Abstract
BACKGROUND
This study assesses the rate and predictors of treatment retention for primary care patients with opioid dependence–prescribed buprenorphine, a long-acting partial opioid agonist.
METHODS
Observational cohort study of patients prescribed buprenorphine/naloxone and followed for 6 months in the period after the adoption of buprenophine/naloxone by a primary care practice in Rhode Island. Practice policy precluded patient discharges due to continuing drug use.
RESULTS
Patients (n=41) had a mean duration of opioid use of 15.7 years and most had a history of heroin use (63.4%). Thirty-nine percent of patients transferred from methadone maintenance. At 24 weeks, 59% remained in treatment. Nearly half of dropouts occurred in the first 30 days. Participants with opiate-positive toxicologies at week 1 were more likely to drop out of the program (P<.01) and had a significantly shorter retention time (P<.01) on average. Among other drug use and drug treatment variables, employment and addiction counseling during treatment were significantly associated with treatment retention (P=.03).
CONCLUSION
Retention rates in a real world, primary care–based buprenorphine maintenance practice reflect those reported in clinical trials. Abstinence during the first week of treatment and receipt of counseling were critical to patient retention.
Keywords: buprenorphine, drug dependence, retention, primary care
The disease burden for the opioid-dependent drug user is substantial, related to overdose, transmission of infectious diseases, and frequent hospitalization.1 The burden to society in terms of crime, law enforcement costs, family disruption, and lost productivity is also notable.2 Managed withdrawal and substitution treatment such as methadone maintenance has been effective in reducing negative consequences of opioid use.3 However, methadone maintenance may not be readily available, attractive to drug users, and may have only limited success in retaining patients in treatment.4
Buprenorphine/naloxone, a long-acting partial opioid agonist, is a maintenance pharmacotherapy for opioid dependence marketed in 2003.5,6 The combination product was designed specifically to minimize diversion, pulverization, and injection, with a safety profile acceptable in the office-based setting. This study describes the early experience of the first buprenorphine/naloxone treatment program in Rhode Island established in a primary care setting. Our aims are to describe: (1) the protocol for selecting appropriate participants; (2) the drug use histories of participants; and (3) our maintenance treatment protocol, retention rate, and predictors of retention.
METHODS
Screening and Eligibility
Beginning in March 2003, 2 physicians at the RI Hospital Primary Care clinic listed their contact information on SAMSHA's buprenorphine web site. These primary care physicians with experience in addiction medicine, HIV, and hepatitis C treatment, but not agonist therapy, worked with a nurse practitioner to provide care. Early clinical questions were referred to national colleagues involved in buprenorphine trials. Callers to the clinic were screened with a series of eligibility questions. Eligibility criteria included: (1) present opioid addiction of at least 6 months or methadone maintenance at doses less than 35 mg/day; (2) alcohol use less than NIAAA hazardous levels7; (3) cocaine use no more than twice weekly; (4) no benzodiazepine dependence; and (5) willingness to remain in treatment for at least 6 months. These criteria were chosen to limit comorbidity and maximize continued participation. If eligible, callers were invited to an anonymous community class to explain the buprenorphine/naloxone program in greater detail.
Community Class
At this 6- to 10-person group session, we provided an explanatory model of how and why treatment works, the schedule of visits, medication costs, the recommendation of psychosocial counseling, and the minimum duration of treatment (6 months) before any requests for detoxification would be considered. Persons still interested in treatment after the 1-hour class left their names with the permission to contact them.
Pretreatment Visit
At a visit within 2 weeks of the class, the medical and psychiatric history was reviewed to confirm eligibility, and a physical examination was performed. Current drug use was assessed, and the patient was asked to return to the next visit in withdrawal from their current opioid, as recommended by treatment guidelines.5
Buprenorphine/Naloxone Induction Visit
Patients provided informed consent, as approved by the RI Hospital Institutional Review Board. The Clinical Institute Narcotics Assessment (CINA) documented withdrawal symptoms prior to treatment.8 The patient walked to a local pharmacy with a prescription for that day's buprenorphine/naloxone, and returned to the office. Under supervision, 4 mg of buprenorphine/naloxone was used sublingually and the patient who remained was observed for 1–2 hours. Additional medication was taken home for use later in the day, with the usual first day dose of 8 mg; 16 mg was frequently used for methadone transfers. Urine toxicology was performed, phone numbers for substance abuse counselors, psychiatric care and self-help groups (all off-site) were offered, abstinence from all drugs was advised, and the patient was scheduled to return the following day.
Patients were seen 3–4 times during the first week, with prescriptions given for 1 or 2 days at a time, to be taken once or twice a day as desired.
Stabilization
During weeks 2–4, patients had 2–3 visits per week. At each visit, the treatment plan was reviewed and advice was dispensed regarding abstinence, modification of lifestyle, and avoidance of drug triggers. Providers also reviewed whether psychosocial counseling had been sought. Off-site individual addiction counseling and 12-step involvement were recommended but not required. Dose adjustments were made based on continued opioid use, craving, and reported withdrawal symptoms. In general, buprenorphine doses in the range 12–24 mg/day were required for stabilization.9 Urine toxicology was performed at the end of week 1, and was performed during weeks 2, 4, 6, 8, 12, and 24.
Discharge
No patient was discharged for continued use of illicit drugs. If drug use continued, more frequent medical visits were scheduled.
Analytic Methods
The Wilcoxon rank-sum and Fisher exact tests were used to compare program completers and dropouts on selected characteristics, and the log-rank test was used to compare the survival curves for treatment retention.
RESULTS
Among 134 phone screens, 65 persons were receiving methadone maintenance and 69 were not in treatment. Ineligibility was due to methadone doses greater than 35 mg/day (n=48), alcohol use (n=9), cocaine use (n=8), and benzodiazepine use (n=7). The remaining 62 persons were invited to the community classes, 52 of whom attended, and 41 enrolled in the buprenorphine program.
Participants averaged 40.1 (±7.83) years of age; the majority (58.5%) were male, and 81% were Caucasian (Table 1). Heroin was the primary drug of choice for 63.4% of the participants; 19.5% and 17.1% reported Vicodin and Oxycontin, respectively. Nearly half had a history of drug injection, 70.7% had ever used cocaine, and 24.4% had used cocaine within the last 30 days. Twenty-nine (70.7%) had ever been on methadone maintenance therapy and 39.0% were in methadone maintenance at entry.
Table 1.
Buprenorphine/Naloxone Program Dropout by Participant Characteristics
| Program Dropout | |||
|---|---|---|---|
| No (n=24) | Yes (n=17) | P* | |
| Mean (SD) age | 40.85 (±6.38) | 39.79 (±9.76) | .967 |
| Gender | |||
| Male | 14 (58.3%) | 10 (41.7%) | 1.00 |
| Female | 10 (58.8%) | 7 (41.2%) | |
| Race | |||
| Caucasian | 20 (66.7%) | 10 (33.3%) | .095 |
| Minority | 2 (28.6%) | 7 (71.4%) | |
| Employed | |||
| Yes | 21 (70.0%) | 9 (30.0%) | .029 |
| No | 3 (27.3%) | 8 (72.7%) | |
| Lives with children | |||
| Yes | 16 (69.6%) | 7 (30.4%) | .125 |
| No | 8 (44.4%) | 10 (55.6%) | |
| Lives with active user | |||
| Yes | 2 (50.0%) | 2 (50.0%) | 1.00 |
| No | 22 (59.5%) | 15 (40.5%) | |
| Insurance type | |||
| None | 3 (75.0%) | 1 (25.0%) | |
| Private | 14 (66.7%) | 7 (33.3%) | .378 |
| Public | 7 (43.8%) | 9 (56.3%) | |
| HIV+ | |||
| Yes | 1 (25.0%) | 3 (75.0%) | .290 |
| No | 23 (62.2%) | 14 (37.8%) | |
| Benzodiazepine use | |||
| Yes | 7 (58.3%) | 5 (41.7%) | 1.00 |
| No | 16 (57.1%) | 12 (43.9%) | |
| Primary drug | |||
| Heroin | 13 (50.0%) | 13 (50.0%) | .195 |
| Other | 11 (73.3%) | 4 (26.7%) | |
| Cocaine use last 30 d | |||
| Yes | 5 (50.0%) | 5 (50.0%) | .714 |
| No | 19 (61.3%) | 12 (38.7%) | |
| Alcohol use last 30 d | |||
| Yes | 5 (45.5%) | 6 (54.6%) | .476 |
| No | 19 (63.3%) | 11 (36.7%) | |
| Cannabis use last 30 d | |||
| Yes | 4 (36.4%) | 7 (63.6%) | .151 |
| No | 20 (66.7%) | 10 (33.3%) | |
| Current methadone | |||
| Yes | 9 (56.3%) | 7 (43.8%) | 1.00 |
| No | 15 (60.0%) | 10 (40.0%) | |
| Week 1 opiate test† | |||
| Positive | 2 (22.2%) | 7 (77.8%) | .002 |
| Negative | 22 (81.5%) | 5 (18.5%) | |
| Maintenance dose† | 17.92 (±5.82) | 19.83 (±8.72) | .696 |
| Mean percentage opiate+test† | 6.56 (±12.69) | 39.17 (±46.10) | .010 |
| Mean counseling Session/week† | .78 (±1.16) | .05 (±.17) | .001 |
| Attended any counseling† | |||
| Yes | 14 (93.3%) | 1 (6.7%) | .005 |
| No | 10 (47.6%) | 11 (52.4%) | |
Reported P values generated by nonparametric (Fisher exact test and Wilxocon rank-sum test) statistical tests.
Five participants dropped out prior to the first follow-up test and were excluded from this comparison.
Twenty-four (58%) participants were still in the buprenorphine/naloxone program at 180 days. Six (35.3%) of the 17 participants who did not complete the program dropped out during the first week of treatment, with employed persons less likely to drop out early (P=.035). Later dropouts occurred more evenly distributed across time, with the last on day 165. There was no evidence that program dropout was systematically associated with age, gender, or ethnicity.
Participants who were employed either part- or full-time (30.0%) were significantly (P=.029) less likely to drop out than the unemployed (72.7%). Participants whose primary drug was heroin (50.0%) appeared to be more likely to drop out than those whose primary drug was some other opiate (26.7%). Program dropout was not associated with recent use of benzodiazepines, cocaine, or marijuana, or with methadone program transfer. However, dropout was associated with positive opioid toxicologies during treatment (P=.01). Receipt of addiction counseling or NA/AA attendance was also significantly associated with retention.
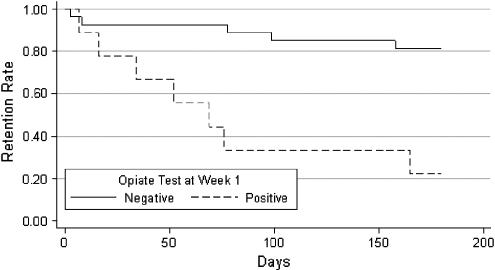
A positive urine opiate test at week 1 was a strong predictor of decreased treatment retention. Seven (77.8%) of the 9 participants with positive opiate toxicologies at 1 week dropped out compared with only 18.5% of those who were opiate negative at 1 week (P=.002). Additionally, the Kaplan-Meier survival curves (Fig. 1) were significantly different (log-rank χ2=13.57, df=1, P<.001) in the 2 groups; those with positive opiate screens at week 1 had significantly shorter time to drop out, on average.
FIGURE 1.
Program retention time by week 1 opiate test.
DISCUSSION
Buprenorphine and methadone have similar efficacies in the management of opioid dependence.10 However, most clinical trials enrolled only heroin users, with protocolized treatment provided in rigid research protocols rather than in primary care settings. Flexible buprenorphine dose schedules have been tested in 6 trials (n=411), with an overall retention rate of 52.7%.11–16 The duration of treatment is predictive of improved patient outcomes; however, none of these studies included an analysis of predictors of retention.3
The current study described our experience with 41 opioid-dependent patients in a primary care clinic treated according to the regulations of the Drug Addiction Treatment Act of 2000. At 24 weeks, 59% of patients remained in treatment. The cohort included active heroin users, persons dependent on oral opioids, and persons transferring care from methadone maintenance treatment programs, most often for convenience or insurance reasons. Our policy dictated that no one was to be discharged for continued drug use during the first 6 months (referrals for counseling increased, as did frequency of clinical visits), and no one was discharged for either behavioral or financial (unable to afford medication) reasons. Our ability to confer with physicians nationally who had more experience with buprenorphine/naloxone provided an important support network during the care of our earliest patients, and underlines the benefits of mentorship.
Most treatment dropout occurred during the first month of care. Indeed, continued opiate use during the first week of buprenorphine/naloxone treatment, as documented by a week 1 urine toxicologic analysis, was predictive of treatment dropout. This early drug use may signal low motivation for treatment or perhaps inadequate dosing, although all patients were receiving at least 12 mg of buprenorphine daily during the first week of care.
Employment, full- or part-time, protected against treatment dropout. Keeping one's job (even if it means temporarily taking time off to attend medical appointments) is likely to be a strong motivation for continuing care. Because many of our patients relied on employer-based health insurance to pay for the buprenorphine/naloxone, continued employment would also seem essential to medication adherence, consistent with previous treatment research.17 No other baseline variables were significantly associated with treatment dropout, but addiction counseling during treatment was protective against dropout and should be strongly encouraged.
Limitations of the study include explicit exclusion of heavy alcohol or cocaine users, patients with uncontrolled psychiatric problems, and those without means to pay for the medication. This situation reflects real-world practice in the United States, where the 30-person limit and lack of universal health insurance create strong incentives to limit access for severely addicted, dual diagnosis, and uninsured patients to office-based buprenorphine programs. The small sample size and observational nature of the study were also limitations.
We conclude that retention rates in a primary care buprenorphine maintenance practice reflects those reported in clinical trials. Abstinence during the first week of treatment and psychosocial counseling are critical to patient retention.
References
- 1.Hulse G, English D, Milne E, Holman C. The quantification of mortality resulting from the regular use of illicit opiates. Addiction. 1999;94:221–9. doi: 10.1046/j.1360-0443.1999.9422216.x. [DOI] [PubMed] [Google Scholar]
- 2.Mark TL, Woody GE, Juday T, Kleber HD. The economic costs of heroin addiction in the United States. Drug Alc Depend. 2001;61:195–206. doi: 10.1016/s0376-8716(00)00162-9. [DOI] [PubMed] [Google Scholar]
- 3.Ward J, Mattick RP, Hall W. Methadone Maintenance Treatment and Other Opioid Replacement Therapies. London: Harwood Press; 1998. [Google Scholar]
- 4.O'Connor PG, Fiellin DA. Pharmacologic treatment of heroin-dependent patients. Ann Int Med. 2000;133:40–54. doi: 10.7326/0003-4819-133-1-200007040-00008. [DOI] [PubMed] [Google Scholar]
- 5.Fiellin DA, Barthwell AG. Center for substance abuse treatment. Guideline development for office-based pharmacotherapies for opioid dependence. J Addict Dis. 2003;22:109–20. doi: 10.1300/j069v22n04_09. [DOI] [PubMed] [Google Scholar]
- 6.Kosten TR, Fiellin DA. Buprenorphine for office-based practice: consensus conference overview. Am J Addiction. 2004;13:S1–7. doi: 10.1080/10550490490440744. [DOI] [PubMed] [Google Scholar]
- 7.National Institute on Alcohol Abuse and Alcoholism. Physicians' Guide To Helping Patients With Alcohol Problems. Vol. 35. Bethesda, MD: NIH Publication; 1995. p. 3769. [Google Scholar]
- 8.Peachey JE, Lei H. Assessment of opioid dependence with naloxone. Br J Addiction. 1988;83:193–201. doi: 10.1111/j.1360-0443.1988.tb03981.x. [DOI] [PubMed] [Google Scholar]
- 9.Center for Substance Abuse Treatment. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2004. Treatment Improvement Protocol (TIP) Series 40. DHHS Publication No. (SMA) 04-3939. [PubMed] [Google Scholar]
- 10.Mattick RP, Ali R, White J, O'Brien S, Wolk S, Danz C. Buprenorphine versus methadone maintenance therapy: a randomized double-blind trial with 405 opioid-dependent patients. Addiction. 2003;98:441–52. doi: 10.1046/j.1360-0443.2003.00335.x. [DOI] [PubMed] [Google Scholar]
- 11.Strain EC, Stitzer ML, Liebson IA, Bigelow GE. Buprenorphine versus methadone in the treatment of opioid-dependent cocaine users. Psychopharmacology. 1994;116:401–6. doi: 10.1007/BF02247469. [DOI] [PubMed] [Google Scholar]
- 12.Strain EC, Stitzer ML, Liebson IA, Bigelow GE. Comparison of buprenorphine and methadone in the treatment of opioid dependence. Am J Psychol. 1994;151:1025–30. doi: 10.1176/ajp.151.7.1025. [DOI] [PubMed] [Google Scholar]
- 13.Fischer G, Gombas W, Eder H, Jagsch R, Peternell A, Stuhlinger G. Buprenorphine versus methadone maintenance for the treatment of opioid dependence. Addiction. 1999;94:1337–47. doi: 10.1046/j.1360-0443.1999.94913376.x. [DOI] [PubMed] [Google Scholar]
- 14.Johnson RE, Chutuape MA, Strain EC, Walsh SL, Stitzer ML, Bigelow GE. A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. N Engl J Med. 2000;343:1290–7. doi: 10.1056/NEJM200011023431802. [DOI] [PubMed] [Google Scholar]
- 15.Petitjean S, Stohler R, Deglon JJ, Livoti S, Waldvogel D, Uehlinger C. Double-blind randomized trial of buprenorphine and methadone in opiate dependence. Drug Alc Depend. 2001;62:97–104. doi: 10.1016/s0376-8716(00)00163-0. [DOI] [PubMed] [Google Scholar]
- 16.Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. 2003. The Cochrane Database of Systematic Reviews. The Cochrane Library Issue 4. www.cochranelibrary.com. [DOI] [PubMed]
- 17.Hser YI, Hoffman V, Grella CE, Anglin MD. A 33-year follow-up of narcotic addicts. Arch Gen Psychol. 2001;58:503–508. doi: 10.1001/archpsyc.58.5.503. [DOI] [PubMed] [Google Scholar]