Abstract
BACKGROUND
Strengthened regulations concerning privacy of health information are affecting large-scale health outcomes research.
OBJECTIVE
To create a data collection system that would facilitate outcomes research, avoid selection bias, and fulfill obligations to protect privacy.
DESIGN
We created a web-based system that uses touch-screen computer technology for longitudinal collection of data. The system provides access to information in deidentified form, enables it to be linked to health services and outcomes data, and allows patients to join a research registry project (RRP) and be placed on a prospective subject list (PSL).
PARTICIPANTS, MEASUREMENTS, AND RESULTS
Pilot testing in 86 consecutive patients who were seen at a large, urban, university-based general medicine practice and had a mean age of 50 years showed that 81 patients had no difficulty, 5 had some difficulty, and none had considerable difficulty using the computer technology to complete a health survey. No patients refused to complete the survey and all patients completed the entire survey. Forty-seven (55%) joined the RRP and 42 of these 47 (89%) joined the PSL. RRP participants were less likely than RRP nonparticipants to be divorced or widowed (P=.03) and less likely to have hypertension (P=.03) but had no other significant differences in sociodemographic or clinical characteristics. PSL participants did not differ from PSL nonparticipants.
CONCLUSIONS
The new system ensures privacy and appears to facilitate research recruitment and avoid selection bias.
Keywords: health services research, computerized data collection, Health Insurance Portability and Accountability Act, research registry, privacy
In 1996, Congress passed the Health Insurance Portability and Accountability Act (HIPAA) to protect the personal health information (PHI) of individuals.1 Accompanying regulations indicate that health care data must be stripped of all identifiable PHI, including an individual's name, social security number, zip code, and dates of office or hospital visits,2 unless the individual provides written informed consent to use this information. The regulations can lead to selection bias in health outcomes research because individuals who consent to participate in research may differ from those who refuse. In one study, for example, individuals who consented were older, were more likely to be male and white, and had poorer physical functioning.3
Instituting the new regulations offered us the challenge to create a system that would facilitate outcomes research while fulfilling ethical and legal protections outlined by HIPAA. Ideally, the system would streamline longitudinal data collection regarding health and health status; centrally store data in a deidentified form; enable data to be linked, without identifiable information, to health services and health outcomes information; enable patients to permit use of their PHI for research purposes; allow patients to identify themselves as potentially willing subjects for future research studies; and function seamlessly within clinical practice.
While researchers are allowed to use PHI without HIPAA authorization in special cases, such as obtaining Institutional Review Board (IRB) waiver or in circumstances when the work is preparatory to research, our approach for meeting the challenge was to invite each of our patients to join a research registry prospectively and be placed on a list of potential research participants. A registry is “an observational, nonexperimental database designed to reflect current patterns of practice without influencing the treatments or interventions prescribed.”4 Most registries collect data regarding a particular disease, but they may also provide data on health-related quality of life (HRQOL).4 As primary care physicians managing individuals with a wide variety of disorders, we designed our registry around HRQOL.
In this article, we describe the system constructed and implemented by the University of Pittsburgh Division of General Internal Medicine. We discuss its acceptance by patients and physicians and its potential contribution to research and clinical care.
METHODS
System Design
We created a web-based data collection system, the Functional Assessment System Tablet (FAST). This system allows patients to use touch-screen computers to complete routine questionnaires that are currently administered in our outpatient practice and elicit information about health, marital status, education, substance use, social support, and HRQOL (RAND-36).5 A summary of the responses is provided to the clinician at the time of the visit and placed in the patient's electronic medical record. The technology allows us to vary time intervals for questions, skip questions that are irrelevant for particular patients, and score instruments in real time. The computers are wirelessly networked, letting patients move through the office while completing questionnaires and thereby minimizing the impact on office flow.
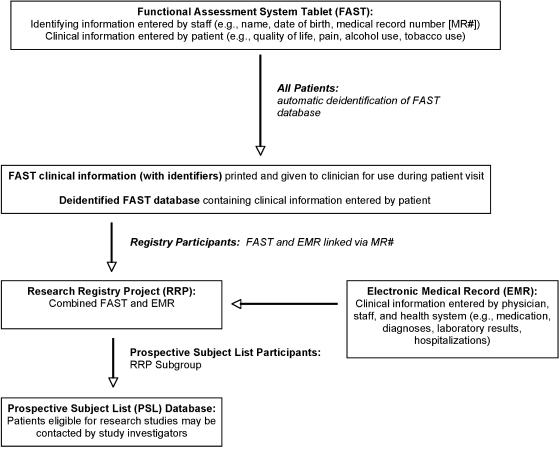
The FAST identifies individuals but automatically segregates their PHI in a separate, password-protected database (Figure 1). This allows longitudinal follow-up, via an electronic honest broker, while storing the majority of data in a deidentified manner. All databases are firewall secured.
FIGURE 1.
Relationship of Functional Assessment System Tablet (FAST), research registry project (RRP), and prospective subject list (PSL). All patients complete the FAST. FAST information is stored in a deidentified database. Patients may consent to participate in the RRP, in which case FAST data are linked to the EMR to create the RRP. RRP participants who agree to participate in the PSL are placed in the PSL database.
The FAST questionnaire asks patients whether they would like to participate in our HRQOL research registry project (RRP) and to join a prospective subject list (PSL) and be notified about studies for which they may be eligible. By affirmatively answering these questions, the patient is “approaching” the research staff, thus removing the obligation for HIPAA authorization.
Signing informed consent for participation in the RRP permits investigators to create a future database with the subject's complete medical record and FAST data in order to “investigate the impact of HRQOL and the presence, absence, risk, or treatment of comorbid medical conditions and symptoms on health services utilization and disease outcomes.” Specific reference is made in both the protocol and consent form as to the inclusion of HIV status and treatment as well as mental health issues. Signing informed consent to participate in the PSL gives investigators permission to query the RRP electronically for eligibility criteria of potential subjects but otherwise respect PHI. PSL participants also consent to allow individual investigators to approach them regarding participation in a specific study. Patients not participating in the PSL may be contacted via usual channels (e.g., approached by personal physician, advertisement).
Investigators who wish to use the RRP must obtain approval from both the IRB and the RRP oversight committee. To use the PSL, the proposal must be additionally approved by the Division's clinical oversight committee. Investigators must be affiliated or collaborating with a member of the Division or the Center for Research on Health Care, all named coinvestigators on the project. All data are controlled by the Division and administered and maintained by the Division's Data Center.
System Implementation
We developed the RRP and PSL as research projects and, after receiving University of Pittsburgh Institutional Review Board approval, pilot tested the system between September 2003 and May 2004 in our large, urban, university-based general medicine practice. Two physicians and 3 medical assistants in our practice agreed to help the principal investigator (PI). When consecutive patients presented for physician appointments, the assistant or PI gave each patient a touch-screen computer, explained how to complete the computerized questionnaire, and remained available to answer queries concerning computer use and noted the time needed to finish the questionnaire.
At the end of the questionnaire, the patient was asked via computer whether they wished to join the RRP and PSL. If the patient agreed, the computer printed a consent form that would be reviewed together by the PI and patient and then signed after the office visit. The final survey question was: “Did you have trouble using the computer to answer these questions?” Patients could respond that they had no difficulty, some difficulty, or considerable difficulty. Patient responses were summarized and immediately printed for the physician to review during the patient encounter. At the end of each session, medical assistants and physicians were asked to comment on concerns or issues that arose during the day.
Data Analysis
We used direct observation to estimate the time needed to complete the questionnaire. We summarized patient characteristics, using means for continuous variables and frequencies for categorical variables. We used the Fisher exact test, Wilcoxon rank-sum test, and Student t test to examine data concerning computer-use difficulties and RRP and PSL participation. We used STATA version 7.0 (Stata Corp., College Station, TX) for all analyses.
In addition to analyzing quantitative data, we asked medical assistants to describe computer-related problems encountered during each session, and we asked physicians to comment on the usefulness of the computer-generated information. We summarize their comments below.
RESULTS
We pilot tested the FAST in 86 consecutive patients, whose sociodemographic and clinical characteristics are shown in Table 1. No patient refused to complete the FAST. Patient age ranged from 19 to 84 years. Similar to the patient panels of the participating physicians, 88% of participants were women. Half were married or in a committed relationship, and over 60% had at least a 4-year college degree. Patients had a variety of medical conditions. On the RAND-36, patients' mean mental health composite (MHC) and physical health composite (PHC) scores were consistent with those found in similar patient populations.6
Table 1.
Sociodemographic and Clinical Characteristics of the 86 Patients Who Completed the Functional Assessment System Tablet (FAST)*
| Characteristic | Number (Percent) |
|---|---|
| Age (y), mean±SD | 50.0±15.6 (range, 19 to 84) |
| Gender | |
| Women | 76 (88) |
| Marital status | |
| Married or in committed relationship | 44 (51) |
| Single | 26 (30) |
| Divorced | 8 (9) |
| Widowed | 8 (9) |
| Highest educational attainment | |
| At least some high school | 9 (10) |
| Some college | 25 (29) |
| Completion of college | 17 (20) |
| Some graduate school | 35 (41) |
| Hazardous drinker† | 14 (16) |
| Current smoker | 8 (9) |
| Comorbid medical conditions | |
| Hypertension | 43 (50) |
| Heart attack | 5 (6) |
| Congestive heart failure | 3 (3) |
| Depression | 31 (36) |
| Lung disease | 8 (9) |
| Arthritis | 27 (31) |
| Cancer | 7 (8) |
| Diabetes | 10 (12) |
| RAND-36 MHC score, mean±SD | 44.4±11.5 (range, 15 to 63) |
| RAND-36 PHC score, mean±SD | 45.9±10.7 (range, 20 to 60) |
| Pain score on a scale of 1 (none) to 10 (worst ever), mean±SD | 2.7±2.1 (range, 1 to 9) |
| Research registry project participant | 47 (55) |
| Prospective subject list participant | 42 (49) |
Values are numbers (percent) unless otherwise indicated. Because of rounding, percentages may not all total 100
Hazardous drinking in women is defined as at least 4 drinks in a day or 7 drinks per week. Hazardous drinking in men is defined as at least 5 drinks in a day or 14 drinks per week
SD, standard deviation; MHC, mental health composite; PHC, physical health composite.
Most patients completed the questionnaire in ≤15 minutes. Only 1 patient needed assistance with more than 2 FAST screens before feeling comfortable using the computer independently. Every patient completed the entire questionnaire, and most finished it before their physician encounter.
Of the 86 patients, 81 reported no difficulty using FAST, 5 reported some difficulty, and none reported considerable difficulty. As shown in Table 2, patients who were older, had higher pain scores, or had lower PHC scores were more likely to report some difficulty using FAST (P=.058 for age, 0.001 for pain, and 0.010 for PHC scores).
Table 2.
Comparison of Patients Reporting No Difficulty and Patients Reporting Some Difficulty in Completing the Functional Assessment System Tablet (FAST)*
| Characteristic | Patients Reporting No Difficulty (n=81) | Patients Reporting Some Difficulty (n=5) | P Value |
|---|---|---|---|
| Age (y), mean±SD | 49.3±15.7 | 61.8±7.9 | .058† |
| Gender | .47‡ | ||
| Men | 9 | 1 | |
| Women | 72 | 4 | |
| Marital status | .58‡ | ||
| Married or in committed relationship | 40 | 4 | |
| Single | 25 | 1 | |
| Divorced or widowed | 16 | 0 | |
| Highest educational attainment | .65‡ | ||
| At least some high school | 8 | 1 | |
| Some college | 23 | 2 | |
| Completion of college | 17 | 0 | |
| Some graduate school | 33 | 2 | |
| Pain score on a scale of 1 (none) to 10 (worst ever), mean±SD | 2.5±1.9 | 6.4±1.9 | .001† |
| Comorbid arthritis | .18‡ | ||
| No | 57 | 2 | |
| Yes | 24 | 3 | |
| RAND-36 MHC score, mean±SD | 44.8±11.5 | 37.0±9.3 | .10† |
| RAND-36 PHC score, mean±SD | 46.6±10.5 | 34.0±4.9 | .01† |
Ease of use response options were no difficulty, some difficulty, and considerable difficulty. No one reported considerable difficulty.
Results of Wilkoxon rank-sum test.
Results of Fisher exact test.
SD, standard deviation; MHC, mental health composite; PHC, physical health composite.
Forty-seven patients (55%) joined the RRP. Of these, 42 (89% of RRP members, 49% overall) also joined the PSL. Because we could access deidentified information for all FAST completers, we could compare characteristics of participants and nonparticipants. RRP participants were less likely than RRP nonparticipants to be divorced or widowed and were less likely to have hypertension (P=.030 for each) but showed no significant differences in age, gender, educational attainment, alcohol or tobacco use, self-reported depression and arthritis, or RAND-36 scores. There were no significant differences between PSL participants and PSL nonparticipants.
Medical assistants reported that FAST altered their workflow by requiring them to answer patients' computer-related questions and to collect computers from patients. These responsibilities increased the workload by about 1–2 minutes per patient. Physicians found FAST information easy to understand and useful for patient care. In particular, the MHC scores were helpful for identifying patients at risk for depression.
DISCUSSION
The new privacy regulations strengthen the protection of human subjects, which is critical to ethical research. Yet, they also make it more difficult to conduct retrospective studies, especially studies requiring the use of identifiers to link multiple data sources, and they make it more complicated to recruit subjects without introducing selection bias.
We believe that a web-based data collection system such as FAST offers a viable solution to these challenges, because it fulfills 5 of the 6 criteria that we identified initially. The system (1) streamlines the longitudinal collection of health and health status data by decreasing the need for manual data entry; (2) provides access to data in a deidentified form; (3) enables the data to be linked to health services and health outcomes information; (4) allows patients to consent to the use of their identifiable information by joining the RRP; and (5) allows patients to indicate their willingness to be contacted regarding future research by participating in the PSL. We are continuing to work with our staff to ensure that the system functions seamlessly within our clinical practice.
Other investigators have designed computerized systems to identify potential subjects to investigators.7 We believe that our system, utilizing the FAST for clinical data collection, the RRP for outcomes research, and the PSL to allow patients to volunteer to be contacted regarding research provides the patient with greater autonomy in the process. Additionally, it allows us to utilize information collected at a previous time point for current research endeavors, including patient recruitment.
By undertaking systematic comparisons of the RRP participants and the overall FAST population, we can use FAST to quantify selection bias in a research sample and better assess its significance. Through the PSL, we can define a volunteer population from which to recruit for future research. In our study, 55% of patients joined the RRP, and 89% of RRP members (49% overall) joined the PSL. These successful participation rates compare favorably with rates reported in other studies8–10 but can likely be improved. In the pilot version of FAST, when patients were asked to join the RRP and PSL, they could only answer yes or no. Adding a “maybe” response will allow patients to obtain additional information before deciding. Repeating the RRP and PSL invitations at subsequent office visits and educating physicians about our project may also enhance participation. Repetitive invitation does raise the risk of patients feeling coerced, and we are following this closely. There have been no complaints to date.
Patients in our study had little difficulty using FAST, consistent with earlier studies that showed good acceptance of touch-screen technology in primary care and subspecialty settings.11 Additionally, we had no incomplete data, corroborating other work demonstrating that computer-based data collection decreased the amount of missing data.11
While we observed few differences between RRP participants and nonparticipants, there are many unmeasured variables yet to be explored. These may include personality type, length of time enrolled as a patient in the practice, trust in the health care system, and more severe illness. We will explore these questions as we move forward with the project.
As with most pilot studies, our study involved a small number of patients, physicians, and staff, making it difficult to generalize our results. However, we are beginning large-scale deployment and will continue to track differences between participant and nonparticipant groups as well as patient difficulties in using the system. We also plan to assess formally how our computer-based survey affects office procedures and health care delivery.
Our new system was developed in the tradition of practice-based research. By allowing physicians to continue with crucial health outcomes research while respecting patients' ethical and legal rights, we believe that the system has the potential to improve health care services on the local level and add to the body of knowledge on the national level.
Acknowledgments
During the time of this work, Dr. Hess was supported by a Veteran's Administration Special Fellowship in Women's Health.
This work was presented in part at the 2004 Society of General Internal Medicine and Academy Health Meetings.
References
- 1. Health Insurance Portability and Acountabilty Act. 1996.
- 2.Department of Health and Human Services. Standards for privacy of individually identifiable health information; proposed rule. The Federal Register. 1999;64:59918–60065. [PubMed] [Google Scholar]
- 3.Woolf SHL, et al. Selection bias from requiring patients to give consent to examine data for health services research. Arch Fam Med. 2000;9:1111–8. doi: 10.1001/archfami.9.10.1111. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy L, Craig AM. Global registries for measuring pharmacoeconomic and quality-of-life outcomes focus on design and data collection, analysis and interpretation. Pharmacoeconomics. 2004;22:551–68. doi: 10.2165/00019053-200422090-00001. [DOI] [PubMed] [Google Scholar]
- 5.Hays RD, Morales LS. The RAND-36 measure of health-related quality of life. Ann Med. 2001;33:350–7. doi: 10.3109/07853890109002089. [DOI] [PubMed] [Google Scholar]
- 6.Hays RD. RAND 36: Health Status Inventory. 1st ed. San Antonio, TX: The Psychological Corporation; 1998. p. 126. [Google Scholar]
- 7.Quinn J, Durski K. A real-time tracking, notification, and web-based enrollment system for emergency department research. Acad Emerg Med. 2004;11:1245–8. doi: 10.1197/j.aem.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 8.Matthews KA, Kelsey SF, Meilahn EN, Kuller LH, Wing RR. Educational attainment and behavioral and biologic risk factors for coronary heart disease in middle-aged women. Am J Epidemiol. 1989;129:1132–44. doi: 10.1093/oxfordjournals.aje.a115235. [DOI] [PubMed] [Google Scholar]
- 9.Sowers M. SWAN a multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, editors. Menopause: Biology and Pathobiology. San Diego: Academic Press; 2000. p. 672. In. eds. [Google Scholar]
- 10.Pradhan AD, Manson JE, Rossouw JE, et al. Inflammatory biomarkers, hormone replacement therapy, and incident coronary heart disease prospective analysis from the women's health initiative observational study. JAMA. 2002;288:980–7. doi: 10.1001/jama.288.8.980. [DOI] [PubMed] [Google Scholar]
- 11.Bliven BD, Kaufman SE, Spertus JA. Electronic collection of health-related quality of life data validity, time benefits, and patient preference. Qual Life Res. 2001;10:15–22. doi: 10.1023/a:1016740312904. [DOI] [PubMed] [Google Scholar]