Abstract
In light of the need for new antifungal regimens, we report that at noncandidacidal concentrations, the lactoferrin-derived peptide hLF(1-11), which is highly active against fluconazole-resistant Candida albicans, acts synergistically with fluconazole against this yeast and a fluconazole-sensitive C. albicans strain as well as C. glabrata, C. krusei, C. parapsilosis, and C. tropicalis. When these yeasts were exposed to hLF(1-11) for 5 min and then incubated with fluconazole, they were killed effectively, while no candidacidal activity was observed when they were incubated first with fluconazole and then exposed to the peptide, indicating that the candidacidal activity is initiated by the peptide while fluconazole is only required during the effector phase. Investigations of the effect of azide, which inhibits mitochondrial respiration, on the activity of combinations of hLF(1-11) and fluconazole against fluconazole-resistant C. albicans revealed that it inhibits this activity, even when added during the effector phase only. As expected, azide inhibited the accumulation of rhodamine 123 in mitochondria and the production and release of ATP by C. albicans that occurred upon exposure to the combination of hLF(1-11) and fluconazole. Accordingly, oxidized ATP (oATP), an antagonist of ATP receptors, completely blocked the candidacidal activity of the hLF(1-11)-fluconazole combination, whereas oATP did not block the activity when its presence was restricted to the effector phase. The candidacidal activity of combinations of hLF(1-11) and fluconazole, which is initiated by the peptide through the involvement of energized mitochondria, renders fluconazole-resistant C. albicans sensitive to this azole.
Candida albicans is an opportunistic yeast that causes mucosal and invasive infections in immunocompromised hosts. Triazole antifungal agents are widely used in treating Candida infections in individuals with severe immunodeficiency (23, 24). Fluconazole, which has a high solubility in water, low toxicity, and wide tissue distribution after oral administration (15), is the most frequently employed agent. However, long-term fluconazole treatment often induces a selective pressure which results in the development of fluconazole-resistant C. albicans strains (25), as has been reported for AIDS patients (29) and individuals with hematological disorders (19, 22). This fluconazole resistance may be associated with (i) upregulation of ERG11, which encodes the drug target enzyme C14α-demethylase (7), (ii) the decreased affinity of azoles to cellular targets, often due to amino acid substitutions in the cytochrome P-450 C14α-demethylase (12, 26), (iii) accumulation of a less toxic ergosterol intermediate, C14α-methylfecosterol, as has been found in cells with an ERG3 mutation (9), and (iv) active efflux of this drug from Candida species. Two types of efflux pumps, i.e., the ATP binding cassette (ABC) transporter superfamily and the major facilitators, are known to contribute to drug resistance (14, 18, 20).
The frequent isolation of fluconazole-resistant C. albicans strains points to a pressing need for the development of new antifungal compounds and/or agents that act in synergy with fluconazole and other currently used antifungals. Human lactoferrin (hLF) is a potential candidate agent, as suggested by a recent report describing the synergistic fungistatic effect exerted by combinations of fluconazole and hLF (11). It has also been reported that the hyphal growth of azole-resistant C. albicans strains is inhibited by combinations of bovine lactoferrin and fluconazole (27, 28). Moreover, lactoferricin H, a cationic peptide released by pepsinolysis of hLF (1), comprises two cationic domains (residues 2 to 5 and 28 to 31) which exhibit a candidacidal activity higher than that of the native protein (16). Furthermore, it has recently been demonstrated that a synthetic peptide corresponding to the N terminus of hLF, hLF(1-11), possesses effective candidacidal activity (16). The possibility that hLF-derived peptides may be more promising candidates than intact lactoferrin for synergistic action with fluconazole should be considered. Indeed, suboptimal concentrations of the bovine lactoferricin, lactoferricin B, and fluconazole act synergistically against C. albicans (28).
The synergistic effects of two or more agents can result from the combination of different killing mechanisms. In this respect, the candidacidal activity of hLF(1-11) involves the energized mitochondrion (16), resulting in synthesis and secretion of ATP (16) and production of reactive oxygen species (17). Both of these mechanisms could be involved in increasing plasma membrane potential and permeability (16). Furthermore, as has been reported previously, the cytochrome P-450-dependent C14α-demethylase, which is involved in the ergosterol biosynthetic pathway, is the cellular target of fluconazole. This antifungal agent causes ergosterol depletion and accumulation of 14α-methyl-sterols in the plasma membrane of Candida species. In light of these considerations, the present study was undertaken (i) to evaluate whether noncandidacidal concentrations of hLF(1-11) and fluconazole act synergistically to kill C. albicans and, if so, (ii) to gain some insight into the mechanism(s) underlying the synergistic action against this yeast. In addition, we evaluated whether this synergistic candidacidal activity was present with other Candida species.
MATERIALS AND METHODS
Materials.
Sodium azide and periodate oxidized ATP (oATP) were purchased from Sigma Chemical Co. (St. Louis, Mo.). A stock of oATP at a concentration of 100 mM was prepared in phosphate-buffered saline (pH 7.5) and stored at −20°C until use. A freshly prepared solution of sodium azide was used where indicated.
Lactoferrin-derived peptides.
A synthetic peptide corresponding to residues 1 to 11 of hLF (GRRRRSVQWCA; Mr, 1,494), designated hLF(1-11), and, as a negative control, hLF(4-11), a peptide lacking the first three N-terminal residues, were prepared and purified as described previously (21). The purity of these peptides usually exceeded 88%, as determined by reverse-phase high-performance liquid chromatography. Stocks of these synthetic peptides at a concentration of 1 mg/ml of 0.01% acetic acid (pH 3.7) were stored at −20°C and, immediately before use, dried in a Speed-Vac (Savant Instruments Inc., Farmingdale, N.Y.).
Fluconazole.
Fluconazole (Pfizer Inc., New York, N.Y.) was dissolved in dimethyl sulfoxide (Fluka Chemie GmbH, Sigma-Aldrich Chemie BV, Zwijndrecht, The Netherlands) at a concentration of 1 mg/ml and stored at −20°C until use. From this stock solution, appropriate concentrations were prepared in 10 mM sodium phosphate buffer (NaPB), pH 7.4. Pilot studies indicated that the concentrations of dimethyl sulfoxide used in the experiments described here were without any effect.
Source of Candida strains.
Fluconazole-resistant C. albicans strain Y01-19 was purchased from Pfizer (Groton, Conn.). A fluconazole-sensitive C. albicans strain as well as C. glabrata, C. krusei, C. parapsilosis, and C. tropicalis were isolated from patients in the ward of the Department of Infectious Diseases, Leiden University Medical Center, Leiden, The Netherlands. Yeasts were identified on the basis of their sugar utilization patterns (API ID 32C; bioMérieux, Marcy l'Etoile, France), and their fluconazole sensitivity (MIC) was evaluated by using the E test (Oxoid Unipath Ltd., Basingstoke, United Kingdom). Yeast cells were cultured overnight in Sabouraud broth (Oxoid) at 37°C and subcultured for 2.5 h on a rotary wheel at 37°C.
Assay for candidacidal activity of hLF-derived peptides and fluconazole.
An in vitro assay was used to assess the candidacidal activity of hLF-derived peptides and/or fluconazole. Briefly, yeast cells were harvested in mid-log phase by centrifugation at 1,500 × g for 10 min, washed twice in NaPB, and diluted to a concentration of 106 CFU/ml of NaPB supplemented with 2% Sabouraud broth. Equal volumes of this suspension were mixed with hLF-derived peptides and/or fluconazole. After a 2-h incubation at 37°C with hLF-derived peptides and/or fluconazole, the number of viable blastoconidia was determined by plating serial dilutions of each sample on Sabouraud agar. Results are expressed as CFU per milliliter.
Exposure of Candida species to peptides and/or fluconazole.
To obtain some insight into the contributions of the different agents to the synergistic effect of hLF(1-11) peptide and fluconazole, Candida cells were preincubated for 5 min with either a noncandidacidal concentration of hLF(1-11) or fluconazole at 37°C, transferred to ice for 5 min, washed twice in NaPB at 4°C, and reincubated with the other agent for 2 h at 37°C and then the number of viable cells was determined microbiologically.
Exposure of fluconazole-resistant C. albicans to sodium azide or oATP prior to addition of hLF(1-11) and/or fluconazole.
Where indicated, fluconazole-resistant C. albicans was incubated with sodium azide (5 mM) or preincubated for 30 min with 300 μM oATP, as described previously (16), prior to the addition of the peptide and/or fluconazole. The optimal concentrations of sodium azide and oATP were determined in preliminary experiments; the selected concentrations were not toxic towards fluconazole-resistant C. albicans, and the binding of oATP to the cells was not affected by the temperature.
ATP bioluminescence assay.
ATP levels in cultures of fluconazole-resistant C. albicans were measured as described previously (16). Briefly, yeast cells were harvested in mid-log phase and washed twice as described above and then diluted to a concentration of 108 CFU/ml of NaPB. Equal volumes of this suspension were mixed with various concentrations of hLF peptides in the presence or absence of fluconazole. Changes in the intracellular ATP (ATPi) and extracellular ATP (ATPe) levels were measured after 2 min of incubation at 37°C, since most variations occurred within the first few minutes. At 2 min postincubation, samples were centrifuged at 10,000 × g at 4°C. Supernatants were collected, and the cells were then resuspended in an equal volume of phosphate-buffered saline (pH 7.4). The cell suspensions were boiled for an additional 3 min. ATPe and ATPi levels were measured by luminometry with an ATP determination kit according to the manufacturer's instructions (Molecular Probes, Eugene, Oreg.). Luciferin-luciferase assay mixture (180 μl) was added to 20 μl of cell lysates or extracellular medium, 150 μl of each sample was transferred into a 96-well microtiter plate, and light emission was monitored by using a model 1420 multilabel counter-Wallac Victor 2 luminometer (EG&G Wallac, Turku, Finland). The results were measured as bioluminescence relative light units, and ATP concentrations were calculated using a standard curve constructed with various concentrations of ATP.
Assay for mitochondrial permeabilization.
Changes in the mitochondrial permeabilization of fluconazole-resistant C. albicans upon exposure to hLF(1-11) and/or fluconazole were monitored with the fluorescent probe rhodamine 123 (Molecular Probes) (3). Rhodamine 123 is a positively charged probe believed to enter cells by diffusion (2). In mammalian systems, it has been shown to accumulate in mitochondria (8), and this accumulation depends on the mitochondrial transmembrane potential. Briefly, fluconazole-resistant C. albicans cells in mid-log phase were resuspended in 1 mM potassium phosphate buffer (PPB), pH 7.0, and preincubated for 10 min at 37°C with a 10 μM concentration of rhodamine 123 in PPB. After washes with PPB, fluconazole-resistant C. albicans cells were treated for 10 min at 37°C with 1 μM hLF(1-11) and/or 100 μg of fluconazole/ml and then subjected to fluorescence-activated cell sorter (FACS) analysis on a FACScan (Becton Dickinson, San Jose, Calif.) equipped with an argon laser at 488 nm. The fluorescence intensity of rhodamine 123 was measured in the second channel. Data acquisition and analysis were controlled by using the Lysis II software and hardware interface. Results are expressed as median fluorescence intensities.
Statistical analysis.
Differences between the results of the various treatments were analyzed using the Mann-Whitney U test. The level of significance was set at a P value of 0.05.
RESULTS
Candidacidal activity of combinations of noncandidacidal concentrations of hLF(1-11) and fluconazole.
Since hLF(1-11) is highly active against fluconazole-resistant C. albicans (16), noncandidacidal concentrations of this peptide were used to determine whether it acts synergistically with fluconazole to kill fluconazole-resistant C. albicans. The results revealed that combinations of noncandidacidal concentrations of hLF(1-11) and fluconazole were highly active (P < 0.05) against fluconazole-resistant C. albicans, whereas these agents used alone produced no effect (Table 1). Furthermore, the hLF(4-11) peptide, which showed no candidacidal effect, showed no significant killing activity when combined with fluconazole (Table 1).
TABLE 1.
Candidacidal activity of noncandidacidal concentrations of hLF(1-11) with or without fluconazolea
| Peptide (concn) | No. of surviving C. albicans cellsb with fluconazole concn (μg/ml) of:
|
|||
|---|---|---|---|---|
| 200 | 150 | 100 | 0 | |
| hLF(1-11) (8 μM) | (14 ± 10) × 103c,d | (1.4 ± 2) × 104c,d | (1.1 ± 1) × 105 | (3.6 ± 3) × 105 |
| hLF(1-11) (4 μM) | (3.4 ± 3) × 104c,d | (3.4 ± 3) × 104c,d | (31 ± 4) × 104c | (7.9 ± 2) × 105 |
| hLF(4-11) (8 μM) | (5.9 ± 2) × 105 | (1.4 ± 1) × 106 | (14 ± 10) × 105 | (12 ± 2) × 105 |
| None | (2.4 ± 3) × 105 | (4.3 ± 3) × 105 | (5.6 ± 3) × 105 | (1 ± 1) × 106 |
106 CFU of fluconazole-resistant C. albicans/ml were incubated with combinations of hLF(1-11) and fluconazole for 2 h at 37°C, and then the number of surviving cells was determined microbiologically. The peptide hLF(4-11) served as the negative control.
Data are means ± SD from at least three independent experiments.
Significantly different (P < 0.05) from values obtained with C. albicans exposed to hLF(1-11) alone.
Significantly different (P < 0.05) from values obtained with C. albicans exposed to fluconazole alone.
Effect of preincubation with noncandidacidal concentrations of hLF(1-11) or fluconazole on the candidacidal activity of fluconazole or hLF(1-11).
To determine which of the two compounds is required to initiate this synergistic effect, fluconazole-resistant C. albicans cells were preincubated for 5 min with a noncandidacidal concentration of hLF(1-11) and, after washing, were incubated with fluconazole, and vice versa. The results showed that the candidacidal activity obtained after preincubation with hLF(1-11) followed by incubation with fluconazole was comparable to that after simultaneous incubation of hLF(1-11) and fluconazole for 2 h (Table 2). In contrast, when fluconazole-resistant C. albicans cells were preincubated with fluconazole for 5 min and then exposed to a noncandidacidal concentration of hLF(1-11) for 2 h, no candidacidal effect was observed (Table 2). Similar results were obtained when fluconazole-resistant C. albicans cells were exposed to fluconazole for up to 1 h prior to the addition of the peptide (data not shown). These results indicate that the candidacidal activity of combinations of hLF(1-11) and fluconazole involves two phases: an initiator phase exerted by hLF(1-11) and an effector phase mediated by fluconazole.
TABLE 2.
Contributions of hLF(1-11) and fluconazole to the synergistic activity against various Candida speciesa
| Preincubation | Treatment | No. of surviving cellsb
|
|||||
|---|---|---|---|---|---|---|---|
| Fluconazole- resistant C. albicans | Fluconazole-sensitive C. albicans | C. glabrata | C. krusei | C. parapsilosis | C. tropicalis | ||
| None | hLF(1-11) | (7.8 ± 4) × 105 | (12 ± 6) × 105 | (2.6 ± 1) × 105 | (6 ± 1) × 104 | (25 ± 2) × 104 | (10 ± 10) × 104 |
| None | Fluconazole | (8.9 ± 2) × 105 | (9.9 ± 5) × 105 | (3.4 ± 3) × 105 | (27 ± 6) × 104 | (7.1 ± 3) × 105 | (1.9 ± 2) × 105 |
| None | hLF(1-11) and fluconazole | (7.3 ± 6) × 103c,d | (5.6 ± 1) × 104c,d | 4 × 103c,d | (3.3 ± 2) × 104c,d | (7.9 ± 3) × 104c,d | (12 ± 3) × 103c,d |
| hLF(1-11) | Fluconazole | (7 ± 9) × 103c,d | 6 × 102c,d,e | (62 ± 4) × 10c,d,e | 6 × 102c,d,e | 6 × 102c,d,e | (1.3 ± 1) × 103c,d,e |
| Fluconazole | hLF(1-11) | (7 ± 3) × 105e,f | (22 ± 7) × 104c,d,e,f | (13 ± 5) × 104c,d,e,f | (3.1 ± 1) × 105c,e,f | (35 ± 6) × 104c,d,e,f | (1.4 ± 1) × 105e,f |
| None | None | (16 ± 5) × 105 | (17 ± 3) × 105 | (10 ± 4) × 105 | (12 ± 2) × 105 | (9.4 ± 2) × 105 | (3.9 ± 1) × 105 |
106 CFU of Candida cells/ml were incubated for 5 min at 37°C with 8 μM hLF(1-11) or 100 μg of fluconazole/ml (except for both C. albicans strains, which were incubated with 200-μg/ml fluconazole), washed, and then incubated for 2 h at 37°C with the above-mentioned concentrations of fluconazole or hLF(1-11), respectively. As controls, Candida cells were incubated for 2 h at 37°C with hLF(1-11), fluconazole, the combination of hLF(1-11) and fluconazole, or no agent, and then the number of surviving Candida cells was determined microbiologically.
Data are means ± SD from at least three independent experiments. The MICs of fluconazole for fluconazole-resistant C. albicans, fluconazole-sensitive C. albicans, C. glabrata, C. krusei, C. parapsilosis, and C. tropicalis are >256, 4, 16, 32, 4, and 16 μg/ml, respectively.
Significantly different (P < 0.05) from values obtained with Candida cells exposed to hLF(1-11) alone.
Significantly different (P < 0.05) from values obtained with Candida cells exposed to fluconazole alone.
Significantly different (P < 0.05) from values obtained with Candida cells exposed to the combination of hLF(1-11) and fluconazole.
Significantly different (P < 0.05) from values obtained with Candida cells exposed to hLF(1-11) and then treated with fluconazole.
Activity of various combinations of noncandidacidal concentrations of hLF(1-11) and fluconazole against a fluconazole-sensitive C. albicans strain and other Candida species.
To investigate whether these candidacidal actions could also be observed with other Candida isolates, in vitro killing assays were performed with a fluconazole-sensitive C. albicans strain as well as other Candida species, including C. glabrata, C. krusei, C. parapsilosis, and C. tropicalis. The results revealed that noncandidacidal concentrations of hLF(1-11) and fluconazole act synergistically (P < 0.05) to kill all of these Candida isolates. Moreover, preincubation for 5 min with hLF(1-11) and then exposure to fluconazole for 2 h resulted in a significantly greater candidacidal effect (P < 0.05) against all strains than that produced by the combination of hLF(1-11) and fluconazole (Table 2). In agreement with this result, preincubation with fluconazole followed by treatment with hLF(1-11) was found to result in significantly less activity (P < 0.05) than simultaneous incubation of hLF(1-11) and fluconazole and preincubation with hLF(1-11) followed by incubation with fluconazole (Table 2).
Effect of oATP and sodium azide on the synergistic candidacidal activity of hLF(1-11) and fluconazole.
Based on the above-mentioned observations and on previously reported results on the mechanism of action of hLF(1-11) against fluconazole-resistant C. albicans (16), we focused our attention on the mechanism underlying the synergistic candidacidal activity of hLF(1-11) and fluconazole. To determine whether receptors for ATPe on fluconazole-resistant C. albicans were involved in the synergistic candidacidal activity of hLF(1-11) and fluconazole, we investigated the effect of oATP, which is an irreversible inhibitor of the ATPe receptors (13), on this activity. The results revealed that preincubation with oATP for 30 min caused a 99% ± 0.6% reduction (mean ± standard deviation [SD]; P < 0.05; n = 4) of the candidacidal effect exerted by combinations of hLF(1-11) and fluconazole.
In order to assess whether the intracellular metabolism of fluconazole-resistant C. albicans was involved in the synergistic effect, killing assays were performed in the presence of sodium azide, which blocks electron transport in the mitochondrial respiratory chain. Incubation with 5 mM sodium azide conferred to fluconazole-resistant C. albicans almost complete (98% ± 0.8%; P < 0.05; n = 4) protection against combinations of hLF(1-11) and fluconazole.
Effect of oATP and sodium azide on the effector phase of the synergism.
In order to investigate whether ligation of receptors for ATPe was involved in the effector phase, fluconazole-resistant C. albicans cells were incubated for 5 min with hLF(1-11), washed, and then incubated with oATP for 30 min at 4°C before being exposed to fluconazole for 2 h prior to assessment of the number of surviving cells. The results revealed that oATP was without effect, indicating that ATPe receptors were not involved in the effector phase of the synergistic candidacidal activity. Next, we investigated whether the effector phase was dependent on the mitochondrial activity. C. albicans cells were preincubated for 5 min with hLF(1-11), washed, resuspended in sodium azide, and then reincubated with fluconazole. The results showed that sodium azide still inhibited the candidacidal activity (99% ± 0.1% reduction; P < 0.05; n = 4) that was present during the effector phase.
Effect of hLF(1-11), fluconazole, and the combination of hLF(1-11) and fluconazole on ATPi and ATPe levels.
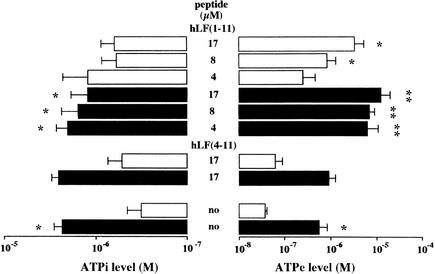
Since ATPe receptors may be involved in the candidacidal activity of hLF(1-11) (16), we measured the changes in the ATPe and ATPi levels of fluconazole-resistant C. albicans in response to the combination of hLF(1-11) and fluconazole as well as to each agent alone. The results of the dose-effect study of hLF(1-11) and fluconazole revealed a 10-fold increase (P < 0.05) in the ATPe level compared with that of unexposed cells. The combination of fluconazole and increasing concentrations of hLF(1-11) resulted in a 100-fold higher ATPe level (P < 0.05) compared with that of unexposed cells and a 10-fold higher level (P < 0.05) compared with that of cells exposed to either hLF(1-11) or fluconazole alone. Finally, the combination of hLF(4-11) and fluconazole did not affect the fluconazole-induced ATPe level (Fig. 1).
FIG. 1.
Effect of hLF(1-11) and/or fluconazole on the ATPe and ATPi levels of fluconazole-resistant C. albicans. The peptide hLF(4-11) served as the negative control. Briefly, approximately 108 CFU of fluconazole-resistant C. albicans/ml were incubated with the peptide in the presence (closed bars) or absence (open bars) of 200 μg of fluconazole/ml. ATP levels at 2 min after stimulation were measured using an ATP determination kit. “no” means no peptide. Data are means plus SD from at least three independent experiments. ∗, significant difference (P < 0.05) compared with results obtained with untreated cells. ∗∗, significant difference (P < 0.05) compared with results obtained with cells exposed to hLF(1-11) or fluconazole alone.
Incubation of fluconazole-resistant C. albicans with fluconazole induced a significant increase (P < 0.05) in the ATPi level compared with that in unexposed cells (Fig. 1). No clear increases in ATPi level were induced by incubation of fluconazole-resistant C. albicans with various concentrations of hLF(1-11). Addition of the combination of hLF(1-11) and fluconazole resulted in ATPi levels that were not different from the fluconazole-induced ATPi level yet significantly higher (P < 0.05) than the ATPi level of unexposed cells.
Moreover, sodium azide did not affect the fluconazole-induced increase in ATPe or ATPi level, while it virtually blocked (P < 0.05) the hLF(1-11)-induced ATPe increase and significantly reduced (P < 0.05) the ATPe increase that occurred upon exposure to combinations of hLF(1-11) and fluconazole (Table 3).
TABLE 3.
Effect of sodium azide on the increase in ATPe level induced by the exposure of fluconazole-resistant C. albicans to hLF(1-11), fluconazole, combinations of these two compounds, or no stimulusa
| Treatment | ATPe level (M) withb:
|
|
|---|---|---|
| No azide | Azide | |
| hLF(1-11) | (2.6 ± 2) × 10−6 | (4.1 ± 2) × 10−8c |
| Fluconazole | (8.1 ± 11) × 10−7 | (1.3 ± 1) × 10−7 |
| hLF(1-11) and fluconazole | (2.1 ± 1) × 10−5 | (3.1 ± 2) × 10−6c |
| No stimulus | (3.9 ± 2) × 10−8 | (3.1 ± 1) × 10−8 |
Approximately 108 CFU of fluconazole-resistant C. albicans/ml were incubated with the various stimuli in the presence or absence of 5 mM sodium azide. At 2 min after stimulation, ATPe levels were measured by using an ATP determination kit.
Data are means ± SD from at least three independent experiments.
Significantly different (P < 0.05) from value obtained without azide treatment.
Effect of the combination of hLF(1-11) and fluconazole on mitochondrial membrane integrity.
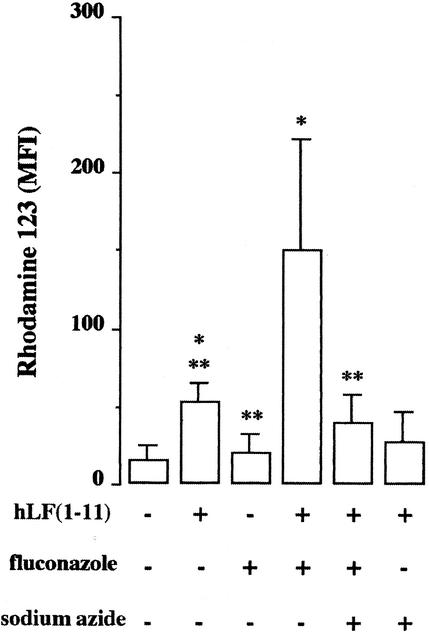
The effect of the combination of a noncandidacidal concentration of hLF(1-11) and fluconazole on mitochondrial membranes in fluconazole-resistant C. albicans was assessed by using the fluorescent probe rhodamine 123 and FACS analysis. The results revealed that noncandidacidal concentrations (1 μM) of hLF(1-11) induced a small but significant increase (P < 0.05) in rhodamine 123 fluorescence in fluconazole-resistant C. albicans compared with that in unexposed cells, while no change in fluorescence in fluconazole-resistant C. albicans upon fluconazole exposure was observed (Fig. 2). Interestingly, the combination of hLF(1-11) and fluconazole induced a significant increase (P < 0.05) in rhodamine 123 fluorescence in fluconazole-resistant C. albicans compared with that induced by noncandidacidal concentrations of hLF(1-11) or fluconazole alone (Fig. 2). In agreement with this result, sodium azide was found to significantly reduce (P < 0.05) the mitochondrial activity induced by the combination of hLF(1-11) and fluconazole (Fig. 2).
FIG. 2.
Effect of the combination of a noncandidacidal concentration of hLF(1-11) and fluconazole or either of these agents alone on the mitochondrial activity of fluconazole-resistant C. albicans. Briefly, rhodamine 123-labeled fluconazole-resistant C. albicans cells were exposed to 1 μM hLF(1-11) and/or 100 μg of fluconazole/ml for 10 min at 37°C, and then the median fluorescence intensity (MFI) of this probe was assessed by FACS analysis. In addition, the effect of 5 mM sodium azide on the stimulation of mitochondrial activity was determined. Data are means plus SD from at least three independent experiments. ∗, significant difference (P < 0.05) compared with results obtained with untreated cells. ∗∗, significant difference (P < 0.05) compared with results obtained with cells exposed to the combination of hLF(1-11) and fluconazole.
DISCUSSION
Three major findings have been made in the present study of the activity exerted by combinations of noncandidacidal concentrations of hLF(1-11) peptide and fluconazole against (fluconazole-sensitive and -resistant) C. albicans and other Candida species, including C. glabrata, C. krusei, C. parapsilosis, and C. tropicalis. First, combinations of hLF(1-11) and fluconazole were highly effective (P < 0.05) against the various Candida species, whereas noncandidacidal concentrations of hLF(1-11) or fluconazole alone were without effect. In agreement with our earlier observation that the first two arginines (residues 2 and 3) of hLF(1-11) are critical for its antimicrobial activities (16, 21), a peptide lacking the first three residues, i.e., hLF(4-11), was found to display no candidacidal activity when combined with fluconazole. Furthermore, our results extend those of a previous report of the inhibition of hyphal growth of azole-resistant C. albicans by the combination of suboptimal concentrations of bovine lactoferrin or lactoferricin and triazoles (28), as we focused on the candidacidal activity and identification in human lactoferricin of a short domain responsible for the synergistic candidacidal activity. Finally, it should be noted that in preliminary experiments a synthetic histatin-derived peptide, which exerts effects on Candida species similar to those seen with hLF(1-11) (5, 6, 10), showed a synergistic activity with fluconazole against fluconazole-resistant C. albicans (data not shown).
The second important finding was that fluconazole-resistant C. albicans cells exposed to noncandidacidal concentrations of hLF(1-11) for 5 min, washed, and then treated with fluconazole were killed effectively, while no candidacidal activity was observed when this yeast was first incubated with fluconazole (even for a prolonged incubation time [1 h]), washed, and then exposed to noncandidacidal concentrations of hLF(1-11). These data indicate that the synergistic activity of combinations of hLF(1-11) and fluconazole is initiated by the peptide, while fluconazole is required for the effector phase only. It is interesting that exposure for 5 min to noncandidacidal concentrations of hLF(1-11) followed by washing and then incubation with fluconazole resulted in even greater activity (P < 0.05) against various Candida species (except fluconazole-resistant C. albicans) than that produced by the simultaneous incubation of hLF(1-11) and fluconazole. Our observation that fluconazole-resistant C. albicans becomes responsive to this azole when applied in combination with the hLF(1-11) peptide is of clinical importance.
The third major finding pertains to the mechanism of action of this synergistic candidacidal activity. We found that the candidacidal activity of combinations of noncandidacidal concentrations of hLF(1-11) and fluconazole was significantly reduced (P < 0.05) by sodium azide, a specific inhibitor of mitochondrial electron transport (30). Interestingly, azide also inhibited the candidacidal activity obtained by preincubation of Candida cells with noncandidacidal concentrations of hLF(1-11) and subsequent treatment with fluconazole when the presence of this inhibitor was restricted to the effector phase only. Although we cannot exclude the possibility that sodium azide has an inhibitory effect on the fluconazole-induced effector phase, we found that this inhibitor had no effect on the fluconazole-induced rise in ATPe levels and that fluconazole had no effect on mitochondrial energization. It should be noted that preliminary data indicated that after exposure of fluconazole-resistant C. albicans to 34 μM hLF(1-11) for 5 min, the killing process was initiated and was still sensitive to the inhibitory effect of sodium azide. Furthermore, the combination of 1 μM hLF(1-11) and fluconazole induced a rapid accumulation (P < 0.05) of the mitochondrial dye rhodamine 123 in mitochondria of fluconazole-resistant C. albicans, whereas this concentration of hLF(1-11) induced only a small, although significant (P < 0.05), increase in mitochondrial activity and, as previously mentioned, fluconazole was completely without effect. Moreover, we found that the ATPe level increased considerably (P < 0.05) when fluconazole-resistant C. albicans was exposed to combinations of noncandidacidal concentrations of hLF(1-11) and fluconazole compared with that seen with either of these compounds alone. As expected, combinations of hLF(4-11) and fluconazole did not induce an increase in ATPe level greater than that induced by fluconazole alone. Our observation that oATP, an irreversible antagonist of receptors for ATPe (10), almost completely blocked the candidacidal activity of combinations of noncandidacidal concentrations of hLF(1-11) and fluconazole, whereas this receptor antagonist was without effect when its presence was restricted to the effector phase, indicated that an increase in the ATPe levels in Candida species is essential in the initiator phase.
Although there is no definitive explanation for our observation that combinations of noncandidacidal concentrations of hLF(1-11) and fluconazole are highly effective against C. albicans and other Candida species, the results presented here suggest the critical involvement of the energized mitochondrion in the initiation of this synergistic candidacidal action by hLF(1-11), while fluconazole is required in the effector phase only. On the other hand, it may be speculated that modification of the mitochondrial (or fungal) membrane sterol composition, which affects the activity of mitochondrial ATPases, as reported for Saccharomyces cerevisiae grown under restricted conditions (4), by the hLF(1-11) peptide is favorable for the action of fluconazole against Candida species. Further studies exploring the mechanism(s) underlying the synergistic effect between hLF(1-11) and fluconazole as well as preclinical studies elucidating the therapeutic potential of such combinations are required.
Acknowledgments
This work was supported by a research grant from the Italian “Ministero dell'Università e della Ricerca Scientifica e Tecnologica” (contracts MM06248147 and 2001064775).
REFERENCES
- 1.Bellamy, W., M. Takase, K. Yamauchi, H. Wakabayashi, K. Kawase, and M. Tomita. 1992. Identification of the bactericidal domain of lactoferrin. Biochim. Biophys. Acta 1121:130-136. [DOI] [PubMed] [Google Scholar]
- 2.Bernal, S. D., T. J. Lampidis, I. C. Summerhayes, and L. B. Chen. 1982. Rhodamine-123 selectively reduces clonal growth of carcinoma cells in vitro. Science 218:1117-1119. [DOI] [PubMed] [Google Scholar]
- 3.Clark, F. S., T. Parkinson, C. A. Hitchcock, and N. A. R. Gow. 1996. Correlation between rhodamine 123 accumulation and azole sensitivity in Candida species: possible role for drug efflux in drug resistance. Antimicrob. Agents Chemother. 40:419-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cobon, G. S., and J. M. Haslam. 1973. The effect of altered membrane sterol composition on the temperature dependence of yeast mitochondrial ATPase. Biochem. Biophys. Res. Commun. 52:320-326. [DOI] [PubMed] [Google Scholar]
- 5.Helmerhorst, E. J., P. Breeuwer, W. van't Hof, E. Walgreen-Weterings, L. C. Oomen, E. C. Veerman, A. V. Amerongen, and T. Abee. 1999. The cellular target of histatin 5 on Candida albicans is the energized mitochondrion. J. Biol. Chem. 274:7286-7291. [DOI] [PubMed] [Google Scholar]
- 6.Helmerhorst, E. J., R. F. Troxler, and F. G. Oppenheim. 2001. The human salivary peptide histatin 5 exerts its antifungal activity through the formation of reactive oxygen species. Proc. Natl. Acad. Sci. USA 98:14637-14642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henry, K. W., J. T. Nickels, and T. D. Edlind. 2000. Upregulation of ERG genes in Candida species by azoles and other sterol biosynthesis inhibitors. Antimicrob. Agents Chemother. 44:2693-2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson, L. V., M. L. Walsh, and L. B. Chen. 1980. Localization of mitochondria in living cells with rhodamine 123. Proc. Natl. Acad. Sci. USA 77:990-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelly, S. L., D. C. Lamb, D. E. Kelly, N. J. Manning, J. Loeffler, H. Hebart, U. Schumacher, and H. Einsele. 1997. Resistance to fluconazole and cross-resistance to amphotericin B in Candida albicans from AIDS patients caused by defective sterol Δ5,6-desaturation. FEBS Lett. 400:80-82. [DOI] [PubMed] [Google Scholar]
- 10.Koshlukova, S. E., M. W. B. Araujo, D. Baev, and M. Edgerton. 2000. Released ATP is an extracellular cytotoxic mediator in salivary histatin 5-induced killing of Candida albicans. Infect. Immun. 68:6848-6856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuipers, M. E., H. G. De Vries, M. C. Eikelboom, D. K. F. Meijer, and P. J. Swart. 1999. Synergistic fungistatic effects of lactoferrin in combination with antifungal drugs against clinical Candida isolates. Antimicrob. Agents Chemother. 43:2635-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamb, D. C., D. E. Kelly, T. C. White, and S. L. Kelly. 2000. The R467K amino acid substitution in Candida albicans sterol 14α-demethylase causes drug resistance through reduced affinity. Antimicrob. Agents Chemother. 44:63-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lammas, D. A., C. Stober, C. J. Harvey, N. Kendrick, S. Panchalingam, and D. S. Kumararatne. 1997. ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z(P2X7) receptors. Immunity 7:433-444. [DOI] [PubMed] [Google Scholar]
- 14.Lopez-Ribot, J. L., R. K. McAtee, L. N. Lee, W. R. Kirkpatrick, T. C. White, D. Sanglard, and T. F. Patterson. 1998. Distinct patterns of gene expression associated with development of fluconazole resistance in serial Candida albicans isolates from human immunodeficiency virus-infected patients with oropharyngeal candidiasis. Antimicrob. Agents Chemother. 42:2932-2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lortholary, O., and B. Dupont. 1997. Antifungal prophylaxis during neutropenia and immunodeficiency. Clin. Microbiol. Rev. 10:477-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lupetti, A., A. Paulusma-Annema, M. M. Welling, S. Senesi, J. T. van Dissel, and P. H. Nibbering. 2000. Candidacidal activities of human lactoferrin peptides derived from the N terminus. Antimicrob. Agents Chemother. 44:3257-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lupetti, A., A. Paulusma-Annema, S. Senesi, M. Campa, J. T. van Dissel, and P. H. Nibbering. 2002. Internal thiols and reactive oxygen species in candidacidal activity exerted by an N-terminal peptide of human lactoferrin. Antimicrob. Agents Chemother. 46:1634-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lyons, C. N., and T. C. White. 2000. Transcriptional analyses of antifungal drug resistance in Candida albicans. Antimicrob. Agents Chemother. 44:2296-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marr, K. A., T. C. White, J. A. H. van Burik, and R. A. Bowden. 1997. Development of fluconazole resistance in Candida albicans causing disseminated infection in a patient undergoing marrow transplantation. Clin. Infect. Dis. 25:908-910. [DOI] [PubMed] [Google Scholar]
- 20.Marr, K. A., C. N. Lyons, T. Rustad, R. A. Bowden, and T. C. White. 1998. Rapid, transient fluconazole resistance in Candida albicans is associated with increased mRNA levels of CDR. Antimicrob. Agents Chemother. 42:2584-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nibbering, P. H., E. Ravensbergen, M. M. Welling, L. A. van Berkel, P. H. van Berkel, E. K. J. Pauwels, and J. H. Nuijens. 2001. Human lactoferrin and peptides derived from its N terminus are highly effective against infections with antibiotic-resistant bacteria. Infect. Immun. 69:1469-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nolte, F. S., T. Parkinson, D. J. Falconer, S. Dix, J. Williams, C. Gilmore, R. Geller, and J. R. Wingard. 1997. Isolation and characterization of fluconazole- and amphotericin B-resistant Candida albicans from blood of two patients with leukemia. Antimicrob. Agents Chemother. 41:196-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Revankar, S. G., W. R. Kirkpatrick, R. K. McAtee, O. P. Dib, A. W. Fothergill, S. W. Redding, M. G. Rinaldi, and T. F. Patterson. 1996. Detection and significance of fluconazole resistance in oropharyngeal candidiasis in human immunodeficiency virus-infected patients. J. Infect. Dis. 174:821-827. [DOI] [PubMed] [Google Scholar]
- 24.Revankar, S. G., W. R. Kirkpatrick, R. K. McAtee, O. P. Dib, A. W. Fothergill, S. W. Redding, M. G. Rinaldi, S. G. Hilsenbeck, and T. F. Patterson. 1998. A randomized trial of continuous or intermittent therapy with fluconazole for oropharyngeal candidiasis in HIV-infected patients: clinical outcomes and development of fluconazole resistance. Am. J. Med. 105:7-11. [DOI] [PubMed] [Google Scholar]
- 25.Rex, J. H., M. G. Rinaldi, and M. A. Pfaller. 1995. Resistance of Candida species to fluconazole. Antimicrob. Agents Chemother. 39:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanglard, D., F. Ischer, L. Koymans, and J. Bille. 1998. Amino acid substitutions in the cytochrome P-450 lanosterol 14α-demethylase (CYP51A1) from azole-resistant Candida albicans clinical isolates contribute to resistance to azole antifungal agents. Antimicrob. Agents Chemother. 42:241-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wakabayashi, H., S. Abe, T. Okutomi, S. Tansho, K. Kawase, and H. Yamaguchi. 1996. Cooperative anti-Candida effects of lactoferrin or its peptides in combination with azole antifungal agents. Microbiol. Immunol. 40:821-825. [DOI] [PubMed] [Google Scholar]
- 28.Wakabayashi, H., S. Abe, S. Teraguchi, H. Hayasawa, and H. Yamaguchi. 1998. Inhibition of hyphal growth of azole-resistant strains of Candida albicans by triazole antifungal agents in the presence of lactoferrin-related compounds. Antimicrob. Agents Chemother. 42:1587-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White, T. C. 1997. Increased mRNA levels of ERG16, CDR, and MDR1 correlate with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob. Agents Chemother. 41:1482-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson, D. F., and B. Chance. 1967. Azide inhibition of mitochondrial electron transport. I. The aerobic steady state of succinate oxidation. Biochim. Biophys. Acta 131:421-430. [DOI] [PubMed] [Google Scholar]