Abstract
An Escherichia coli strain exhibiting decreased susceptibility to carbapenems was isolated from a hospitalized patient in Greece. The strain carried a self-transferable plasmid coding for metallo-β-lactamase VIM-1. blaVIM-1, along with aacA7, dhfrI, and aadA, was included as a gene cassette in a novel class 1 integron. A Citrobacter freundii ampC-derived gene, not associated with the integron, was also located in the same plasmid.
The clinical significance of the carbapenem-hydrolyzing metallo-β-lactamases (MBLs) of the IMP and VIM types is increasing. The respective bla genes are spread among various gram-negative microorganisms in the Far East and Europe (8). The VIM-type MBLs (VIM-1 through -3) have been found mostly in Pseudomonas aeruginosa and also in P. putida, P. stutzeri, Acinetobacter spp., Achromobacter xylosoxidans, and Serratia marcescens (2, 3, 9, 10, 15-17). In this report, we describe an Escherichia coli strain with reduced susceptibility to imipenem due to acquisition of a self-transferable, VIM-1-encoding plasmid.
E. coli V541 was isolated in November 2001 from a urine specimen of a patient treated in the “Tzanion” general hospital in Piraeus, Greece. E. coli K-12 strain 26R793 (Rifr) was used as the recipient in conjugation experiments. E. coli DH5α was used as the host for transformation. E. coli DH5α strains producing the β-lactamases VIM-2 (5) and LAT-1 (13) were used as controls.
MICs of β-lactams were determined by an agar dilution technique (6). Susceptibility to β-lactams in the presence of Ro 48-1220, a penicillanic acid sulfone exhibiting potent inhibitory activity against both class A and class C β-lactamases (12), was also determined. Susceptibility to other antimicrobials was assessed by disk diffusion (7). To detect MBL production, a synergy test using imipenem- and EDTA-containing disks was employed (1).
Conjugation was carried out in mixed broth cultures. Transconjugant clones were selected on Mueller-Hinton agar containing ampicillin (50 μg/ml) plus rifampin (200 μg/ml). Extraction of plasmid DNA and transformation experiments were performed by standard techniques (11).
blaVIM genes were detected by PCR with primers VIM-F (5′-AGTGGTGAGTATCCGACAG-3′) and VIM-R (5′-ATGAAAGTGCGTGGAGAC-3′), corresponding to nucleotides (nt) 1339 to 1357 and 1599 to 1582, respectively, of the blaVIM-1 integron (GenBank accession no. Y18050). Detection of Citrobacter freundii ampC-derived genes by PCR was performed as described previously (14). DNA fragments obtained in these assays were labeled with a digoxigenin DNA labeling and detection kit (Roche Diagnostics, Mannheim, Germany) and used as probes in hybridization experiments. Mapping of class 1 integrons was performed by PCR with a set of primers including 5′-CS, 3′-CS (4), and INT-F (5′-CGTTCCATACAGAAGCTG-3′), which corresponds to an intI1 sequence preceding the promoter region of class 1 integrons (from nt 657 to nt 674 of the blaVIM-1 integron). Nucleotide sequences of the PCR products were determined on both strands with an ABI Prism 377 DNA sequencer (Perkin-Elmer Applied Biosystems Division, Foster City, Calif.).
β-Lactamases were extracted by ultrasonic treatment of bacterial cell suspensions and clarified by centrifugation. The rate of imipenem hydrolysis was measured by UV spectrophotometry (2) and expressed as units of activity (1 U was defined as the amount of enzyme hydrolyzing 1 pmol of substrate/min/μg of protein at 30°C). Inhibition of imipenem hydrolysis by Ro 48-1220 (10 μg/ml) and EDTA (2 mM) was also assessed. Hydrolysis and inhibition experiments were performed in triplicate. Analytical isoelectric focusing was performed with polyacrylamide gels containing ampholytes (pH range, 3.5 to 9.5; AP Biotech, Piscataway, N.J.). β-Lactamase activity was visualized with nitrocefin (Oxoid Ltd., Basingstoke, United Kingdom) and by the iodine-starch method with imipenem as the substrate (9).
E. coli V541 was collected because of its decreased susceptibility to imipenem. It was resistant to penicillins, penicillin-inhibitor combinations, cefoxitin, and expanded-spectrum cephalosporins. MICs of aztreonam, imipenem, and meropenem, although below the respective resistance breakpoints, indicated decreased susceptibility to these agents (Table 1). The strain also exhibited resistance to aminoglycosides, trimethoprim, and sulfonamides. Resistance was readily transferable to E. coli 26R793 recipients at a relatively high frequency (2 × 10−2 transconjugant per donor cell). Transconjugants exhibited a resistance phenotype similar to that of the donor. Plasmid DNA analysis indicated transfer of a plasmid approximately 50 kb in size that was designated p541. Transformation of E. coli DH5α with purified p541 produced resistant clones that were able to transfer resistance by conjugation. All p541-carrying strains were positive by the EDTA disk synergy test.
TABLE 1.
MICs of β-lactam antibiotics tested against VIM-1-producing and control E. coli strains
| Antibiotic(s)a | MIC (μg/ml) for E. coli strainb:
|
||||||
|---|---|---|---|---|---|---|---|
| V541 wt | 26R793 trc (VIM-1 + AmpC) | 26R793 | DH5α trf (VIM-1 + AmpC) | DH5α (VIM-2) | DH5α (LAT-1) | DH5α | |
| Ampicillin | >256 | >256 | 2 | >256 | >256 | >256 | 1 |
| Ticarcillin | >256 | >256 | 1 | >256 | >256 | >256 | 0.5 |
| Ticarcillin + CLA | >256 | >256 | 1 | >256 | >256 | >256 | 1 |
| Piperacillin | >256 | >256 | 1 | >256 | 128 | >256 | 0.5 |
| Piperacillin + TAZ | >256 | >256 | 0.5 | >256 | 128 | 64 | 0.25 |
| Piperacillin + Ro | >256 | 256 | 1 | 256 | 128 | 2 | 0.5 |
| Cefoxitin | >128 | >128 | 2 | >128 | >128 | >128 | 2 |
| Cefoxitin + Ro | >128 | 64 | 2 | 64 | >128 | 2 | 2 |
| Cefotaxime | 128 | >128 | 0.12 | 128 | 32 | 32 | ≤0.06 |
| Cefotaxime + Ro | 128 | 128 | ≤0.06 | 64 | 32 | ≤0.06 | ≤0.06 |
| Cefazidime | >128 | >128 | 0.12 | >128 | 64 | 128 | 0.12 |
| Ceftazidime + Ro | >128 | >128 | 0.12 | >128 | 64 | 0.5 | 0.12 |
| Cefepime | 64 | 32 | ≤0.06 | 32 | 2 | 0.5 | ≤0.06 |
| Cefepime + Ro | 64 | 32 | ≤0.06 | 32 | 1 | ≤0.06 | ≤0.06 |
| Aztreonam | 8 | 4 | ≤0.06 | 4 | 0.25 | 16 | ≤0.06 |
| Aztreonam + Ro | 0.25 | 0.12 | 0.12 | 0.12 | 0.25 | 0.12 | ≤0.06 |
| Imipenem | 8 | 8 | ≤0.06 | 8 | 2 | 0.5 | ≤0.06 |
| Imipenem + Ro | 8 | 8 | ≤0.06 | 8 | 2 | ≤0.06 | ≤0.06 |
| Meropenem | 2 | 2 | ≤0.06 | 1 | 1 | 0.12 | ≤0.06 |
| Meropenem + Ro | 2 | 2 | ≤0.06 | 1 | 1 | ≤0.06 | ≤0.06 |
CLA, clavulanic acid (2 μg/ml); TAZ, tazobactam (4 μg/ml); Ro, Ro 48-1220 (10 μg/ml).
wt, wild-type; trc, transconjugant; trf, transformant. The β-lactamas(es) produced by each strain is in parentheses.
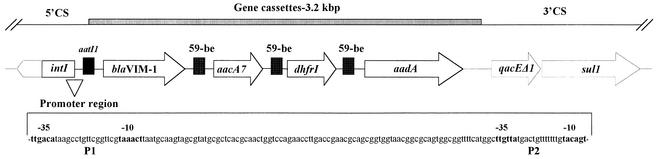
DNA preparations from E. coli V541 and the p541-containing laboratory strains were positive by the blaVIM-specific and C. freundii ampC-specific PCR assays. Also, p541 hybridized with the blaVIM and ampC probes (data not shown). PCR mapping indicated that blaVIM was part of a class 1 integron. DNA sequencing showed a gene cassette array that included (from 5′ to 3′) blaVIM-1, aacA7, dhfrI, and aadA. This structure was preceded by a strong P1 promoter (TTGACAN17TAAACT) and the inactive form of P2 (TTGTTAN14TACAGT) located at the 5′ end of an integrase 1 gene (Fig. 1). The blaVIM-1 cassette was identical to that originally described in P. aeruginosa (2). The DNA sequence of a 325-bp ampC amplicon was 98% homologous with the respective segment of the blaCMY-2 gene (nt 2679 to 3003; GenBank accession no. X91840).
FIG. 1.
Schematic presentation of the blaVIM-1-containing class 1 integron carried by plasmid p541. Part of the variable region and the 3′ conserved sequence (dashed lines) were postulated on the basis of PCR assays. The sequence of the promoter region is also shown. CS, conserved segment.
In the p541-containing strains, Ro 48-1220 decreased the MICs of aztreonam by five doubling dilutions. A decrease in the MICs of piperacillin, cefoxitin, and cefotaxime was also observed, while susceptibility to carbapenems was not affected by this inhibitor, as was observed for the VIM-2-producing E. coli control strain. In contrast, Ro 48-1220 rendered the cephalosporinase-producing E. coli control strain susceptible to β-lactams (Table 1).
Two β-lactamase species focusing at pH values of 9.0 (AmpC) and 5.2 (VIM-1) were observed by isoelectric focusing. VIM-1 activity was detectable by the iodine-starch method but not with nitrocefin (data not shown). β-Lactamase preparations from E. coli V541 exhibited imipenem-hydrolyzing activity (532 ± 21 U) that was inhibited by EDTA (<10 U) but was not significantly reduced by Ro 48-1220 (480 ± 35 U).
Production of a VIM MBL by an E. coli strain is documented here for the first time. Notably, VIM-1 was encoded by a self-transferable plasmid. Conjugal transfer of blaVIM was suggested in P. aeruginosa strains from Korean hospitals, but the respective plasmids could not be identified (3). blaVIM-1, along with aacA7, dhfrI, and aadA, constitutes the gene cassette region of a class 1 integron the structure of which was different from that of the previously described VIM-encoding integrons. Also, the putative promoter was a strong P1 while the blaVIM-1 genes described previously in P. aeruginosa and A. xylosoxidans were under the control of a weak P1 promoter and a hybrid P1 promoter, respectively (2, 10). The different phylogeny and diverse genetic locations of these integrons suggest a widespread environmental reservoir of the blaVIM genes. Acquisition of these determinants by self-transferable plasmids that can be established in enterobacteria will facilitate their further dissemination.
Plasmid p541 also encoded a class C β-lactamase that, most likely, did not substantially contribute to the levels of resistance to carbapenems, as indicated by susceptibility testing and hydrolysis experiments with the inhibitor Ro 48-1220. The carbapenem MICs for E. coli V541 were below the resistance breakpoints, as has been observed in E. coli strains expressing cloned blaVIM genes (2, 5, 9, 15). Detection of such strains may be facilitated by EDTA synergy tests.
REFERENCES
- 1.Arakawa, Y., N. Shibata, K. Shibayama, H. Kurokawa, T. Yagi, H. Fujiwara, and M. Goto. 2000. Convenient test for screening metallo-β-lactamase-producing Gram-negative bacteria by using thiol compounds. J. Clin. Microbiol. 38:40-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lauretti, L., M. L. Riccio, A. Mazzariol, G. Cornaglia, G. Amicosante, R. Fontana, and G. M. Rossolini. 1999. Cloning and characterization of blaVIM, a new integron-borne metallo-β-lactamase gene from a Pseudomonas aeruginosa clinical isolate. Antimicrob. Agents Chemother. 43:1584-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee, K., J. B. Lim, J. H. Yum, D. Yong, Y. Chong, J. M. Kim, and D. M. Livermore. 2002. blaVIM-2 cassette-containing novel integrons in metallo-β-lactamase-producing Pseudomonas aeruginosa and Pseudomonas putida isolates disseminated in a Korean hospital. Antimicrob. Agents Chemother. 46:1053-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lévesque, C., L. Piché, C. Larose, and P. H. Roy. 1995. PCR mapping of integrons reveals several novel combinations of resistance genes. Antimicrob. Agents Chemother. 39:185-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mavroidi, A., A. Tsakris, E. Tzelepi, S. Pournaras, V. Loukova, and L. S. Tzouvelekis. 2000. Carbapenem-hydrolysing VIM-2 metallo-β-lactamase in Pseudomonas aeruginosa from Greece. J. Antimicrob. Chemother. 46:1041-1043. [DOI] [PubMed] [Google Scholar]
- 6.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed. Approved standard M7-A4 (M100-S7). National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 7.National Committee for Clinical Laboratory Standards. 1997. Performance standards for antimicrobial disk susceptibility tests, 6th ed. Approved standard M2-A6 (M100-S7). National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 8.Nordmann, P., and L. Poirel. 2002. Emerging carbapenemases in Gram-negative aerobes. Clin. Microbiol. Infect. 8:321-331. [DOI] [PubMed] [Google Scholar]
- 9.Poirel, L., T. Naas, D. Nicolas, L. Collet, S. Bellais, J.-D. Cavallo, and P. Nordmann. 2000. Characterization of VIM-2, a carbapenem-hydrolyzing metallo-β-lactamase and its plasmid- and integron-borne gene from a Pseudomonas aeruginosa clinical isolate in France. Antimicrob. Agents Chemother 44:891-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riccio, M. L., L. Pallecchi, R. Fontana, and G. M. Rossolini. 2001. In70 of plasmid pAX22, a blaVIM-1-containing integron carrying a new aminoglycoside phosphotransferase gene cassette. Antimicrob. Agents Chemother. 45:1249-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 12.Tzouvelekis, L. S., M. Gazouli, E. E. Prinarakis, E. Tzelepi, and N. J. Legakis. 1997. Comparative evaluation of the inhibitory activities of the novel penicillanic acid sulfone Ro 48-1220 against β-lactamases that belong to groups 1, 2b, and 2be Antimicrob. Agents Chemother. 41:475-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tzouvelekis, L. S., E. Tzelepi, and A. F. Mentis. 1994. Nucleotide sequence of a plasmid-mediated cephalosporinase gene (blaLAT-1) found in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 38:2207-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villa, L., C. Mammina, V. Miriagou, L. S. Tzouvelekis, P. T. Tassios, A. Nastasi, and A. Carattoli. 2002. Multidrug and broad-spectrum cephalosporin resistance among Salmonella enterica serotype Enteritidis clinical isolates in Southern Italy. J. Clin. Microbiol. 40:2662-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan, J.-J., P.-R. Hsueh, W.-C. Ko, K.-T. Luh, S.-H. Tsai, H.-M. Wu, and J.-J. Wu. 2001. Metallo-β-lactamases in clinical Pseudomonas isolates in Taiwan and identification of VIM-3, a novel variant of the VIM-2 enzyme. Antimicrob. Agents Chemother. 5:2224-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yum, J. H., K. Yi, H. Lee, D. Yong, K. Lee, J. M. Kim, G. M. Rossolini, and Y. Chong. 2002. Molecular characterization of metallo-β-lactamase-producing Acinetobacter baumannii and Acinetobacter genomospecies 3 from Korea: identification of two new integrons carrying the blaVIM-2 gene cassettes. J. Antimicrob. Chemother. 49:837-840. [DOI] [PubMed] [Google Scholar]
- 17.Yum, J. H., D. Yong, K. Lee, H. S. Kim, and Y. Chong. 2002. A new integron carrying VIM-2 metallo-beta-lactamase gene cassette in a Serratia marcescens isolate. Diagn. Microbiol. Infect. Dis. 42:217-219. [DOI] [PubMed] [Google Scholar]