Abstract
BACKGROUND
The last 5 years of trial data demonstrate the ineffectiveness of hormone replacement therapy (HRT). The impact of these trials on age-specific HRT use, HRT discontinuation, and regional HRT variation has not been evaluated extensively.
OBJECTIVE
To characterize the relation between HRT trial dissemination and age-specific HRT use, HRT discontinuation, and regional HRT variation before and after the trials' publication.
DESIGN
Using the Medco Health database, we analyzed HRT prescription filling, discontinuation, and regional variation among women ≥55 years from May 1998 to May 2003.
MEASUREMENTS AND MAIN RESULTS
Approximately 340,000 women were eligible for Medco benefits each month. Within 3 months of the Women's Health Initiative (WHI), HRT prescriptions declined from 12.5% to 9.4%, P≤.0001. When stratified by age, a statistically significant decline in HRT post-WHI occurred in all age groups, with the biggest decline among women ≥55 to 64 (18% to 11%, P≤.0001). Among HRT users, we found statistically significant increases in discontinuation in 2002 (67%) compared with 2001 (53%, P<.0001). Prior to the WHI there was substantial regional variation in HRT use, with the West South Central and mid-Atlantic having the highest and lowest proportions, respectively (19% vs 6%, P≤.0001). Despite a relative decline in HRT use of 25% to 42% across all regions, substantial geographic variation remained.
CONCLUSIONS
Hormone replacement therapy use decreased significantly immediately post-WHI, suggesting that trial results can have a rapid effect on practice. Marked regional variation in HRT use persisted after the WHI, suggesting that local practice patterns exert a strong effect on clinical behavior even after new evidence is available.
Keywords: hormone replacement therapy, estrogen, clinical trials, Women's Health Initiative
Translating research into practice has become a major focus of national efforts aimed at improving the quality of care.1,2 Hormone replacement therapy (HRT) provides an ideal opportunity to explore the speed of the integration of new knowledge into clinical practice. Before 1998, a large body of prospective observational evidence favored the use of HRT in postmenopausal women.3–7 However, a series of randomized clinical trials (RCTs) failed to support this position.8–10 Two of these studies, the Heart and Estrogen/progestin Replacement Study (HERS) and the Women's Estrogen for Stroke Trial (WEST), showed that combined estrogen/medroxyprogesterone acetate or combined hormone replacement therapy (cHRT) and unopposed estradiol or estrogen replacement therapy (ERT), respectively, were not effective for the secondary prevention of cerebrovascular events in postmenopausal women.8,9 In 2002, an interim analysis of the National Institute of Health's Women's Health Initiative (WHI) revealed an increased risk of breast cancer, coronary heart disease, stroke, and venous thromboembolism in women randomized to cHRT, leading to an early termination of that aspect of the trial.10,11 More recently, researchers halted the ERT arm of the WHI after concluding that estrogen alone increases the risk of stroke in postmenopausal women.12
Previous work from Canada demonstrated an increase in HRT prescriptions after HERS, with a decline in existing prescriptions following the WHI in older women.13 Studies from the United States confirm an increase in overall prescriptions following HERS, with a decline in overall HRT use after the WHI.14,15 These studies together demonstrate the WHI's general effect on prescription filling. However, there are several important issues that remain unclear. The previous studies did not evaluate the impact of these trials among women of different age groups, i.e., women who may be taking HRT for different reasons. We also do not know how the trial affected the discontinuation of HRT among current users. The trial may have discouraged the initiation of treatment but we do not know how it affected those already taking the medication. Finally, we do not know whether the trial affected practice similarly across the country or whether there were important regional differences. The objective of this study, therefore, was to characterize the temporal trends in age-specific prescription-filling behavior, discontinuation of prescriptions for HRT therapies, and regional HRT in the periods around the release of 3 recent trials.
METHODS
Data Source
We used a database from Medco Health Solutions Inc. (Franklin Lakes, NJ), a pharmacy benefits management (PBM) company serving approximately 60 million members. The Medco Health Solutions Inc. database allows for the examination of prescription-filling behavior of a large number of patients in community medical practice, is updated frequently with a<2-week lag time, and has the ability to distinguish between prescriptions filled for the beneficiary member or a member's dependent. All prescriptions filled by beneficiaries from both retail and mail-order pharmacies are part of the database. Given that we analyzed aggregate trends in medication use and did not have access to personal health information, Institutional Review Board approval was not obtained.
HRT
For this longitudinal analysis, we analyzed HRT prescription filling among postmenopausal women, defined as women ≥55 years, from May 1998 through May 2003. The age of 55 years was chosen to maximize the probability of capturing only postmenopausal women, as the mean age of menopause in Western countries is 50 to 51 years.16 We examined both unopposed estrogen and any estrogen/progestin combination. We used a claims-based search strategy, which identified a woman as using combination HRT if she filled a prescription for both estrogen and progesterone in the same month. We included all medications in the same specific therapeutic class and products with the relevant ingredient combinations. We excluded women using transdermal formulations, selective estrogen receptor modulators, other bone resorption inhibitors, and vaginal estrogen preparations because these medications were rarely prescribed and not evaluated in the clinical trials.
HRT Use
HRT Prescriptions
For these repeated cross-sectional analyses, HRT prescriptions were defined as the proportion of women who were eligible for Medco benefits who had filled a prescription for HRT in a given month.
HRT Discontinuation
This was a longitudinal cohort analysis of HRT discontinuation, measured among women previously taking HRT. It was defined as an absence of HRT prescription refills for greater than a consecutive 3-month period. We identified a cohort of women ≥55 years who had filled at least 1 HRT prescription during a 3-month interval before each prespecified time period, and were continuously eligible for Medco benefits for 365 days after those 3 months. We analyzed the calendar years 2001 and 2002 to understand the impact of the WHI.
Statistical Analyses
Descriptive statistics are reported as percentages and proportions. To test for an impact of the publications on prescription filling, we compared the proportion of women filling prescriptions 3 months prior to the month of each publication to the proportion of women 3 months after these publications. Chi-square tests were used to test associations between the proportions of women using/discontinuing hormone therapy before and after study publication; the resulting P values were 2 sided.
We also performed time-series analyses by comparing actual and predicted claims immediately following the WHI publication (July 17, 2002). To establish a trend line for predictive purposes we used weekly data from the time period 5 months to 2 weeks prior to publication of the WHI (February 15, 2002 to July 1, 2002). We examined models of linear trend, linear trend with first-level autocorrelation, and quadratic trend. The regression line was extrapolated from prediction of weekly claims-post event. Two-sided 95% confidence intervals (CIs) were calculated for trend line predictions. Observed data points falling outside these 95% CIs were considered statistically significant. All analyses were performed with SAS statistical software (SAS Institute Inc., Cary, NC), and the time series was conducted using the SAS time series forecasting system.17
To test for differences in HRT utilization by age, we compared baseline HRT prescription filling as well as the change in mean prescriptions after the WHI publication across age groups (55 to 64 years old; 65 to 74 years old; ≥75 years old). The change in mean HRT prescriptions was determined by taking the difference between the average proportion of women filling HRT for the period prior to the WHI (May 1998 to June 2002) and subtracting the average prescription filling proportion for the period post-WHI (July 2002 to May 2003) for each age group. Chi-square test was used for all proportions; the resulting P-values were 2 sided.
We also used χ2 to test for differences in HRT discontinuation, during each 365-day period of interest. Subgroup analysis was performed to explore age variations in HRT discontinuation within and across each calendar year.
To test for regional differences in HRT use, an extremal quotient (EQ), the ratio of the highest to lowest proportions was calculated as a measure of variability.18 We compared baseline HRT utilization as well as the change in HRT utilization pre- and post-WHI in all regions. The change in HRT use was determined by taking the difference between the average prescriptions for the period prior to the WHI (May 1998 to June 2002) and subtracting the average prescriptions for the period post-WHI (July 2002 to May 2003). An indirect age adjustment was then performed by multiplying the age-specific rates of HRT use by the number of women in each age group in each region. We then summed this age-adjusted number of women in each age group in the same region and divided by the total number of women in that region to arrive at an expected rate of HRT use for each region. Population estimates of women in each region were derived from the U.S. Census data for 2000. We then compared this expected rate to the observed rate derived from the Medco regional data. Chi-square test was used for all proportions; the resulting P values were 2-sided.
RESULTS
HRT Prescriptions
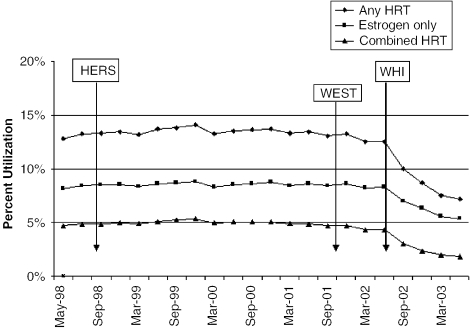
There were approximately 337,375 women ≥55 years who were eligible and had any prescription filled for Medco benefits in any given month from May 1998 to May 2003 (Fig. 1). Hormone replacement therapy prescriptions remained relatively constant from May 1998 to May 2002, with approximately 13% of eligible women filling prescriptions for HRT. Approximately 8% of eligible women filled a prescription for an unopposed estrogen product, and 5% filled prescriptions for combination therapy. Hormone replacement therapy prescriptions steadily declined from June 2002 through May 2003, with approximately 8% of eligible women taking some type of HRT (6% taking ERT and 2% taking cHRT) by the end of the study period.
FIGURE 1.
Hormone Replacement Therapy (HRT) Utilization 1998 to 2003. Heart and Estrogen/progestin Replacement Study (HERS), randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women; Women's Estrogen for Stroke Trial (WEST), a clinical trial of estrogen replacement therapy after ischemic stroke; Women's Health Initiative (WHI), risks and benefits of estrogen plus progestin in health postmenopausal women principal results from the Women's Health Initiative randomized controlled trial.
The proportion of women filling HRT prescriptions 3 months before the release of HERS and WEST was not statistically different from the proportion after the release of the publications (13.16 vs 13.21, P=.47, HERS; 13.04 vs 13.09, P=.56, WEST). However, a clear decline was observed in HRT prescriptions after the release of WHI (12.53 vs 9.37, P≤.0001). Similar trends were observed for both ERT and cHRT.
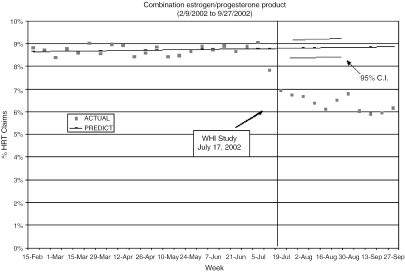
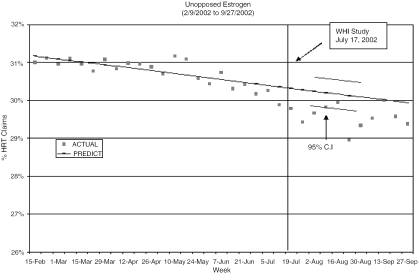
Figures 2 and 3 compare predicted and observed cHRT and ERT claims as a proportion of all HRT claims for each of the 10 weeks following the WHI publication. Although prescriptions for cHRT were beginning to decline several weeks prior to the WHI, claims drop beneath the 95% CI in the first week following the WHI. Similarly, ERT showed a decline beginning 6 weeks prior to the WHI, but observed claims fall below the 95% CI immediately after its publication. Although the observed ERT claims do approach the predicted range 1 month after the WHI, they again dip below the expected values 5 weeks post-WHI, demonstrating a statistically significant decline.
FIGURE 2.
Plot of the observed and predicted percent of hormone replacement therapy (HRT) prescription claims for combined HRT before and immediately after the Women's Health Initiative.
FIGURE 3.
Plot of observed and predicted percent of HRT prescription claims for unopposed estrogen before and immediately after the Women's Health Initiative.
When stratified by age, we found that a higher proportion of women ≥55 to 64 years used HRT (18%) than women ≥65 to 74 years (15%) and women ≥75 years (8%; P value for all pairwise comparisons between age groups ≤.0001) from May 1998 to June 2002. The overall pattern of HRT use decreased from 18% to 11% in the ≥55- to 64-year-olds, from 15% to 9% in the ≥65- to 74-year-olds, and from 8% to 5% in the women ≥75 years from the pre-WHI period (May 1998 to June 2002) compared with the post-WHI period (July 2002 to May 2003). This represents a relative decrease of approximately 37% to 38% in all 3 age groups (P<.0001 for all pre-post comparisons within each age group). After the WHI, a statistically significant difference (P<.0001) in mean prescription proportions was observed for all pairwise comparisons across age groups.
Discontinuation of HRT
The number of women discontinuing their HRT prescriptions increased substantially in 2002. During 2001, 53% of HRT users discontinued HRT within 1 year of initiating therapy. In comparison, 67% of women who had initiated HRT in 2002 had discontinued it within 1 year, (P≤.0001). When same age comparisons were made between the calendar years 2001 and 2002, significant differences were observed for each same age pairing at P<.0001, as seen in Table 1.
Table 1.
HRT Discontinuation by Age: The Proportion of Women using HRT in January Who Discontinued HRT by December of the Same Calendar Year
| 2001 n (%) | 2002 n (%) | P Value | |
|---|---|---|---|
| 55 to 64 years | 15,245 (52) | 166,899 (66) | <.0001 |
| 65 to 74 years | 12,613 (54) | 13,533 (68) | <.0001 |
| ≥75 years | 10,000 (55) | 9,885 (67) | <.0001 |
HRT, hormone replacement therapy
Regional Variation
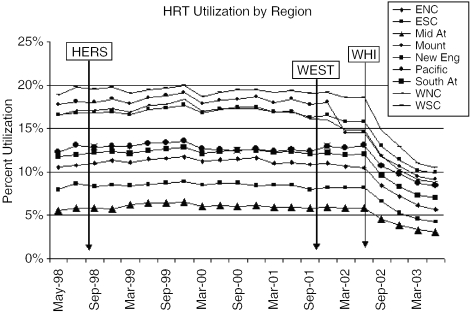
When stratified by region, we found that the West South Central had the highest proportions of HRT use (19%) from May 1998 to June 2002, with the mid-Atlantic having the lowest (6%; P≤.0001), as seen in Figure 4. The ratio of prescription proportions for all regions when compared with the mid-Atlantic ranged from a low of 1.4 to an EQ of 3.2 pre-WHI. The EQ of >3 represents a >3-fold variation between the highest and lowest regions.19 The overall pattern of HRT use decreased from 19% to 13% in the West South Central region and from 6% to 4% in the mid-Atlantic following publication of the WHI results, with declines in other regions also ranging from 2% to 8%. This represents a 34% and 37% relative decrease in the West South Central and mid-Atlantic regions, respectively, with a range of relative decrease of 25% to 42% in the other regions. In the period following the release of the WHI, the ratio of prescription proportions ranged from 1.1 to an EQ of 3.3, with the mid-Atlantic again having the lowest proportion of HRT prescriptions. A statistically significant difference in HRT use persisted between the West South Central (13%) and the mid-Atlantic (4%; P≤.0001), with the West South Central continuing to exhibit the highest proportions of HRT use. These results did not change after indirect age adjustment.
FIGURE 4.
Hormone Replacement Therapy (HRT) Utilization, According to Geographic Region. Heart and Estrogen/progestin Replacement Study (HERS), randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women; Women's Estrogen for Stroke Trial (WEST), a clinical trial of estrogen replacement therapy after ischemic stroke; Women's Health Initiative (WHI), risks and benefits of estrogen plus progestin in health postmenopausal women principal results from the Women's Health Initiative randomized controlled trial; ENC, East North Central; ESC, East South Central; Mid At, Mid-Atlantic; Mount, Mountain; New Eng, New England; Pacific, Pacific; South At, South Atlantic; WNC, West North Central; WSC, West South Central.
DISCUSSION
The WHI demonstrates one of the most remarkable success stories in rapidly translating the findings of a clinical trial into practice. Despite the release of 2 large randomized, controlled secondary prevention trials since 1998, it was not until the publication of the WHI investigation that a decline in HRT use was noted at the population level. Using a large database that documents actual prescription-filling behavior, we found a statistically significant decline in HRT utilization within each age group after the WHI was disseminated. We also found that the WHI was associated with a greater proportion of discontinuation among HRT users. This suggests that the decline in overall prescription use in the U.S. population can be explained by both decreased “incident” use as well as decreased “prevalent” use. We can only speculate about the third of women who continued HRT despite the WHI trial. One explanation is that they may have been using HRT for treatment of menopausal symptoms rather than as primary or secondary prevention of cardiovascular disease or osteoporosis. This hypothesis is strengthened by the fact that among women who had been using HRT, more 55- to 64-year-olds continued therapy than the older age groups in our analysis. Our discontinuation proportion of any HRT in 2002 (either ERT or cHRT) of 65% is consistent with Roumie et al.,20 who found a 70% overall discontinuation of cHRT among female veterans after the use of patient and physician interventions to alert them to the results of the WHI.
Based on actual prescription-filling behavior of a nationwide sample of an average of 337,375 insured women per month, our findings are consistent with those of Hersh et al. but differ slightly, with earlier work based on self-reported HRT among women in a mammography registry in San Francisco.14,15 These investigators found a modest decline in HRT use post-HERS as well as a larger decline in HRT post-WHI, while we found no change in HRT prescription proportions after HERS.
The rapid decline in any HRT use after the WHI trial can be explained by many reasons, including the fact that this study was the third in a series of RCTs investigating the role of HRT. The initial publications of HERS and WEST may have built anticipation for a definitive trial of primary prevention. In addition, the release of HERS II, also in July 2002, may have influenced the temporal decrease in HRT prescriptions.21 Heart and Estrogen/progestin Replacement Study II demonstrated that cardiovascular benefits found in the final years of HERS did not persist after 6.8 years of follow-up and recommended that HRT not be used for the secondary prevention of coronary heart disease. Because the WHI was a primary prevention study, however, the results applied to a larger number of women than the previous secondary prevention trials. The decision to stop the trial early in May 2002 along with the express publication of the WHI results and a press release from the National Heart, Lung, and Blood Institute 1 week prior to the study's publication, as well as the WHI's demonstration of increased risk of cardiovascular disease, rather than the absence of HRT benefit shown in HERS II, may have also heightened the urgency with which its results were interpreted.
Given that 50 million American women are ≥50 years old, it is not surprising that the long-awaited results of the WHI received a cascade of media attention.22 Despite this attention, however, we found that statistically significant regional differences of HRT use persisted both before and after its release. This demonstrates that local practice style and physician habit powerfully influence clinical behavior despite widely publicized and “convincing” evidence.
Although we cannot determine the indication for HRT use in each region, this finding is consistent with previous work demonstrating that regional variations in medication use persist despite adjustments for comorbidity and age.23 Proposed explanations of geographic variation in the use of other health care services have included uncertainty about indications for certain procedures or medications.24 Physician uncertainty as to whether or not HRT was appropriate for their patients seems an unlikely explanation for our observations of HRT prescription filling given the same trend of decline across all regions evaluated. Furthermore, publication of the U.S. Preventive Services Task Force (USPTF) recommendations advising against the use of combined HRT for primary prevention of chronic disease in November 2002 should have clarified the role of HRT for providers.25
The “enthusiasm hypothesis” is another explanation that has been proposed to explain regional variation in health care services.26 This theory suggests that providers who are “enthusiasts” for particular services may be driving local patterns of use. In the case of HRT, patient as well as provider “enthusiasm” or concern regarding HRT side effects may also have contributed to the regional variation we observed. Although a credible explanation for the geographic variation was found, this particular theory was beyond the scope of our study to examine. Our observation does, however, contribute to the growing body of literature that the quality of care delivered in some geographic regions may be clinically suboptimal and demonstrates the variable penetration of new knowledge.27 Marked regional variations in HRT use provide information about where to target educational campaigns about the risks and benefits of HRT.
Despite the conclusion of the WHI that only combined estrogen/progestin therapy conferred increased risk to patients, we observed the same pattern for unopposed estrogen therapy prior to the WHI's decision to stop the ERT arm in March 2004. This is consistent with the findings of Haas and Hersh that unopposed estrogen use declined in similar fashion to cHRT after the WHI release. The speed with which the WHI results were translated into action may have been fueled by an overestimation of the perceived risk of heart disease as documented by Sullivan, who notes that among a largely menopausal cohort of women, 31% believed their risk of heart disease increased by 10% to 30% per year of use, when the WHI data suggest the risk increased by <0.1% per year of use. Together, the speed and overestimation of risk may account for its indiscriminate application, as evidenced by the decline of ERT as well as cHRT prior to the published findings of the ERT arm in April 2004.28–30
This work has several important caveats. We were able to study only prescription-filling behavior, not actual adherence to a medication regimen. Also, our data do not allow us to determine whether prescription patterns were motivated by patients or health care providers or whether HRT was prescribed for primary or secondary prevention of cardiovascular/cerebrovascular disease. It should also be noted that the analysis for HRT prescription filling measures refill activity and not necessarily “drug on hand.” Therefore, it is possible that women would be credited for filling a prescription only in the month that they literally refilled or initiated the drug, rather than each month they had active drug available. However, given the 5-year duration of our data, this is unlikely to explain the decrease in prescription-filling behavior around the release of the WHI. Lastly, our cross-sectional analysis does involve women moving in and out of eligibility for Medco benefits. Bias could be introduced if the departing women were much more likely to be using HRT than the incoming women. However, the stable HRT prescription filling rates during the 4 years before the WHI suggest that the subsequently observed changes were more likely due to the WHI publication than this potential systematic bias.
Our study shows that the results of large clinical trials can translate quickly into behavior change; however, the application of this knowledge to patients remains highly variable and influenced by local practice patterns and standards of care. To ensure that studies appropriately influence practice, the dissemination and application of these publications should be a parallel effort to the investigation itself. Mechanisms to assess the impact of trials must be instituted if we are to improve the translation of research into practice in a serious and scientific manner.
Acknowledgments
Dr. Kim's work was supported by the Robert Wood Johnson Clinical Scholars Program and the Fellowship in Geriatric Medicine and Clinical Epidemiology training grant at the Yale University School of Medicine (T32AG019134). Dr. Gross's efforts were supported by a Cancer Prevention, Control and Population Sciences Career Development Award (1K07CA-90402) and the Claude D. Pepper Older Americans Independence Center at Yale (P30AG21342).
References
- 1.Lenfant C. Clinical research to clinical practice—lost in translation? N Engl J Med. 2003;349:868–74. doi: 10.1056/NEJMsa035507. [DOI] [PubMed] [Google Scholar]
- 2.Berwick DM. Disseminating innovations in health care. JAMA. 2003;289:1969–75. doi: 10.1001/jama.289.15.1969. [DOI] [PubMed] [Google Scholar]
- 3.Col NF, Eckman MH, Karas RH, et al. Patient-specific decisions about hormone replacement therapy in postmenopausal women. JAMA. 1997;277:1140–7. [PubMed] [Google Scholar]
- 4.Stampfer MJ, Colditz GA, Willett WC, et al. Postmenopausal estrogen therapy and cardiovascular disease: ten-year follow-up from the Nurses' Health Study. N Engl J Med. 1991;325:756–62. doi: 10.1056/NEJM199109123251102. [DOI] [PubMed] [Google Scholar]
- 5.Grady D, Gebretsadik T, Kerlikowske K, Ernster V, Petitte D. Hormone replacement therapy and endometrial cancer risk: a meta-analysis. Obstet Gynecol. 1995;85:304–13. doi: 10.1016/0029-7844(94)00383-O. [DOI] [PubMed] [Google Scholar]
- 6.Stampfer MJ, Colditz GA. Estrogen replacement therapy and coronary heart disease: a quantitative assessment of the epidemiologic evidence. Prev Med. 1991;20:47–63. doi: 10.1016/0091-7435(91)90006-p. [DOI] [PubMed] [Google Scholar]
- 7.Colditz GA, Willett WC, Stampfer MJ, Rosner B, Speizer FE, Hennekens CH. Menopause and the risk of coronary heart disease in women. N Engl J Med. 1987;316:1105–10. doi: 10.1056/NEJM198704303161801. [DOI] [PubMed] [Google Scholar]
- 8.Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–13. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 9.Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med. 2001;345:1243–9. doi: 10.1056/NEJMoa010534. [DOI] [PubMed] [Google Scholar]
- 10.Writing Group for the Women's Health Initiative Investigators Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 11.Manson JE, Hsia J, Cushman M, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523–34. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 12.The Women's Health Initiative Steering Committee Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004;291:1701–12. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 13.Austin PC, Mamdani MM, Tu K, Jaakkimainen L. Prescriptions for estrogen replacement therapy in Ontario before and after publication of the Women's Health Initiative Study. JAMA. 2003;289:3241–2. doi: 10.1001/jama.289.24.3241. [DOI] [PubMed] [Google Scholar]
- 14.Haas JS, Kaplan CP, Gerstenberger EP, Kerlikowske K. Changes in the use of postmenopausal hormone therapy after the publication of clinical trial results. Ann Intern Med. 2004;140:184–8. doi: 10.7326/0003-4819-140-3-200402030-00009. [DOI] [PubMed] [Google Scholar]
- 15.Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy—annual trends and response to recent evidence. JAMA. 2004;291:47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- 16.Velde ER, Pearson PL. The variability of female reproductive ageing. Reprod Update. 2002;8:141–54. doi: 10.1093/humupd/8.2.141. [DOI] [PubMed] [Google Scholar]
- 17.Brunt ME, Murray MD, Hui SL, Kesterson J, Perkins AJ, Tierney WM. Mass media release of medical research results. An analysis of antihypertensive drug prescribing in the aftermath of the calcium channel blocker scare of March 1995. J Gen Intern Med. 2003;18:84–94. doi: 10.1046/j.1525-1497.2003.20502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anis AH, Carruthers SG, Carter AO, Kierulf J. Variability in prescription drug utilization: issues for research. Can Med Assoc J. 1996;154:635–40. [PMC free article] [PubMed] [Google Scholar]
- 19.Feasby TE, Quan H, Ghali WA. Geographic variation in the rate of carotid endarterectomy in Canada. Stroke. 2001;32:2417–22. doi: 10.1161/hs1001.096196. [DOI] [PubMed] [Google Scholar]
- 20.Roumie CL, Grogen EL, Falbe W, Awad J, Speroff T. A three-part intervention to change the use of hormone replacement therapy in response to new evidence. Ann Intern Med. 2004;141:118–25. doi: 10.7326/0003-4819-141-2-200407200-00010. [DOI] [PubMed] [Google Scholar]
- 21.Writing Group for the HERS Research Group Cardiovascular disease outcomes during 6.8 years of hormone therapy—Heart and estrogen/progestin replacement study follow-up (HERS II) JAMA. 2002;288:49–57. doi: 10.1001/jama.288.1.49. [DOI] [PubMed] [Google Scholar]
- 22.Mosca L, Collins P, Herrington DM, et al. Hormone replacement therapy and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 2001;104:499–503. doi: 10.1161/hc2901.092200. [DOI] [PubMed] [Google Scholar]
- 23.Hogan DB, Maxwell CJ, Fun TS, Ebly EM. Regional variation in the use of medications by older Canadians—a persistent and incompletely understood phenomena. Pharmacoepidemiol Drug Saf. 2003;12:575–82. doi: 10.1002/pds.803. [DOI] [PubMed] [Google Scholar]
- 24.Wennberg JE, Barnes BA, Zubkoff M. Professional uncertainty and the problem of supplier-induced demand. Soc Sci Med. 1982;16:811–24. doi: 10.1016/0277-9536(82)90234-9. [DOI] [PubMed] [Google Scholar]
- 25.U.S. Preventive Services Task Force Postmenopausal hormone replacement therapy for primary prevention of chronic conditions: recommendations and rationale. Ann Intern Med. 2002;137:834–9. doi: 10.7326/0003-4819-137-10-200211190-00013. [DOI] [PubMed] [Google Scholar]
- 26.Chassin MR. Explaining geographic variations: the enthusiasm hypothesis. Med Care. 1993;31(suppl):37–44. doi: 10.1097/00005650-199305001-00006. [DOI] [PubMed] [Google Scholar]
- 27.Hirth RA, Tedeschi PJ, Wheeler JRC. Extent and sources of geographic variation in Medicare end-stage renal disease expenditures. Am J Kidney Dis. 2001;38:824–31. doi: 10.1053/ajkd.2001.27702. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan MG. Women overestimate hormones' risks, benefits. Internal Medicine News. 2004;28 March 1. [Google Scholar]
- 29.Bozkurt B, Agoston I, Knowlton AA. Complications of inappropriate use of spironolactone in heart failure: when an old medicine spirals out of new guidelines. J Am College Cardiol. 2003;41:211–4. doi: 10.1016/s0735-1097(02)02694-3. [DOI] [PubMed] [Google Scholar]
- 30.Gross CP, Steiner CA, Bass EB, Powe NR. Relation between prepublication release of clinical trial results and the practice of carotid endarterectomy. JAMA. 2000;284:2886–93. doi: 10.1001/jama.284.22.2886. [DOI] [PubMed] [Google Scholar]