 | 0.32 (.748) |
| Race | −0.06 | −0.48 (.628) |
| Asthma history (Yes) | 0.05 | 0.42 (.677) |
| COPD history (Yes) | −0.12 | −0.53 (.597) |
| FTND | 0.08 | 1.40 (.162) |
| Years smoked | 0.04 | 0.33 (.740) |
| Cigarette change | 0.29 | 5.16 (.000) |
Coefficients estimates using iteratively reweighted least squares.
COPD, chronic obstructive pulmonary disease; FTND, Fagerström test for nicotine dependence.
Motivation to quit smoking (or to stay quit) assessed at the 3-month follow-up was positively and significantly associated (r=.30, P<.001) with a reduction in average number of cigarettes smoked per day between baseline and follow-up. However, reduction in smoking-related health symptom severity between baseline and 3 months was not associated (r=.01, P=.918) with level of motivation to quit smoking at follow-up.
DISCUSSION
The great majority of heavy smokers in our study of a behavior-based nicotine replacement cessation intervention, even when they tried to stop and failed, reduced their number of cigarettes smoked per day. In doing so, they reduced their smoking-related symptoms. This is the first study to demonstrate an association between subjective short-term health changes and reduction in smoking.
The use of nicotine patch as a replacement for a percentage of cigarettes likely contributed to the reduction in daily smoking. Concomitant NRT and tobacco use appears to have minimal adverse risks and may have long-term acceptability.4,18 Whether study participants had reduction, rather than cessation, as an actual goal, or whether reduction became a goal is unknown. The reasons that smokers attempt to quit are various and complex. Often, concern about health consequences predominate, including the risks of cancer, lung disease, and cardiovascular disease.19 Concurrent with these are the barriers to smoking cessation: nicotine dependence and symptoms of withdrawal, lack of social support, and co-morbid mental health disorders. Persons using methadone may have different expectancies around quitting because of beliefs about the interaction of smoking and other drug use and thus may have additional barriers to quitting.20
Ninety percent of study participants made quit attempts. Nearly all of these persons abstained temporarily, and then returned to a reduced quotient of cigarettes per day. Those unwilling or unable to quit, who may have tried to “control” their smoking, and were most successful at reducing their cigarette consumption had higher motivation to quit at follow-up. Cigarette reduction did not blunt desire to quit. Smokers who obtained symptom relief with reduction, in particular, felt no less cause to attempt or re-attempt cessation. While we were concerned that for some smokers, feeling better might be an adequate outcome, this did not seem to be the case. Motivation to quit cigarette use did not lose salience when symptoms improved.
We speculate that symptom reduction in itself may be an important motivator for individuals attempting cigarette cessation. Persons with greater symptoms at baseline may be more motivated to change their behaviors. Success in symptom reduction may further motivate smokers and could serve as an important lever in encouraging quit attempts among those initially unwilling to quit in some instances. However, in the Lung Health Study of smokers with early-stage COPD, Hughes et al.6 reported that greater CPD reduction at year 1 predicted neither more quit attempts or abstinence between years 2 and 5. Neither our study nor the study by Hughes et al.6 was designed to examine whether reduction that occurs in the setting of a cessation intervention undermines or promotes subsequent quit attempts or cessation success.
We reported symptoms as related to both percent reduction and absolute number of cigarettes reduced because we do not know which measure of smoking reduction is more meaningful. Bolliger et al.21 demonstrated that persons who met a 50% reduction criteria at 6 weeks were significantly more likely to maintain reduction at 24 months compared with those who reduced by lesser amounts. Hughes et al.6 reported that among participants still smoking at the first year follow-up, 27% smoked the same, 43% smoked 1% to 49% fewer, and 30% smoked at least 50% fewer cigarettes per day. About half of the less than 50% reducers and one-fifth of the at least 50% reducers maintained or exceeded this reduction over the next 4 years.
Riley et al.22 reported that reductions in smoking by approximately 10 cigarettes per day were associated with modest reductions in CO levels. We are unaware of any data elucidating how much of a reduction in CPD or the duration of reduction must occur before disease benefits accrue. But we found no specific threshold of CPD where all smokers had minimal or no symptoms; an individual's symptom score was likely based on duration of smoking, type of cigarettes used, and underlying lung disease. Of note, methadone dose did not affect symptom score, although opiates are known to suppress the cough reflex, and therefore might have served to reduce coughing and phlegm production, 2 of the symptoms measured.
We are aware that a reduction in cigarettes per day may lead to secondary changes in smoking behavior. For instance, switching to low-tar cigarettes can lead to compensatory smoking (adjusting inhalation, more frequent puffs, smoking to shorter butts, blocking ventilation holes), which may increase the delivery of tobacco-specific carcinogens and thereby lead to the rising incidence of lung adenocarcinoma.23 However, at least 1 study has shown that a reduction in the daily number of cigarettes is possible without substantial compensatory smoking.21 The relationship of smoking reduction to actual disease states and long-term health effects needs further study.
Our study had several limitations. Participants were not screened for appropriateness of a smoking reduction goal. All data on symptoms and smoking status were self-reported, other than CO confirmation of smoking cessation at the 3-month follow-up. Self-reports of smoking prevalence have been found to be reasonably accurate, but reduction estimates are more difficult to confirm.24 Validation procedures for reduced smoking via either biochemical or observational methods have not yet been established. Because symptom reduction is subjective and not blinded to smoking reduction, participant expectancies of a decrease in symptoms as a result of smoking less might bias reports of symptoms. Of note, the small number of participants who did not reduce their smoking had a 1 unit decline in symptom severity, suggesting the possibility of measurement error or a repeated assessment effect. In addition, cigarette number was reported over the 28 days prior to the assessment while respiratory symptoms were reported for the 3 months prior, but this discordance should be mitigated by our use of change scores for these measures. Finally, our findings may not be generalizable to smokers in others methadone programs, or to other smoking populations with lower nicotine dependence or shorter durations of use.
The harm reduction literature in smoking has not traditionally included measurement of smoking-related symptoms, and only 1 study has included a quality-of-life measure.20 Smokers may perceive cigarette reduction as a sufficient behavior change if it improves smoking-related symptoms. Smoking interventions should measure symptoms and use improvement or lack of improvement in symptoms as a motivation for cessation.
Future work in this area might include longer follow-up of methadone participants to determine if reduction ultimately leads to abstinence. In addition, smoking reduction in nonmethadone populations might yield different results. The inclusion of spirometry or other objective measures of lung function would allow correlation of perceived symptoms reduction and actual functional status. Finally, use of ecological approaches to measure daily smoking and symptoms might offer insights beyond retrospection.
Acknowledgments
This study was funded by the National Cancer Institute (R01 CA84392). Dr. Stein is a recipient of a NIDA Mid-Career Investigator Award (K24 DA00512).
References
- 1.Abrams DB, Orleans CT, Niaura RS, Goldstein MG, Velicer W, Prochaska JO. Treatment issues: towards a stepped-care model. Tob Control. 1993;2:S17–37. [Google Scholar]
- 2.Irvin JE, Brandon TH. The increasing recalcitrance of smokers in clinical trials. Nicotine Tob Res. 2000;2:79–84. doi: 10.1080/14622200050011330. [DOI] [PubMed] [Google Scholar]
- 3.Hughes JR. Applying harm reduction to smoking. Tob Control. 1995;4:S33–8. [Google Scholar]
- 4.Jimenez-Ruiz C, Kunze M, Fagerstrom KO. Nicotine replacement: a new approach to reducing tobacco-related harm. Eur Respir J. 1998;11:473–9. doi: 10.1183/09031936.98.11020473. [DOI] [PubMed] [Google Scholar]
- 5.Paoletti P, Fornai E, Maggiorelli F, et al. Importance of baseline cotinine plasma values in smoking cessation: results from a double-blind study with nicotine patch. Eur Respir J. 1996;9:643–51. doi: 10.1183/09031936.96.09040643. [DOI] [PubMed] [Google Scholar]
- 6.Hughes JR, Lindgren PG, Connett JE, Nides MA. Smoking reduction in the lung health study. Nicotine Tob Res. 2004;6:275–80. doi: 10.1080/14622200410001676297. [DOI] [PubMed] [Google Scholar]
- 7.Fagerstrom KO, Tejding R, Westin A, Lunnell E. Aiding reduction of smoking with nicotine replacement medications: hope for the recalcitrant smokers. Tob Control. 1997;6:311–6. doi: 10.1136/tc.6.4.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolliger CT, Zellweger JP, Danielsson T, et al. Smoking reduction with oral nicotine inhalers: double-blind, randomized clinical trial of efficacy and safety. Br Med J. 2000;321:329–33. doi: 10.1136/bmj.321.7257.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke J, Stein M, McGarry K, Gogineni A. Interest in smoking cessation among injection drug users. Am J Addict. 2001;10:159–66. doi: 10.1080/105504901750227804. [DOI] [PubMed] [Google Scholar]
- 10.Hser YI, McCarthy WJ, Anglin MD. Tobacco use as a distal predictor of mortality among long-term narcotic addicts. Prev Med. 1994;23:61–9. doi: 10.1006/pmed.1994.1009. [DOI] [PubMed] [Google Scholar]
- 11.Manley M, Epps RP, Husten C, Glynn T, Shoplande D. Clinical interventions in tobacco control. A national cancer institute training program for physicians. JAMA. 1991;266:3172–3. [PubMed] [Google Scholar]
- 12.Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. 2nd edn. New York: Guilford Press; 2002. [Google Scholar]
- 13.Ferris BG. Epidemiology standardization project (American Thoracic Society) Am Rev Respir Dis. 1978;118:1–120. [PubMed] [Google Scholar]
- 14.Helsing KJ, Comstock GW, Speizer FE, et al. Comparison of three standardized questionnaires on respiratory symptoms. Am Rev Respir Dis. 1979;120:1221–31. doi: 10.1164/arrd.1979.120.6.1221. [DOI] [PubMed] [Google Scholar]
- 15.Sobell LC, Sobell MB. Timeline Follow-Back Users Guide: A Calendar Method for Assessing Alcohol and Drug Use. Toronto, ON, Canada: Addiction Research Foundation; 1996. [Google Scholar]
- 16.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstorm KO. The fagerström test for nicotine dependence: a revision of the fagerström tolerance questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 17.StataCorp. Stata Statistical Software: Release 8.0. College Station, Tex: Stata Corporation; 2003. [Google Scholar]
- 18.Warner KE, Slade J, Sweanor LLB. The emerging market for long-term nicotine replacement. JAMA. 1997;278:1087–92. [PubMed] [Google Scholar]
- 19.Hayaki J, Anderson BJ, Stein MD. Perceptions of health risk susceptibility among methadone maintained smokers. J Addictive Dis. 2005;24:73–84. doi: 10.1300/J069v24n01_07. [DOI] [PubMed] [Google Scholar]
- 20.Stein MD, Anderson BJ. Nicotine and drug interaction expectancies among methadone maintained cigarette smokers. J Subst Abuse Treat. 2003;24:357–61. doi: 10.1016/s0740-5472(03)00030-8. [DOI] [PubMed] [Google Scholar]
- 21.Bolliger CT, Zellweger JP, Danielsson T, et al. Influence of long-term smoking reduction on health risk markers and quality of life. Nicotine Tob Res. 2002;4:433–9. doi: 10.1080/1462220021000018380. [DOI] [PubMed] [Google Scholar]
- 22.Riley W, Jerome A, Behar A, Weil J. Computer and manual self-help behavioral strategies for smoking reduction: initial feasibility and one-year follow-up. Nicotine Tob Res. 2002:S183–8. doi: 10.1080/1462220021000032762. [DOI] [PubMed] [Google Scholar]
- 23.Stellman SD, Muscat JE, Thomson S, Hoffman D, Wynder EL. Risk of squamous cell carcinoma and adenocarcinoma of the lung in relation to lifetime filter cigarette smoking. Cancer. 1997;80:382–8. doi: 10.1002/(sici)1097-0142(19970801)80:3<382::aid-cncr5>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 24.Patrick DL, Cheadle A, Thompson DC, Diehr P, Koepsell T, Kinne S. The validity of self-reported smoking: a review and meta-analysis. Am J Public Health. 1994;84:1086–93. doi: 10.2105/ajph.84.7.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]