Abstract
Objective
Our goal was to establish whether psychosocial risk factors for nonadherence, previously identified as negative predictors of warfarin prescribing, are predictors of adverse events for patients with nonvalvular atrial fibrillation receiving warfarin.
Design
Retrospective cohort analysis.
Setting
Ohio Medicaid administrative database.
Patients
We studied Ohio Medicaid recipients with nonvalvular atrial fibrillation receiving warfarin to determine whether a history of substance abuse, psychiatric illness, or social factors (identified as conditions perceived to be barriers to adherence) are predictors of adverse events, including stroke, intracranial hemorrhage, and gastrointestinal bleeding. Multivariable risk ratios were calculated for each risk factor using Cox proportional hazards models.
Results
9,345 patients were identified as having nonvalvular atrial fibrillation and receiving 2 or more warfarin prescriptions between 1997 and 2002. The event rates for the sample as a whole were 1.5 strokes, 0.7 intracranial hemorrhages, and 4.3 gastrointestinal bleeds per 100 person-years of follow-up. Subjects with substance abuse had the highest adjusted risk ratio, 2.4 (95% confidence interval [CI]: 1.4, 4.0) for an intracranial hemorrhage while receiving warfarin, followed by subjects with psychiatric illness, adjusted risk ratio of 1.5 (95% CI: 1.04, 2.1). Subjects with psychiatric illness also had an adjusted risk ratio of 1.4 (95% CI: 1.1, 1.7) for stroke. Patients in all 3 identified risk groups were at a significantly increased risk of gastrointestinal bleeding.
Conclusion
Patients with nonvalvular atrial fibrillation treated with warfarin who have psychosocial risk factors for nonadherence have an increased risk of adverse events.
Keywords: atrial fibrillation, warfarin, risk factors, psychosocial, stroke, hemorrhage
Patients with nonvalvular atrial fibrillation have a fivefold increase in the incidence of thromboembolic stroke.1,2 Several factors have been identified that increase the risk of an embolic event in patients with nonvalvular atrial fibrillation including increasing age, prior transient ischemic event or stroke, hypertension, diabetes, and left ventricular dysfunction.2,3 While anticoagulation with warfarin has been shown to reduce the incidence of embolic events,3–7 there is an increased risk of bleeding.
A number of clinical risk factors for bleeding while receiving anticoagulation have been identified, such as an international normalized ratio (INR) greater than 3, poorly controlled hypertension, history of gastrointestinal bleeding, and increasing age,8–14 but no published models have included psychosocial risk factors in addition to the known clinical risk factors. Several clinical reasons for an elevated INR have been identified including drug-drug interactions, noncompliance with drug or diet, worsened heart failure, and impaired liver function.15,16 Current alcohol abuse has been shown to be associated with major bleeding and an elevated INR in several small studies 14,17 and in 1 large study 18 while others have not shown such an association.16,19 No studies have examined the effect of psychiatric illness on bleeding risk.
Despite the evidence supporting the benefits of anticoagulating patients with nonvalvular atrial fibrillation, only 30% to 55% of patients without contraindications are currently receiving warfarin.20–22 One of the potential barriers to prescribing warfarin may be physician concern about psychosocial factors that may impair effectiveness and present barriers to therapy. A recent study of Ohio Medicaid patients identified several psychosocial factors that were negative predictors of warfarin use in newly diagnosed cases of atrial fibrillation.23 Johnston and colleagues demonstrated in this study that patients with substance abuse had an adjusted odds ratio of 0.59 (95% confidence interval (CI) 0.35, 0.99) for receiving warfarin at the time of diagnosis. Patients with other perceived barriers to compliance, including psychiatric illness and social risk factors, had an adjusted odds ratio of 0.84 (95% CI 0.73, 0.97) for receiving warfarin. The social risk factors included no caregiver for the patient, homelessness and previous documentation of noncompliance. In this study psychiatric illness was defined as schizophrenia, affective psychosis (including major depressive disorder, manic disorder, or bipolar affective disorder), paranoia or other nonorganic psychosis as determined by International Classification of Diseases, Ninth Revision, Clinical Modification codes (ICD-9-CM) as these were considered to be likely predictors of nonadherence. No other psychiatric diagnoses were considered. It has yet to be determined whether these negative predictors of warfarin prescribing are risk factors for adverse outcomes in patients receiving warfarin.
The specific aim of this study is to identify and quantify the psychosocial risk factors leading to thromboembolic and hemorrhagic complications in Ohio Medicaid patients who received warfarin for nonvalvular atrial fibrillation. We hypothesize that substance abuse, psychiatric disease, and the social factors that are perceived barriers to adherence increase the risk of both thromboembolic and hemorrhagic events in these patients.
SUBJECTS AND METHODS
Basic Design and Data Source
We performed a retrospective cohort analysis of Ohio Medicaid patients from January 1, 1997, through May 31, 2002. Data were collected from the Ohio Medicaid administrative claims database which has been well described elsewhere.23–25 Briefly, the database includes fee-for-service data from all institutions, providers, and pharmacies that provide services to Ohio Medicaid enrollees. The 2 Ohio Medicaid categories are people who are Aged, Blind, or Disabled and Covered Families and Children (CFC). All enrollees must meet income eligibility requirements: elderly or disabled recipients must earn no more than 64% of the federal poverty level. Recipient identification numbers link the data. The Institutional Review Board at the University of Cincinnati approved the use of this database.
Patient Selection
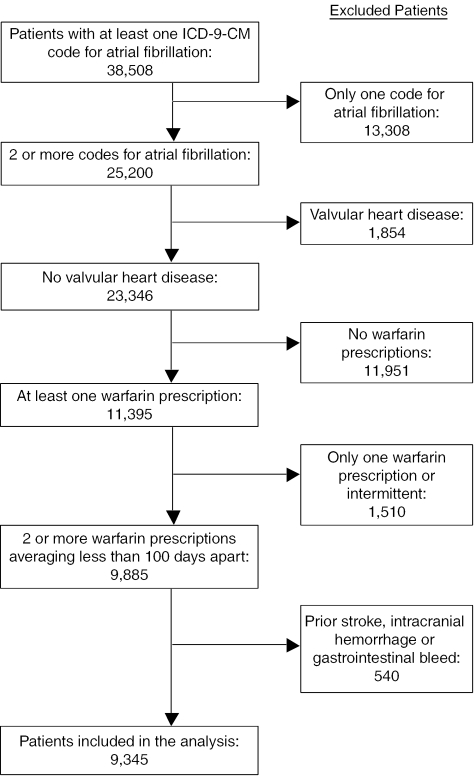
We identified all subjects with 2 or more claims containing an ICD-9-CM for atrial fibrillation (427.31) during the study period of January 1, 1997, through May 31, 2002. Two claims were required for inclusion to ensure that we did not include subjects with transient atrial fibrillation. Exclusion criteria are described in Figure 1.
FIGURE 1.
Flow diagram of subjects. We excluded all subjects with a history of valvular heart disease (2 or more claims with International Classification of Diseases, Ninth Revision, Clinical Modification codes for mitral valve disease (394.x), aortic valve disease (395.x), mitral and aortic valve disease (396.x), heart valve transplant (V42.2), or heart valve replacement (V43.3), or a procedure code for mitral or aortic valve repair or replacement (35.10–35.14, 35.20–35.28)). Subjects were excluded if they had a previous diagnosis of stroke (434.x, 438.x), intracranial hemorrhage (430.x, 431.x, 432.0, 432.1, 432.2, 432.9, 851-854), or gastrointestinal bleeding (530.82, 531.2, 531.4, 531.6, 532.2, 532.4 532.6, 533.2, 533.4, 533.6, 534.2, 534.4, 534.6, 535.x1, 537.83, 562.02, 562.03, 562.12, 562.13, 569.3, 578.x) prior to starting warfarin therapy.
We used pharmacy claims data to determine when patients filled prescriptions for warfarin, and we examined all warfarin prescriptions filled. Searching for National Drug Codes corresponding to warfarin and the ICD-9-CM codes indicating warfarin use identified warfarin prescriptions for these patients. Patients were included in the study only if they filled 2 or more prescriptions for warfarin and if the average time between prescription refills was less than 100 days. We chose the 100-day window because some patients, on a stable dose of anticoagulation, may receive up to a 3-month supply per refill.
Risk Factors
We identified the psychosocial features of interest from the database using ICD-9-CM codes and then categorized them as being present or absent for each patient. Psychiatric illness was defined as schizophrenia (ICD-9-CM code 295.x), affective psychosis (296.x), paranoia (297.x), or other nonorganic psychosis (298.x). Affective psychosis includes major depressive disorder, manic disorder, and bipolar affective disorder. Substance abuse was defined as alcohol dependence (303.x), drug dependence (304.x), or nondependent abuse (305.x excluding 305.1-tobacco use disorder). Social risk factors included lack of housing (V60.0), inadequate housing (V60.1), inadequate material resources (V60.2), persons living alone (V60.3), no other household member able to render care (V60.4), or noncompliance with medical treatment (V15.81).
Covariates, which, a priori, were believed to confer independent risks for the outcomes, were identified from the dataset using ICD-9 CM codes. Hypertension (401-405), diabetes mellitus (250.x), chronic heart failure (402.x1, 404.x1, 404.x3, 428), liver disease (070.2-6, 070.9, 570, 571.x, 572.2-4, 572.8), and renal disease (584.x, 585.x, 586.x) were the potential confounders identified for each patient as being present or absent. We also included a history of deep vein thrombosis (451.1) in the modeling process as another indication for warfarin therapy. Demographic data were used to obtain the age, sex, and race for each subject. Another potential covariate included in the analysis was frequency of warfarin refills, thought to be a crude measure of adherence. This proxy for adherence was considered present if the average time between prescription refills for a subject was greater than 32 days (the median prescription refill time) and absent if less than or equal to 32 days.
Outcomes
We defined adverse events based on ICD-9-CM codes that were recorded on inpatient hospitalization claims. Inpatient hospitalization codes were required to improve the reliability of the claim and the diagnosis. The primary adverse events considered were stroke (434.x), intracranial hemorrhage (430.x, 431.x, 432.0, 432.1, 432.2, 432.9, 851-854), and gastrointestinal bleeding (530.82, 531.2, 531.4, 531.6, 532.2, 532.4 532.6, 533.2, 533.4, 533.6, 534.2, 534.4, 534.6, 535.x1, 537.83, 562.02, 562.03, 562.12, 562.13, 569.3, 578.x). For gastrointestinal bleeding, we only considered major bleeding as defined as bleeding requiring hospitalization. Other studies using administrative data have used a similar approach.18 Date of death was used to adjust the time at risk for each patient and was considered a secondary end point, although cause of death was not available in the dataset.
Time at Risk
The date of a patient's initial warfarin prescription claim was used to define the start of the patient's at-risk period. A patient was then considered to be at risk until an adverse event occurred. Patients without adverse outcomes were censored at 30 days following their last prescription for warfarin, their date of disenrollment from Medicaid or on their date of death. A 30-day window was chosen to allow patients to finish taking their last prescription refill.
Analysis
We used descriptive statistics including the t-test and the χ2-test to characterize the study sample, stratified by risk factor. Event rates for each outcome (stroke, intracranial hemorrhage, and gastrointestinal bleeding) were calculated for each risk factor group and for the cohort as a whole.
Cox proportional hazards models were used to evaluate the univariate relationship between each risk group and the potential confounders with each outcome. We then selected the variables that were significant predictors at P <.05 to be included in the multivariable models. Because of the large sample size and the large number of outcomes, each adjusted model included all of the significant covariates in order to maintain the face validity of the results. SAS version 8.2 (Cary, NC) was used for all analyses.
RESULTS
Patient Characteristics
25,200 patients were identified as having 2 or more ICD-9-CM codes for atrial fibrillation. After the inclusion and exclusion criteria were applied, 9,345 patients remained in the analysis. These patients were followed for a mean of 740 days and filled an average of 25 warfarin prescriptions during this time.
The mean age (SD) of the study sample was 72 (13.8) years (Table 1) A significant majority of patients were women and were white. In general, the sample had numerous comorbid conditions, particularly hypertension and chronic heart failure. The subjects with substance abuse tended to be younger and male, and were more likely to be African American. They were also more likely to have liver disease and hypertension than the other subgroups. The subjects with social risk factors for nonadherence tended to be older and were more likely to have chronic heart failure, diabetes mellitus, and renal disease (Table 1).
Table 1.
Characteristics of Cohort *
| Total (n=9,345) | No Risk Factors (n=4,994) | Substance Abuse †(n=435) | Psychiatric Illness ‡(n=2,108) | Social Risk Factors §(n=2,619) | |
|---|---|---|---|---|---|
| Age ∥ | 73±13.8 | 72±14.4 | 59±13.4¶ | 73±10.7¶ | 76±10.7¶ |
| Sex, Female | 6,371 (68) | 3,303 (66) | 122 (28)¶ | 1,447 (69)¶ | 2,052 (78)¶ |
| Race, White | 7,847 (84) | 4,252 (85) | 297 (68)¶ | 1,822 (86) | 2,124 (81)¶ |
| Risk factors | |||||
| Substance abuse † | 435 (5) | NA | NA | 152 (7) | 96 (4) |
| Psychiatric illness ‡ | 2,108 (23) | NA | 152 (35) | NA | 609 (23) |
| Social factors § | 2,619 (28) | NA | 96 (22) | 609 (29) | NA |
| Comorbid conditions | |||||
| Hypertension | 7,045 (75) | 3,575 (72) | 357 (82)¶ | 1,672 (79)¶ | 2,150 (82)¶ |
| Chronic heart failure | 7,140 (76) | 3,565 (71) | 357 (82)¶ | 1,681 (80)¶ | 2,232 (85)¶ |
| Diabetes mellitus | 4,290 (46) | 2,143 (43) | 174 (40) | 1,028 (49)¶ | 1,370 (52)¶ |
| Liver disease | 421 (5) | 182 (4) | 85 (20) | 114 (5)¶ | 115 (4) |
| Renal disease | 2,033 (22) | 935 (19) | 112 (26)¶ | 522 (25)¶ | 711 (27)¶ |
| Deep vein thrombosis | 1,064 (11) | 496 (10) | 77 (18) | 298 (14) | 346 (13) |
| Refill time (d)∥ | 33±16 | 32±16 | 36±19¶ | 29±15¶ | 35±16¶ |
| Follow-up (d)∥ | 740±593 | 724±594 | 703±599 | 751±587 | 772±593¶ |
| Warfarin prescriptions ∥ | 25±22 | 25±22 | 21±18¶ | 29±25¶ | 24±20¶ |
There were 106 patients with substance abuse and psychiatic illness, 50 patients with substance abuse and social risk factors, 563 patients with psychiatric illness and social risk factors, and 46 patients with all 3 risk factors. Results are presented as number (%) unless otherwise noted
Substance abuse—alcohol dependence, alcohol abuse, or drug dependence
Psychiatric illness—schizophrenia, affective psychosis (including major depressive disorder, manic disorder, or bipolar affective disorder), paranoia or other psychosis
Social factors—no caregiver for patient, documented noncompliance or homelessness
Mean±SD
P<.05 when compared with no risk factor group, byχ2- or t-test where appropriate
NA, not applicable.
With regard to the average time between prescription refills (Table 1), subjects with substance abuse and with social risk factors averaged significantly more time between prescription refills than subjects without risk factors for nonadherence. However, subjects with psychiatric illness filled prescriptions more frequently than any other risk group examined.
The percentage of subjects with each outcome and the baseline event rates are presented for each risk group in Table 2. Significantly more strokes occurred in the subjects with psychiatric illness and in the subjects with social risk factors for nonadherence. Significantly more intracranial hemorrhages occurred in subjects with substance abuse and psychiatric illness. All 3 risk groups had significantly more gastrointestinal bleeding events than the group with no risk factors.
Table 2.
Adverse Events Across Risk Factor Groups *
| Total (n=9,345) | No Risk Factors (n=4,994) | Substance Abuse (n=435) | Psychiatric Illness (n=2,108) | Social Risk Factors (n=2,619) | |
|---|---|---|---|---|---|
| Adverse event | |||||
| Stroke | 308 (3.3) | 141 (2.8) | 13 (3.0) | 87 (4.1) | 108 (4.1)† |
| Intracranial hemorrhage | 158 (1.7) | 68 (1.4) | 16 (3.7)† | 49 (2.3)† | 44 (1.7) |
| Gastrointestinal bleed | 864 (9.5) | 397 (8.0) | 55 (12.6)† | 245 (11.6) | 314 (12.0)† |
| Event rates (/100 person-years) | |||||
| Stroke | 1.5 | 1.3 | 1.4 | 1.8 | 1.8 |
| Intracranial hemorrhage | 0.7 | 0.6 | 1.7 | 1.0 | 0.7 |
| Gastrointestinal Bleed | 4.3 | 3.7 | 6.2 | 5.2 | 5.3 |
Results are presented as number(%) unless otherwise noted
P<.05 when compared with no risk factor group usingχ2.
Risk Ratios
In the univariate analysis (Table 3), there were several significant predictors of increased risk for stroke at the P <.05 level including psychiatric illness, social risk factors for nonadherence, hypertension, diabetes mellitus, renal disease, and African-American race. Only 2 variables conferred an increase risk of intracranial hemorrhage at the P <.05 level: substance abuse, and psychiatric illness. Multiple variables significantly increased the risk of gastrointestinal bleeding at the P <.05 level: substance abuse, psychiatric illness, social risk factors, chronic heart failure, diabetes mellitus, liver disease, renal disease, and refill time less than or equal to 32 days.
Table 3.
Unadjusted and Adjusted Hazard Ratios for Adverse Events
| Variable | Stroke Hazard Ratio (95% Confidence Interval) | Intracranial Hemorrhage Hazard Ratio (95% Confidence Interval) | Gastrointestinal Bleed Hazard Ratio (95% Confidence Interval) | |||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusted * | Unadjusted | Adjusted * | Unadjusted | Adjusted * | |
| Substance abuse | 0.97 (0.55, 1.68) | 2.47 (1.48, 4.15) | 2.35 (1.40, 3.95) | 1.48 (1.12, 1.94) | 1.41 (1.07, 1.87) | |
| Psychiatric illness | 1.34 (1.04, 1.71) | 1.36 (1.06, 1.74) | 1.52 (1.08, 2.13) | 1.46 (1.04, 2.05) | 1.30 (1.12, 1.51) | 1.19 (1.03, 1.39) |
| Social risk factors | 1.31 (1.04, 1.66) | 1.20 (0.95, 1.52) | 0.93 (0.66, 1.32) | NA | 1.34 (1.17, 1.54) | 1.28 (1.12, 1.48) |
| Hypertension | 1.47 (1.08, 2.01) | 1.20 (0.87, 1.65) | 1.40 (0.91, 2.15) | NA | 1.13 (0.95, 1.34) | NA |
| Chronic heart failure | 1.19 (0.89, 1.57) | NA | 0.85 (0.59, 1.22) | NA | 1.53 (1.28, 1.83) | 1.31 (1.09, 1.58) |
| Diabetes mellitus | 1.39 (1.11, 1.75) | 1.28 (1.01, 1.61) | 0.94 (0.69, 1.28) | NA | 1.17 (1.02, 1.33) | 1.03 (0.90, 1.18) |
| Liver disease | 1.01 (0.58, 1.76) | NA | 0.90 (0.40, 2.03) | NA | 1.56 (1.18, 2.04) | 1.31 (0.99, 1.74) |
| Renal disease | 1.47 (1.15, 1.89) | 1.27 (0.98, 1.64) | 1.30 (0.91, 1.86) | NA | 1.78 (1.54, 2.05) | 1.61 (1.39, 1.87) |
| Deep vein thrombosis | 0.94 (0.66, 1.34) | NA | 0.94 (0.58, 1.54) | NA | 1.35 (1.13, 1.62) | 1.22 (1.02, 1.47) |
| Refill time >32 d | 1.00 (0.80, 1.25) | NA | 1.01 (0.74, 1.38) | NA | 0.85 (0.75, 0.97) | 0.79 (0.69, 0.91) |
| Age (per decade) | 0.99 (0.91, 1.08) | NA | 1.04 (0.92, 1.17) | NA | 1.03 (0.99, 1.09) | NA |
| Sex, male | 0.99 (0.78, 1.27) | NA | 1.10 (0.78, 1.53) | NA | 1.07 (0.93, 1.24) | NA |
| Race, white | 0.47 (0.37, 1.60) | 0.49 (0.38, 0.63) | 0.82 (0.55, 1.22) | NA | 0.86 (0.72, 1.02) | NA |
Adjusted models only include the variables significant at a P <.05 value in the unadjusted models
NA, not applicable
In the final adjusted Cox proportional hazards model (Table 3) for stroke that included all the variables significant at the P <.05 level in the univariate models, only psychiatric illness, diabetes mellitus, and African-American race were significant predictors of increased risk for stroke. The final model for intracranial hemorrhage included both psychiatric illness and substance abuse as significantly increasing the risk. In the adjusted model for gastrointestinal bleeding, the risk was significantly increased by substance abuse, psychiatric illness, social risk factors for nonadherence, chronic heart failure, liver disease, and renal disease. Averaging greater than 32 days between prescription refills was associated with a significantly lower risk of gastrointestinal bleeding.
As there was some overlap between the risk groups studied, we also calculated risk ratios based upon the total number of psychosocial risk factors for each patient. Overall, 53% of the patients had none of the risk factors, 38% had 1 risk factor, 8% had 2 risk factors, and 0.5% of the patients had all 3. For the outcome of gastrointestinal bleeding in the adjusted models, the risk ratio for having any 1 of the psychosocial risk factors was 1.19 (95% CI 1.02, 1.37), for having any 2 risk factors the risk ratio was 1.57 (95% CI 1.26, 1.95), and for a patient having all 3 risk factors the risk ratio was 2.85 (95% CI 1.56, 5.21). This trend was significant with a P value less than .001. For the outcomes of stroke and intracranial hemorrhage the trends towards increasing risk with increasing number of risk factors were significant with P values of .02 for both, however, the individual risk ratios were not all significant.
DISCUSSION
We have shown in the Ohio Medicaid population that psychosocial risk factors for nonadherence, previously identified as negative predictors of warfarin prescribing, are strong predictors of adverse events in patients with nonvalvular atrial fibrillation receiving warfarin compared with patients without these risk factors. Subjects with psychiatric illnesses had a 36% increase in the risk of stroke, a 46% increase in the risk of intracranial hemorrhage, and a 19% increase in the risk of gastrointestinal bleeding. Substance abusers had a 135% increase in the risk of intracranial hemorrhage and a 41% increase in the risk of gastrointestinal bleeding. Subjects with social risk factors for nonadherence had a 28% increased risk for gastrointestinal bleeding.
Previous studies have identified clinical predictors for bleeding in patients receiving warfarin.8,9,13 and risk factors for embolic events from atrial fibrillation.2,3 Several of these previously identified predictors of adverse events were not significant in our multivariable models but were significant in the univariate analyses. We can only speculate as to why some of the other known risk factors for stroke were not predictive in this analysis. Wang et al.26 have described prior stroke or TIA, diabetes, and hypertension as predictors of stroke. In our cohort we have excluded patients with a prior stroke or TIA in order to increase the validity of our outcomes (so that they will not be incorrectly coded as “prior stroke or TIA”). Our cohort has a fairly high proportion of comorbid conditions and it is likely we no longer have the same discriminatory power for some of the other predictors including hypertension. Our incidence rates for the outcomes of stroke, intracranial hemorrhage, and gastrointestinal bleeding are similar to what others have reported in the literature for patients receiving anticoagulation for atrial fibrillation.14,27,28
McMahan et al.,14 demonstrated that alcohol abuse was associated with a 2.7-fold increased risk of major bleeding in a study of 579 veterans, and White et al.18 demonstrated that an admission for an alcohol-related diagnosis was associated with a 2.6-fold increased risk of major bleeding in a study of over 21,000 patients. Our results are similar to these findings, particularly for intracranial hemorrhage, where we have shown patients have 2.5 times the risk for intracranial hemorrhage if they are substance abusers, which includes drug abuse in addition to alcohol. To our knowledge, there are no previously reported studies examining the effect of psychiatric illness or the social risk factors we identified on adverse events.
The validity of using the Ohio Medicaid administrative database for the diagnosis of atrial fibrillation and the use of warfarin has not been directly established. However, Borzecki et al.29 have validated the use of administrative data in the Veterans Administration for the diagnosis of atrial fibrillation. They demonstrated an observed agreement of 0.98 between chart review and administrative data-based algorithms when only 1 administrative diagnosis was required. They report the best strategy was to require 2 diagnoses over a period of at least 2 years. In our analysis, we required 2 diagnostic codes for atrial fibrillation as well in an effort to increase the validity.
Other studies have documented the reliability of Medicaid administrative claims data when compared with medical record diagnoses or expert chart review. Walkup et al.,30 demonstrated a 100% concordance rate for both psychiatric diagnoses and secondary substance abuse diagnoses in the New York State's Medicaid Management Information System compared with the medical record. In a similar study, Lurie et al.,31 demonstrated that the diagnosis of schizophrenia in Medicaid claims data actually agreed with an expert chart review by two psychiatrists in 87% of patients. In the Maryland Medicaid claims database, both the diagnosis and the date of service were shown to be in agreement with the medical record 82% of the time across a broad range of patients.32 Bright et al.,33 demonstrated that Medicaid pharmacy claims correspond to medical records 94% of the time, making them quite reliable.
Our study has several limitations. Prior studies of atrial fibrillation have generally included fewer women and individuals of higher socioeconomic status than in the current study. However, the results of this study are probably applicable to other Medicaid populations. We are aware of no studies that examine the validity of warfarin prescriptions in administrative data. As mentioned above, while administrative billing data has reasonable reliability, chart review would enhance the accuracy of diagnoses and of warfarin use. There is likely a severity of illness bias in using ICD-9-CM codes for diagnosing the psychosocial risk factors limiting the generalizability to only those patients with diagnosed conditions. The results are not adjusted for patient compliance with warfarin use nor for the intensity of anticoagulation therapy because of lack of laboratory results. If a patient had prior events or conditions not documented during the study years, our results would not reflect this information or be adjusted accordingly. However, given the large cohort size and length of follow-up, this information is likely to have minimal effects on our conclusions.
While this study cannot address the question of whether or not patients with these risk factors should be anticoagulated, these patients have an increased risk of adverse events and if they are anticoagulated they should be monitored closely to ensure that INRs remain within the therapeutic range. Furthermore, as patients with psychiatric illness were at an increased risk of both thromboembolic and hemorrhagic complications, it is reasonable to suspect that their level of anticoagulation may alternate between subtherapeutic and supratherapeutic ranges. Another possibility is that these patients are placed at an increased risk of drug interactions with warfarin because of additional antipsychotic medications leading to fluctuations in the INR although this study does not address this. Clearly, further evaluation needs to be performed in order to determine whether these patients benefit from anticoagulation or are only placed at increased risk of adverse outcomes.
In conclusion, our study of Ohio Medicaid patients with nonvalvular atrial fibrillation receiving warfarin demonstrates that psychosocial factors that were previously identified as negative predictors of warfarin prescribing are also risk factors for adverse events in patients receiving warfarin. Patients with psychiatric illnesses had an increased risk of both thromboembolic and hemorrhagic complications, while substance abusers and patients with social risk factors for nonadherence were at increased risk for hemorrhagic complications. Closer monitoring of these patients during anticoagulation is required to ensure the efficacy and safety of warfarin therapy.
Acknowledgments
This project was conducted under an interagency agreement with the University of Cincinnati and the Ohio Department of Jobs and Family Services through the Ohio Medicaid Technical Assistance Policy and Program (MEDTAPP). Results and opinions expressed do not necessarily represent the official views of the Ohio Department of Jobs and Family Services.
References
- 1.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke, the Framingham Study. Stroke. 1991;22:983–8. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 2.Britton M, Gustafsson C, et al. Non-rheumatic atrial fibrillation as a risk factor for stroke. Stroke. 1985;16:182–8. doi: 10.1161/01.str.16.2.182. [DOI] [PubMed] [Google Scholar]
- 3.Ezekowitz MD, Bridgers SL, James KE. Warfarin in the prevention of stroke associated with nonrheumatic atrial fibrillation. Veterans Affairs Stroke Prevention in Nonrheumatic Atrial Fibrillation Investigators. N Engl J Med. 1992;327:1406–12. doi: 10.1056/NEJM199211123272002. [DOI] [PubMed] [Google Scholar]
- 4.Stroke Prevention in Atrial Fibrillation Study. Final results. Circulation. 1991;84:527–39. doi: 10.1161/01.cir.84.2.527. [DOI] [PubMed] [Google Scholar]
- 5.Connolly SJ, Laupacis A, Gent M, Roberts RS, Cairns JA, Joyner C. Canadian Atrial Fibrillation Anticoagulation (CAFA) Study. J Am Coll Cardiol. 1991;18:349–55. doi: 10.1016/0735-1097(91)90585-w. [DOI] [PubMed] [Google Scholar]
- 6.Petersen P, Boysen G, Godtfredsen J, Andersen ED, Andersen B. Placebo-controlled, randomised trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation. The Copenhagen AFASAK study. Lancet. 1989;1:175–9. doi: 10.1016/s0140-6736(89)91200-2. [DOI] [PubMed] [Google Scholar]
- 7.The Boston Area Anticoagulation Trial for Atrial Fibrillation Investigators. The effect of low-dose warfarin on the risk of stroke in patients with nonrheumatic atrial fibrillation. N Engl J Med. 1990;323:1505–11. doi: 10.1056/NEJM199011293232201. [DOI] [PubMed] [Google Scholar]
- 8.Hylek EM, Heiman H, Skates SJ, Sheehan MA, Singer DE. Acetaminophen and other risk factors for excessive warfarin anticoagulation. JAMA. 1998;279:657–62. doi: 10.1001/jama.279.9.657. [DOI] [PubMed] [Google Scholar]
- 9.Landefeld CS, Goldman L. Major bleeding in outpatients treated with warfarin incidence and prediction by factors known at the start of outpatient therapy. Am J Med. 1989;87:144–52. doi: 10.1016/s0002-9343(89)80689-8. [DOI] [PubMed] [Google Scholar]
- 10.Landefeld CS, Cook EF, Flatley M, Weisberg M, Goldman L. Identification and preliminary validation of predictors of major bleeding in hospitalized patients starting anticoagulant therapy. Am J Med. 1987;82:703–13. doi: 10.1016/0002-9343(87)90004-0. [DOI] [PubMed] [Google Scholar]
- 11.Landefeld CS, Rosenblatt MW, Goldman L. Bleeding in outpatients treated with warfarin relation to the prothrombin time and important remediable lesions. Am J Med. 1989;87:153–9. doi: 10.1016/s0002-9343(89)80690-4. [DOI] [PubMed] [Google Scholar]
- 12.Man-Son-Hing M, Laupacis A. Anticoagulant-related bleeding in older persons with atrial fibrillation physicians' fears often unfounded. Arch Intern Med. 2003;163:1580–6. doi: 10.1001/archinte.163.13.1580. [DOI] [PubMed] [Google Scholar]
- 13.The Stroke Prevention in Atrial Fibrillation Investigators Bleeding during antithrombotic therapy in patients with atrial fibrillation. Arch Intern Med. 1996;156:409–16. [PubMed] [Google Scholar]
- 14.McMahan DA, Smith DM, Carey MA, Zhou XH. Risk of major hemorrhage for outpatients treated with warfarin. J Gen Intern Med. 1998;13:311–6. doi: 10.1046/j.1525-1497.1998.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Penning-van Beest FJ, van Meegen E, Rosendaal FR, Stricker BH. Characteristics of anticoagulant therapy and comorbidity related to overanticoagulation. Thromb Haemost. 2001;86:569–74. [PubMed] [Google Scholar]
- 16.Penning-van Beest FJ, Geleijnse JM, van Meegen E, Vermeer C, Rosendaal FR, Stricker BH. Lifestyle and diet as risk factors for overanticoagulation. J Clin Epidemiol. 2002;55:411–7. doi: 10.1016/s0895-4356(01)00485-1. [DOI] [PubMed] [Google Scholar]
- 17.Brigden ML, Kay C, Le A, Graydon C, McLeod B. Audit of the frequency and clinical response to excessive oral anticoagulation in an out-patient population. Am J Hematol. 1998;59:22–7. doi: 10.1002/(sici)1096-8652(199809)59:1<22::aid-ajh5>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 18.White RH, Beyth RJ, Zhou H, Romano PS. Major bleeding after hospitalization for deep-venous thrombosis. Am J Med. 1999;107:414–24. doi: 10.1016/s0002-9343(99)00267-3. [DOI] [PubMed] [Google Scholar]
- 19.Gitter MJ, Jaeger TM, Petterson TM, Gersh BJ, Silverstein MD. Bleeding and thromboembolism during anticoagulant therapy a population-based study in Rochester, Minnesota. Mayo Clin Proc. 1995;70:725–33. doi: 10.4065/70.8.725. [DOI] [PubMed] [Google Scholar]
- 20.Stafford RS, Singer DE. Recent national patterns of warfarin use in atrial fibrillation. Circulation. 1998;97:1231–3. doi: 10.1161/01.cir.97.13.1231. [DOI] [PubMed] [Google Scholar]
- 21.Go AS, Hylek EM, Borowsky LH, Phillips KA, Selby JV, Singer DE. Warfarin use among ambulatory patients with nonvalvular atrial fibrillation the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Ann Intern Med. 1999;131:927–34. doi: 10.7326/0003-4819-131-12-199912210-00004. [DOI] [PubMed] [Google Scholar]
- 22.Fang MC, Stafford RS, Ruskin JN, Singer DE. National trends in antiarrhythmic and antithrombotic medication use in atrial fibrillation. Arch Intern Med. 2004;164:55–60. doi: 10.1001/archinte.164.1.55. [DOI] [PubMed] [Google Scholar]
- 23.Johnston JA, Cluxton RJ, Jr., Heaton PC, Guo JJ, Moomaw CJ, Eckman MH. Predictors of warfarin use among Ohio medicaid patients with new-onset nonvalvular atrial fibrillation. Arch Intern Med. 2003;163:1705–10. doi: 10.1001/archinte.163.14.1705. [DOI] [PubMed] [Google Scholar]
- 24.Koroukian SM, Cooper GS, Rimm AA. Ability of Medicaid claims data to identify incident cases of breast cancer in the Ohio Medicaid population. Health Serv Res. 2003;38:947–60. doi: 10.1111/1475-6773.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shireman TI, Olson BM, Dewan NA. Patterns of antidepressant use among children and adolescents. Psychiatr Serv. 2002;53:1444–50. doi: 10.1176/appi.ps.53.11.1444. [DOI] [PubMed] [Google Scholar]
- 26.Wang TJ, Massaro JM, Levy D, et al. A risk score for predicting stroke or death in individuals with new-onset atrial fibrillation in the community the Framingham Heart Study. JAMA. 2003;290:1049–56. doi: 10.1001/jama.290.8.1049. [DOI] [PubMed] [Google Scholar]
- 27.Fihn SD, Callahan CM, Martin DC, McDonell MB, Henikoff JG, White RH. The risk for and severity of bleeding complications in elderly patients treated with warfarin. The National Consortium of Anticoagulation Clinics. Ann Intern Med. 1996;124:970–9. doi: 10.7326/0003-4819-124-11-199606010-00004. [DOI] [PubMed] [Google Scholar]
- 28.Go AS, Hylek EM, Chang Y, et al. Anticoagulation therapy for stroke prevention in atrial fibrillation how well do randomized trials translate into clinical practice? JAMA. 2003;290:2685–92. doi: 10.1001/jama.290.20.2685. [DOI] [PubMed] [Google Scholar]
- 29.Borzecki AM, Wong AT, Hickey EC, Ash AS, Berlowitz DR. Identifying hypertension-related comorbidities from administrative data what's the optimal approach? Am J Med Qual. 2004;19:201–6. doi: 10.1177/106286060401900504. [DOI] [PubMed] [Google Scholar]
- 30.Walkup JT, Boyer CA, Kellermann SL. Reliability of Medicaid claims files for use in psychiatric diagnoses and service delivery. Admin Policy Ment Health. 2000;27:129–39. doi: 10.1023/a:1021308007343. [DOI] [PubMed] [Google Scholar]
- 31.Lurie N, Popkin M, Dysken M, Moscovice I, Finch M. Accuracy of diagnoses of schizophrenia in Medicaid claims. Hosp Community Psychiatr. 1992;43:69–71. doi: 10.1176/ps.43.1.69. [DOI] [PubMed] [Google Scholar]
- 32.Steinwachs DM, Stuart ME, Scholle S, Starfield B, Fox MH, Weiner JP. A comparison of ambulatory Medicaid claims to medical records a reliability assessment. Am J Med Qual. 1998;13:63–9. doi: 10.1177/106286069801300203. [DOI] [PubMed] [Google Scholar]
- 33.Bright RA, Avorn J, Everitt DE. Medicaid data as a resource for epidemiologic studies strengths and limitations. J Clin Epidemiol. 1989;42:937–45. doi: 10.1016/0895-4356(89)90158-3. [DOI] [PubMed] [Google Scholar]