Abstract
The benznidazole (BZ) and itraconazole (ITC) susceptibilities of a standard set of Trypanosoma cruzi natural stocks were evaluated during the acute phase and the chronic phase of experimental chagasic infection in BALB/c mice. Twenty laboratory-cloned stocks representative of the total phylogenetic diversity of T. cruzi, including genotypes 20 and 19 (T. cruzi I) and genotypes 39 and 32 (T. cruzi II), were analyzed. Our results demonstrate important differences among stocks that could be pointed out as markers of biological behavior. Members of the T. cruzi I group were highly resistant to both BZ and ITC, whereas members of the T. cruzi II group were partially resistant to both drugs, despite their susceptibilities to ITC during the chronic phase of infection. The resistance to BZ observed for T. cruzi I was mainly triggered by genotype 20 isolates, whereas resistance to ITC was due to both genotype 20 and 19 isolates. Two polar patterns of response to BZ observed for genotype 39 isolates had a major impact on the partial resistance pattern observed for members of the T. cruzi II group. Genotype 32 isolates showed a typical profile of susceptibility. The correlation between the response to treatment and phylogenetic classification of T. cruzi stocks was clearer for ITC than for BZ. In conclusion, the data presented show a correlation between phylogenetic divergence among T. cruzi stocks and their susceptibilities to chemotherapeutic agents in vivo. Our results warn of the necessity to take into account the lesser genetic subdivisions of T. cruzi stocks since the upper subdivisions (T. cruzi I and II) show a great deal of heterogeneity for in vivo drug susceptibility.
American trypanosomiasis, caused by the protozoan parasite Trypanosoma cruzi, is widespread in Latin America, where 16 million to 18 million people are estimated to be infected and more than 50 million people are at risk of infection (32). Although specific treatment for Chagas' disease is available, the use of benznidazole (BZ; Roche) and nifurtimox (NFX; Bayer) shows controversial results during the acute phase (AP) and the chronic phase (CP) of infection (9, 12). Moreover, notorious differences in the efficacies of chemotherapy are observed, especially when therapeutic screenings are performed in distinct geographic areas (3). During the last 20 years, the susceptibilities to BZ and NFX of many T. cruzi strains isolated from different hosts and geographic areas have been determined (1, 3, 15, 31, 39). In this context, 56.0% of T. cruzi strains have been considered susceptible to BZ, 16.82% have been considered partially susceptible, and 27.1% have been considered resistant. One factor that may contribute to these differences during treatment for Chagas' disease could be the type of strain predominant in each geographic area. A well-known feature involved in resistance to chemotherapy is the high degree of biological and genetic diversity of T. cruzi strains (1, 2, 4, 31, 33). The clonal evolution model postulated for T. cruzi (36) predicts a correlation between the phylogenetic divergence of T. cruzi clonal genotypes and their biological properties including their drug sensitivities. This has been verified for several experimental parameters, including in vitro drug sensitivity (33). The goal of the present study was to test this working hypothesis for in vivo drug sensitivity, which is more relevant than in vitro susceptibility from a medical point of view. Two drugs, BZ and itraconazole (ITC), were tested, as the latter has recently shown promise as treatment for human Chagas' disease (6).
MATERIALS AND METHODS
Parasites.
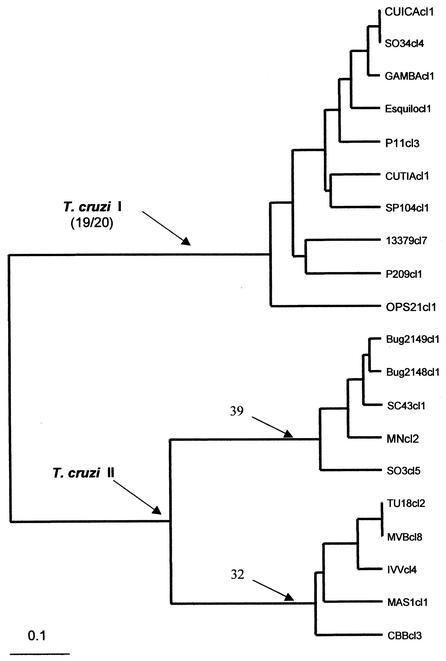
A standardized sample of 20 T. cruzi stocks were selected so that they were representative of the whole phylogenetic diversity of the parasite (36). They all corresponded to the major “clonets” (widespread clonal genotypes) 19, 20, 39, and 32 (36). The stocks were cloned by micromanipulation, with visual verification under a microscope. They were fully characterized by multilocus enzyme electrophoresis (MLEE) at 15 loci (36) and then by both MLEE at 22 different loci (7) and randomly amplified polymorphic DNA analysis with 10 primers (37). According to the recently proposed nomenclature (30), major clonets 20 and 19 are included within the T. cruzi I group (zymodeme 1 of Miles et al. [25], major lineage 1 of Tibayrenc [38], lineage 2 of Souto et al. [35], biodema III of Andrade and Magalhães [4]), whereas both clonets 39 and 32 are included in the T. cruzi II group (zymodeme 2 of Miles et al. [25], major lineage 2 of Tibayrenc[38], lineage 1 of Souto et al. [35], biodema II of Andrade and Magalhães [4]). Clonet 39 is equivalent to lineages 1 and 2 of Souto et al. (35) and is considered a hybrid clonal genotype (21). Information on the laboratory codes, hosts, and geographic origins for these stocks is given in Table 1. Clonal genotypes 20, 19, 39, and 32 illustrate different phylogenetic relationships. Genotypes 20 and 19 are more closely related to each other, whereas genotypes 39 and 32 are more distantly related to both genotypes 20 and 19 but are closely related to each other (Fig. 1). Genotypes 20 and 19 differ by very few characters, in particular for the 6PGDH isoenzyme locus, which is heterozygous in genotype 20 and homozygous in genotype 19. Handling of live T. cruzi parasites was performed according to established guidelines (16).
TABLE 1.
Laboratory codes, hosts, and geographic origins of the 20 T. cruzi stocks used in the present study
| T. cruzi group | Clonal genotype | Stock | Host | Country | Region |
|---|---|---|---|---|---|
| I | 19 | SP104 cl1 | Triatoma spinolai | Chile | Choquimbo |
| I | Cutia cl1 | Dasyprocta agudi | Brazil | Espirito Santo | |
| I | Gambá cl1 | Didelphis azarae | Brazil | São Paulo | |
| I | 13379 cl7 | Human, AP | Bolívia | Santa Cruz | |
| I | OPS21 cl11 | Human, AP | Venezuela | Cojedes | |
| I | 20 | SO34 cl4 | Triatoma infestans | Bolívia | Potosi |
| I | Cuica cl1 | Opossum cuica philander | Brazil | São Paulo | |
| I | P209 cl1 | Human, CP | Bolívia | Sucre | |
| I | Esquilo cl1 | Sciurus aestuans ingramini | Brazil | São Paulo | |
| I | P11 cl3 | Human, CP | Bolívia | Cochabamba | |
| II | 39 | SC43 cl1 | Triatoma infestans | Bolívia | Santa Cruz |
| II | Bug2148 cl1 | Triatoma infestans | Brazil | Rio Grande do Sul | |
| II | Bug2149 cl10 | Triatoma infestans | Brazil | Rio Grande do Sul | |
| II | SO3 cl5 | Triatoma infestans | Bolívia | Potosi | |
| II | MN cl2 | Human, CP | Chile | Santiago | |
| II | 32 | MAS cl1 | Human, CP | Brazil | Brasilia |
| II | CBB cl3 | Human, CP | Chile | Tulahuen | |
| II | TU 18 cl2 | Triatoma infestans | Bolívia | Tupiza | |
| II | IVV cl4 | Human, CP | Chile | Santiago | |
| II | MVB cl8 | Human, CP | Chile | Santiago |
FIG. 1.
Dendrogram depicting the phylogenetic relationships among the 20 T. cruzi stocks studied by assay of 22 isoenzyme loci. The dendrogram was obtained by the unweighted pair group method with arithmetic averages (37). The cluster at the top corresponds to clonal genotypes 19 and 20 (T. cruzi I), the cluster in the middle corresponds to clonal genotype 39 (T. cruzi II), and the cluster at the bottom corresponds to clonal genotype 32 (T. cruzi II). The scale indicates genetic distances estimated by use of the Jaccard index (17).
Infection of mice.
Groups of 40 female isogenic BALB/c mice (age, 28 to 30 days; Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais) were inoculated intraperitoneally with 10,000 blood-form trypomastigotes (per animal) of each of the 20 stocks studied. The number of parasites was determined as described by Brener (8). Inocula were obtained from breast-fed Swiss mice that had previously been inoculated with a large number of metacyclic trypomastigotes from late-stationary-phase culture in liver-infusion tryptose (LIT) medium, purified as described by Deane et al. (13).
Treatment scheme.
After detection of the infection, the mice were divided into two groups: 20 treated mice and 20 mice used as untreated controls. Ten mice from the first group were treated by the oral route with BZ (Roche): 5 mice during AP starting 10 days after inoculation and 5 mice during CP starting 90 days after inoculation. Both groups were treated for 20 consecutive daily doses (15). Likewise, 10 mice were treated with ITC (Biolab), but for 60 consecutive daily doses (24). Both compounds were resuspended in water and were administered by gavage at 100 mg/kg of body weight.
Parasitological tests. (i) Fresh blood examination.
Blood collected from the tails of the mice was examined microscopically for living trypomastigotes. The level of parasitemia was counted daily from the 5th day after inoculation as described by Brener (8). This examination was performed to confirm the presence of infection before the start of treatment and, subsequently, to access cure during treatment during AP.
(ii) Hemoculture.
Thirty days after the end of treatment, during either AP or CP, an orbital sinus blood sample was inoculated into 5 ml of LIT medium, in duplicate, as described by Filardi and Brener (15). The hemocultures were maintained at 28°C. Thirty, 60, 90, and 120 days later, each tube was examined for the detection of parasites.
Serological tests. (i) Conventional serology (enzyme-linked immunosorbent assay).
Samples of serum were collected 3 and 6 months after the end of AP treatment and 6 and 9 months after CP treatment. Sera were tested at a dilution of 1:40 in phosphate-buffered saline. T. cruzi-specific antibodies were detected by the technique described by Voller et al. (42). Enzyme-linked immunosorbent assay plates were sensitized with T. cruzi antigen prepared by alkaline extraction of the Y strain obtained at exponential growth in LIT medium. Antibody binding was detected by using peroxidase-labeled anti-mouse immunoglobulin G after the absorbance was read in a spectrophotometer with a 490-nm filter (model 3550; Bio-Rad). The mean absorbance for 10 negative control serum samples plus 2 standard deviations was used as the cutoff to discriminate positive and negative results. Positive and negative controls were processed in parallel for each assay.
(ii) Anti-live trypomastigote antibody.
Similar to conventional serology, serum samples were collected 3 and 6 months after the end of AP treatment and 6 and 9 months after CP treatment. Immunofluorescence staining for detection of anti-live trypomastigote antibodies was carried out as described by Martins-Filho et al. (23), but modified for 96-well U-bottom plates, as introduced by Cordeiro et al. (11). Briefly, after incubation with mouse serum diluted 1:1,500 and 1:3,000 in phosphate-buffered saline-fetal bovine serum, the binding of antibodies to trypomastigotes was detected with phycoerythrin (PE)-conjugated anti-mouse immunoglobulin G diluted 1:400. The PE-labeled parasites were fixed before they were run in a flow cytometer. Flow cytometric measurements were performed on a FACScalibur flow cytometer (Becton Dickinson, San Jose, Calif.). The Cell-Quest software package was used for both data storage and analysis. Trypomastigotes were first identified on the basis of their specific forward (size) and side (granularity) light-scattering properties. The relative PE fluorescence intensity for each parasite preparation after incubation with an individual serum sample was analyzed by using a single histogram. A marker was set up on the internal control for unspecific binding and was used to determine the percentage of parasites positive for fluorescence (PPFP). The samples were considered negative when PPFP was ≤20% and positive when PPFP was >20%. Positive and negative controls were run routinely.
Cure assessment.
Cure criteria were based on both parasitological and serological methods. Animals presenting negative results by all parasitological and serological tests used were considered cured.
Drug resistance and susceptibility criterion.
The cure rates were calculated by determination of the ratio (number of mice cured/total number of mice infected and treated during AP or CP) × 100. The stocks were classified as resistant (cure rates, between 0 and 33%), partially resistant (cure rates, between 34 and 67%), and susceptible (cure rates, greater than 67%).
Triplicate experiments were carried out to assess the reproducibilities of the experiments by using 2 of the 20 stocks, including SO34 cl4 of genotype 20 and Cutia cl1 of genotype 19. Compatible results regarding drug resistance or susceptibility were obtained in all three experiments. Animals infected with the SO34 cl4 stock were always 100% resistant to BZ and ITC. Likewise, mice infected with the Cutia cl1 stock were always partially resistant to BZ (40% [two of five] of the infected mice were cured) and resistant to ITC. Considering the high degree of reproducibility of the triplicate experiments, we continued the experiments using five animals for each experimental group.
Statistical analysis.
The cure rates were compared by the chi-square test. Comparative analyses were performed between T. cruzi groups I and II and between each clonal genotype (19, 20, 32, 39). Moreover, the comparison also accounted for AP and CP and for each chemotherapeutic agent (BZ and ITC) used.
We also performed a global analysis of the data by the nonparametric Mantel test (22), which makes it possible to evaluate a putative correlation between genetic distances measured by either MLEE or randomly amplified polymorphic DNA analysis on the one hand and the susceptibility of the stocks to chemotherapy on the other. Contrary to the classical correlation test, this randomization procedure does not need any assumptions about the number of degrees of freedom for each parameter studied: susceptibility to BZ during AP, susceptibility to BZ during CP, susceptibility to ITC during AP, and susceptibility to ITC during CP. This procedure gives equal weights to each biological parameter in the overall biological distance.
RESULTS
The in vivo susceptibilities of T. cruzi to BZ and ITC during AP and CP are shown by considering three levels of comparison: (i) between each major phylogenetic subdivision (T. cruzi I and II), (ii) between each clonal genotype, and (iii) between each stock studied.
Treatment with BZ. (i) Comparison between T. cruzi I and II isolates.
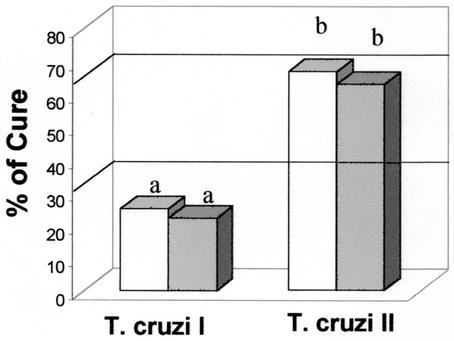
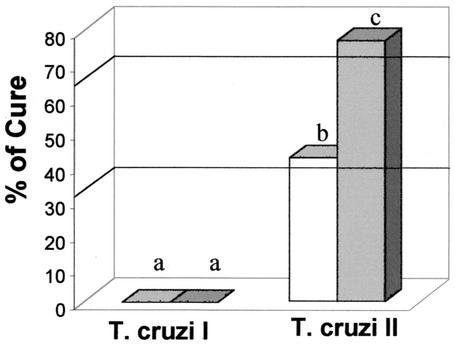
After treatment with BZ, the cure rates for animals infected with T. cruzi I isolates were 25 and 22% during AP and CP, respectively. Thus, T. cruzi I isolates were considered resistant to BZ (Fig. 2). On the other hand, the cure rates for mice infected with T. cruzi II isolates were 66.67 and 62.75% when they were treated during AP and CP, respectively. Thus, T. cruzi II isolates were considered partially resistant to BZ (Fig. 2). T. cruzi I isolates were significantly more resistant to BZ than T. cruzi II isolates (P < 10−3) when they were treated during both AP and CP.
FIG. 2.
Percent cure for BALB/c mice infected with stocks of T. cruzi I (n = 119) or T. cruzi II (n = 102) after treatment with BZ (100 mg/kg/day for 20 days) during AP (open bars) and CP (shaded bars). Different letters (a and b) represent statistically significant differences (P < 0.0001).
(ii) Comparison between clonal genotypes.
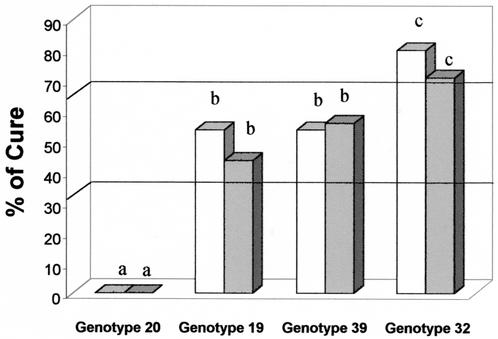
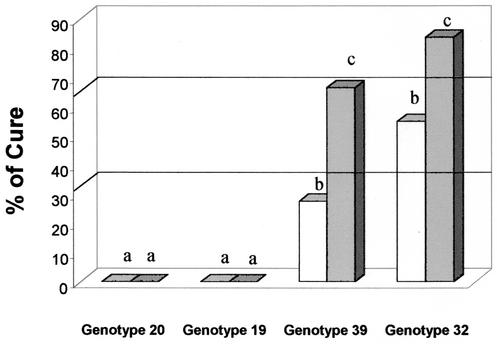
The cure rates were always 0% for animals infected with genotype 20, and therefore, genotype 20 isolates were considered resistant to BZ (Fig. 3). The cure rates were significantly lower (P < 10−3) for mice infected with genotype 20 than for mice infected with any of the other genotypes.
FIG. 3.
Percent cure for BALB/c mice infected with T. cruzi isolates of genotype 20 (n = 61), 19 (n = 58), 39 (n = 51), or 32 (n = 51) after treatment with BZ (100 mg/kg/day for 20 days) during AP (open bars) and CP (shaded bars). Different letters (a, b, and c) represent statistically significant differences (P < 0.05) except for those for isolates of genotypes 39 and 32 during CP.
The cure rates for mice infected with genotype 19 were 53.83% (AP) and 43.75% (CP), and therefore, genotype 19 isolates were considered partially resistant to BZ (Fig. 3). The cure rates for mice infected with genotype 19 were higher than those for mice infected with genotype 20 during both phases of infection (P < 10−3) and lower than those for mice infected with genotype 32 (P < 0.05 for AP and P = 0.05 for CP).
The cure rates for mice infected with genotype 39 were 53.85% (AP) and 56% (CP), and therefore, genotype 39 isolates were considered partially resistant to BZ (Fig. 3). The cure rates were significantly higher for mice infected with genotype 39 than for mice infected with genotype 20 (P < 10−3) and did not differ significantly from those for mice infected with genotype 19 during both phases of the infection. Comparative analysis of the results for mice infected with genotype 39 and those infected with genotype 32 showed a marginal difference (P = 0.048) during AP and no significant difference during CP.
The cure rates for mice infected with genotype 32 were 80% (AP) and 69.23% (CP), and therefore, genotype 32 isolates were considered susceptible to BZ (Fig. 3). Isolates of this genotype were considered more susceptible to BZ during AP than isolates of the other three genotypes. The susceptibilities of isolates of this genotype to BZ during CP were significantly higher than those of isolates of genotype 20 (P < 10−3), marginally significantly higher than those of isolates of genotype 19 (P = 0.052), and not significantly higher than those of isolates of genotype 39.
Comparative analyses of the cure rates during AP and CP did not show significant differences within each clonal genotype.
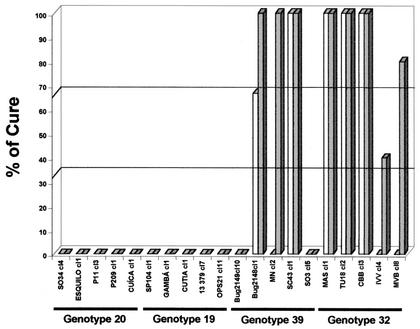
(iii) Analysis between stocks of a given clonal genotype.
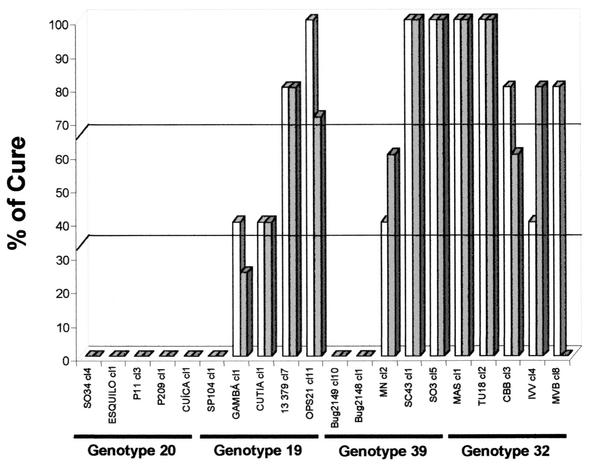
Analysis of stocks of genotype 20 demonstrated homogeneous results, with all stocks being 100% resistant to BZ during both AP and CP (Fig. 4).
FIG. 4.
Percent cure for BALB/c mice infected with 20 T. cruzi stocks of genotype 20, 19, 39, or 32 after treatment with BZ (100 mg/kg/day for 20 days) during AP (open bars) and CP (shaded bars).
During AP, we identified one stock of genotype 19 (SP104 cl1) resistant to BZ, two stocks of genotype 19 (Cutia cl1 and Gamba cl1) partially resistant to BZ, and two stocks of genotype 19 (OPS21 cl11 and 13379 cl7) susceptible to BZ. During CP two stocks of genotype 19 (Gamba cl1 and SP104 cl1) were considered resistant to BZ, one stock (Cutia cl1) was considered partially resistant to BZ, and two stocks (OPS21 cl11 and 13379 cl7) were considered susceptible to BZ (Fig. 4).
Analysis of genotype 39 isolates with BZ showed two resistant stocks (Bug2149 cl10 and Bug2148 cl1), one partially resistant stock (MN cl2), and two susceptible stocks (SC43 cl1 and SO3 cl5) during both phases of infection (Fig. 4).
During AP, one stock of genotype 32 (IVV cl4) was considered partially resistant BZ and four stocks (MAS cl1, TU18 cl2, CBB cl3, and MVB cl8) were considered susceptible. During CP, one stock of genotype 32 (MVB cl8) was resistant to BZ, one stock (CBB cl3) was partially resistant to BZ, and three stocks (MAS cl1, TU18 cl2, and IVV cl4) were susceptible to BZ (Fig. 4).
In general, there were no significant differences between the resistance and susceptibilities of isolates of each stock during AP and CP. Only one exception was observed with stock MVB cl8 of genotype 32, classified as susceptible (80%) during AP and resistant (0%) during CP (Fig. 4).
Treatment with itraconazole. (i) Comparison between T. cruzi I and II isolates.
After treatment with ITC, animals infected with T. cruzi I isolates showed cure rates of 0% during both phases of the infection. Thus, T. cruzi I isolates were considered resistant to ITC (Fig. 5). The cure rates were 42.1 and 76.74% for mice infected with T. cruzi II isolates during AP and CP, respectively. Thus, T. cruzi II isolates were considered partially resistant during AP and susceptible during CP (Fig. 5). Animals infected with T. cruzi I isolates showed significantly lower (P < 10−4) levels of susceptibility to ITC than mice infected with T. cruzi II isolates during both phases of infection.
FIG. 5.
Percent cure for BALB/c mice infected with stocks of T. cruzi I (n = 94) and T. cruzi II (n = 81) after treatment with ITC (100 mg/kg/day for 60 days) during AP (open bars) and CP (shaded bars). Different letters (a, b, and c) represent statistically significant differences (P < 0.005).
(ii) Comparison between clonal genotypes.
The cure rates were 0% for animals infected with genotypes 20 and 19 when the mice were treated during both AP and CP. Thus, isolates of these genotypes were considered resistant to ITC (Fig. 6). The cure rates were lower for genotype 20 and 19 isolates than for genotype 39 and 32 isolates during both phases of infection (P < 10−4)
FIG. 6.
Percent cure for BALB/c mice infected with T. cruzi isolates of genotype 20 (n = 49), 19 (n = 45), 39 (n = 36), or 32 (n = 45) after treatment with ITC (100 mg/kg/day for 60 days) during AP (open bars) and CP (shaded bars). Different letters (a, b, and c) represent statistically significant differences (P < 0.05).
The cure rates for mice infected with genotype 39 were 27.8 and 66.7% during AP and CP, respectively. Thus, isolates of this genotype were considered resistant during AP and partially resistant during CP (Fig. 6). The cure rates for mice infected with genotype 39 isolates were higher than those for mice infected with genotype 20 and 19 isolates during both phases of infection (P < 0.05).
The cure rates were 55 and 84% for animals infected with genotype 32 isolates when they were treated during AP and CP, respectively. Thus, isolates of this genotype were considered partially resistant during AP and susceptible during CP (Fig. 6). The cure rates were higher than those for mice infected with isolates of genotypes 20 and 19 during both phases of infection (P < 10−3).
Comparative analysis within each clonal genotype showed that the cure rates were significantly higher (P < 0.05) during CP in animals infected with genotypes 39 and 32 (Fig. 6).
(iii) Analysis between stocks of a given clonal genotype.
Analysis of genotype 20 and 19 isolates showed homogeneous results, with all stocks being resistant to ITC during both phases of infection (Fig. 7).
FIG. 7.
Percent cure for BALB/c mice infected with 20 T. cruzi stocks of genotype 20, 19, 39, or 32 after treatment with ITC (100 mg/kg/day for 60 days) during AP (open bars) and CP (shaded bars).
During AP, three stocks (Bug2149 cl10, SO3 cl5, and MN cl2) of genotype 39 were resistant to ITC, one stock (Bug2148 cl1) was partially resistant to ITC, and one stock (SC43 cl1) was susceptible to ITC. During CP, two stocks (Bug2149 cl10 and SO3 cl5) were resistant to ITC and three stocks (MN cl2, Bug2148 cl1 and SC43 cl1) were susceptible to ITC (Fig. 7).
Genotype 32 stock MVB cl8 was resistant to ITC during AP and susceptible during CP. Three of five stocks (MAS cl1, CBB cl3, and TU18 cl2) were considered susceptible to ITC during both phases of infection. Isolates of stock IVV cl4 were resistant during AP and partially resistant during CP (Fig. 7).
In general, there were no significant differences between the resistance and susceptibilities of isolates of each stock during AP and CP. Four exceptions were observed, and these pertained exclusively to stocks of T. cruzi II isolates: genotype 39 stock MN cl2, classified as resistant (0%) during AP and susceptible (100%) during CP; stock Bug2148 cl1, also of genotype 39, classified as partially resistant (66%) during AP and susceptible (100%) during CP; genotype 32 stock MVB cl8, classified as resistant (0%) during AP and susceptible (80%) during CP; and genotype 32 stock IVVcl4, classified as resistant (0%) during AP and partially resistant (40%) during CP (Fig. 7).
Mantel test.
Global analysis of all data by the Mantel test showed a coefficient of correlation of 0.4717 between the matrices of the four parameters considered (susceptibility to BZ during AP, susceptibility to BZ during CP, susceptibility to ITC during AP, susceptibility to ITC during CP) on the one hand and the genetic distances (Jaccard index) on the other for all pairs of stocks (P < 10−4).
DISCUSSION
Several studies have paid attention to the hypothesis of a linkage between the phylogenetic diversity of T. cruzi natural clones and the relevant biological properties of this parasite, such as its behavior in vitro and in vivo, including its vectors and murine hosts (10, 14, 18, 19, 26) and the clinical manifestations of the disease (20, 26).
Andrade and colleagues (1, 2, 4) have also investigated the association between phylogenetic diversity and the response to chemotherapy. In those studies, T. cruzi strains from different hosts and geographic areas of Latin America, corresponding to T. cruzi I (equivalent to zymodeme 1 and biodema III), T. cruzi II (equivalent to zymodeme 2 and biodema II), and T. cruzi (equivalent to zymodeme 2b and biodema I) (30) were evaluated. It was observed that during AP T. cruzi (zymodeme 2b) strains were highly susceptible and T. cruzi II strains were partially to highly susceptible to BZ and NFX, whereas T. cruzi I strains were highly resistant to BZ and NFX (1). Interestingly, during CP a different association between the zymodeme and the drug resistance pattern was found. Even strains highly resistant to BZ during AP, like T. cruzi I strains, were shown to be partially susceptible during CP, while no such differences were observed for T. cruzi II strains (2). Although the studies cited above have made significant contributions, they did not rely on a rigorous population genetics framework. Differently, our study was based on the clonal evolution model of T. cruzi and on the working hypothesis that biological differences are proportional to the degree of evolutionary divergence among the clonal genotypes.
Our results confirm the previous hypothesis that there is an association between phylogenetic diversity and the chemotherapeutic response for the two major groups of T. cruzi. These differences can be considered important markers of the biological behavior of this parasite: T. cruzi I isolates are resistant to BZ and ITC during both AP and CP, whereas T. cruzi II isolates are partially resistant to both drugs, despite their susceptibilities to ITC during CP.
The resistance to BZ observed for T. cruzi I was predominantly due to genotype 20, since genotype 19 isolates were partially resistant. It is interesting that the apparent partial resistance of genotype 19 isolates was actually a consequence of variable responses to BZ. On the other hand, isolates of both genotypes 20 and 19 were resistant to ITC, with homogeneous results for all stocks tested. Our data suggest that stocks of genotype 20 might present multiple-drug-resistance-conferring genes that could explain their resistance to both drugs used in the present study. This possible association increases the interest in studies of T. cruzi stocks of this genotype to test new chemotherapeutic agents potentially active against T. cruzi, such as the new triazole derivatives (inhibitors of the parasite's sterol C14-alpha-demethylase) (41). Detailed studies on the capacities of triazole derivatives to overcome the natural resistance to nitroimidazoles and nitrofurans of T. cruzi I strains have recently been published (28, 29).
Data for T. cruzi II isolates demonstrated that genotype 39 isolates had a major impact on the pattern of partial resistance to BZ observed. Interestingly, two polar patterns of response to BZ led to this result, with BUG2149 cl10 and BUG2148 cl1 strains being resistant and SC43 cl1 and SO3 cl5 isolates being susceptible. This dual behavior was also observed among other biological properties of stocks of genotype 39, including the level of parasitemia, the percentage of positive hemoculture, and histopathology during AP and CP (40). Perhaps the hybrid nature of this genetic group, which harbors a combination of genes of two putative parental genotypes (21), could explain these results. Isolates of genotype 32 showed a typical profile of susceptibility, with a few exceptions, including IVV cl4 during AP and MVB cl8 and CBB cl3 during CP. There was a clear indication that genotype 32 isolates had a major influence on the mixed pattern of partial resistance to ITC during AP and susceptibility to ITC during CP. However, analysis of the data for individual stocks demonstrated a clear tendency for the three stocks (MAS cl1, CBB cl3, and TU18 cl2) to be susceptible to ITC during AP and CP and, additionally, for MVB cl8 to be susceptible to ITC during CP. A predominance of resistance was observed for genotype 39 isolates (BUG2149 cl10, SO3 cl5, and MN cl2) during AP. Interestingly, BUG2148 cl1, MN cl2, and SC43 cl1 isolates of genotype 39 pushed the therapeutic results toward susceptibility during CP. In fact, data from the ITC treatments showed a better association with the creation of a phylogenetic hierarchy of the T. cruzi genotypes than BZ treatments did. With ITC, no cure was detected in mice infected with isolates of genotypes 20 and 19 during either phase of infection. This homogeneous result allows consideration of both genotypes as a unique category of isolates. On the other hand, despite the wide range of resistance and susceptibility, stocks of T. cruzi II showed a tendency toward susceptibility, mainly due to genotype 32.
In general, we found similar therapeutic responses between AP and CP. A therapeutic discrepancy was observed for Gamba cl1 of genotype 19, which was partially resistant during AP and which was resistant during CP. Discrepant data were also observed for BUG2148 cl1 and MN cl2 of genotype 39 and MVB cl8 and IVV cl4 of genotype 32. Similar results were previously described by Andrade et al. (2), who demonstrated that T. cruzi I strains that were highly resistant to BZ and NFX during AP were partially susceptible to both drugs during CP.
Stocks of T. cruzi II were more susceptible to treatment than stocks of T. cruzi I, leading to therapeutic responsiveness after treatment with BZ and ITC. This result is in agreement with those of studies conduced by Apt and colleagues (6) in Chile, where over 50% of chronically infected patients were cured after ITC chemotherapy. It was previously described that the majority of T. cruzi stocks isolated from these human patients were classified as T. cruzi II (zymodeme 2 from Miles et al. [27]) (5). Murta et al. (31) also reported that T. cruzi zymodeme B strains (T. cruzi II) were highly susceptible to BZ and NFX.
In conclusion, the data presented here fit the predictions of the clonal evolution model of T. cruzi (36) and demonstrate for the first time a correlation between phylogenetic divergence (Jaccard distance) (17) among T. cruzi clonal genotypes and their in vivo susceptibilities to chemotherapeutic agents. This correlation was highly significant by the Mantel test and was observed during AP and CP for both drugs tested. Moreover, our results corroborate previous observations of the responses to the drugs in vitro in studies with acellular and cellular cultures (33). Those studies detected similar patterns, with genotypes 20 and 19 being more resistant to BZ and NFX and genotypes 39 and 32 being more susceptible to the two drugs. Our results also warn of the necessity to take into account the lesser phylogenetic subdivisions. As a matter of fact, the upper subdivisions, T. cruzi I and II, proved to be heterogeneous for the parameters studied here. A remarkable example of this feature was the case of T. cruzi I genotypes 19 and 20, which are phylogenetically very close but which nevertheless showed drastic differences in their levels of resistance to BZ.
Acknowledgments
This work received financial support from the Fundação de Amparo à Pesquisa do Estado de Minas Gerais and from the Coordenadoria de Aperfeiçoamento de Pessoal de Nível Superior.
REFERENCES
- 1.Andrade, S. G., J. B. Magalhães, and A. L. Pontes. 1985. Evaluation of chemotherapy with benznidazole and nifurtimox in mice infected with Trypanosoma cruzi strains of different types. Bull. W. H. O. 63:721-726. [PMC free article] [PubMed] [Google Scholar]
- 2.Andrade, S. G., J. B. Magalhães, and A. L. Pontes. 1989. Terapêutica da fase crönica da infecção experimental pelo Trypanosoma cruzi com o benzonidazol e o nifurtimox. Rev. Soc. Bras. Med. Trop. 22:113-118. [DOI] [PubMed] [Google Scholar]
- 3.Andrade, S. G., A. Rassi, J. B. Magalhães, F. Ferrioli Filho, and A. O. Luquetti. 1992. Specific chemotherapy of Chagas' disease: a comparison between the response in patients and experimental animals inoculated with the same strains. Trans. R. Soc. Trop. Med. Hyg. 86:624-626. [DOI] [PubMed] [Google Scholar]
- 4.Andrade, S. G., and J. B. Magalhães. 1997. Biodemes and zymodemes of Trypanosoma cruzi strains: correlations with clinical data and experimental pathology. Rev. Soc. Bras. Med. Trop. 30:27-35. [DOI] [PubMed] [Google Scholar]
- 5.Apt, B. W., X. Aguilera, A. Arribada, L. Gomez, M. A. Miles, and G. Widmer. 1987. Epidemiology of Chagas' disease in northern Chile: isoenzyme profiles of Trypanosoma cruzi from domestic and sylvatic transmission cycles and their association with cardiopathy. Am. J. Trop. Med. Hyg. 37:302-307. [DOI] [PubMed] [Google Scholar]
- 6.Apt, B. W., X. Aguilera, A. Arribada, C. Pérez, C. Miranda, G. Sanchez, I. Zulantay, P. Cortés, J. Rodriguez, and D. Juri. 1998. Treatment of chronic Chagas' disease with itraconazole and allopurinol. Am. J. Trop. Med. Hyg. 59:133-138. [DOI] [PubMed] [Google Scholar]
- 7.Barnabé, C., S. Brisse, and M. Tibayrenc. 2000. Population structure and genetic typing of Trypanosoma cruzi, the agent of Chagas disease: a multilocus enzyme electrophoresis approach. Parasitology 150:513-526. [DOI] [PubMed] [Google Scholar]
- 8.Brener, Z. 1962. Therapeutic activity and criterion of cure on mice experimentally infected with Trypanosoma cruzi. Rev. Inst. Med. Trop. São Paulo 4:389-396. [PubMed] [Google Scholar]
- 9.Cançado, J. R. 2002. Long term evaluation of etiological treatment of Chagas disease with benznidazole. Rev. Inst. Med. Trop. São Paulo 44:29-37. [PubMed] [Google Scholar]
- 10.Carneiro, M., A. J. Romanha, and E. Chiari. 1991. Biological characterization of Trypanosoma cruzi strains from different zymodemes and schizodemes. Mem. Inst. Oswaldo Cruz 86:387-393. [DOI] [PubMed] [Google Scholar]
- 11.Cordeiro, F. D., O. A. Martins-Filho, M. O. da Costa Rocha, S. J. Adad, R. Correia-Oliveira, and A. J. Romanha. 2001. Anti-Trypanosoma cruzi immunoglobulin G1 can be a useful tool for diagnosis and prognosis of human Chagas' disease. Clin. Diagn. Lab. Immunol. 8:112-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coura, J. R., and S. L. Castro. 2002. A critical review on Chagas disease chemotherapy. Mem. Inst. Oswaldo Cruz 97:3-24. [DOI] [PubMed] [Google Scholar]
- 13.Deane, M. P., H. L. Lenzi, and A. Jansen. 1984. Trypanosoma cruzi: vertebrate and invertebrate cycles in the same mammal host, the opossum Didelphis marsupialis. Mem. Inst. Oswaldo Cruz 79:513-515. [DOI] [PubMed] [Google Scholar]
- 14.Dvorak, J. A., D. L. Hartman, and M. A. Miles. 1980. Trypanosoma cruzi: correlation of growth kinetics to zymodeme type in clones derived from various sources. J. Protozool. 27:472-474. [Google Scholar]
- 15.Filardi, L. S., and Z. Brener. 1987. Susceptibility and natural resistance of Trypanosoma cruzi strains to drugs used clinically in Chagas disease. Trans. R. Soc. Trop. Med. Hyg. 81:755-759. [DOI] [PubMed] [Google Scholar]
- 16.Hudson, L., F. Grover, W. E. Gutteridge, R. A. Klein, W. Peters, R. A. Neal, M. A. Miles, M. T. Scott, R. Nourish, and B. P. Ager. 1983. Suggested guidelines for work with live Trypanosoma cruzi. Trans. R. Soc. Trop. Med. Hyg. 77:416-419. [DOI] [PubMed] [Google Scholar]
- 17.Jaccard, P. 1908. Nouvelles recherches sur la distribution florale. Bull. Soc. Vaudoise Sci. Naturelles 44:223-270. [Google Scholar]
- 18.Lana, M., A. S. Pinto, C. Barnabé, V. Quesney, S. Noël, and M. Tibayrenc. 1998. Trypanosoma cruzi: compared vectorial transmissibility of three major clonal genotypes by Triatoma infestans. Exp. Parasitol. 89:1-5. [DOI] [PubMed] [Google Scholar]
- 19.Laurent, J. P., C. Barnabé, V. Quesney, S. Noël, and M. Tibayrenc. 1997. Impact of clonal evolution on the biological diversity of Trypanosoma cruzi. Parasitology 114:213-218. [DOI] [PubMed] [Google Scholar]
- 20.Lauria-Pires, L., A. R. Bogliolo, and A. R. L. Teixeira. 1996. Diversity of Trypanosoma cruzi stocks and clones derived from Chagas disease patients II. Isozyme and RFLP characterizations. Exp. Parasitol. 82:182-190. [DOI] [PubMed] [Google Scholar]
- 21.Machado, C. A., and F. J. Ayala. 2001. Nucleotide sequences provide evidence of genetic exchange among distantly related lineages of Trypanosoma cruzi. Proc. Natl. Acad. Sci. USA 98:7396-7401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mantel, N. 1967. The detection of disease clustering and a generalized regression approach. Cancer Res. 27:209-220. [PubMed] [Google Scholar]
- 23.Martins-Filho, O. A., M. E. S. Pereira, J. Carvalho, J. R. Cançado, and Z. Brener. 1995. Flow cytometry, a new approach to detect anti-live trypomastigotes antibody and monitor the efficacy of specific treatment in human Chagas' disease. Clin. Diagn. Lab. Immunol. 2:569-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCabe, R., J. Remington, and F. Araújo. 1986. In vitro and in vivo effects of itraconazole against Trypanosoma cruzi. Am. J. Trop. Med. Hyg. 35:280-284. [DOI] [PubMed] [Google Scholar]
- 25.Miles, M. A., M. M. Povoa, A. A. De Souza, R. Lainson, J. J. Shaw, and D. S. Ketteridge. 1981. Chagas' disease in the Amazon Basin. II. The distribution of Trypanosoma cruzi zymodemes 1 and 3 in Para state, north Brazil. Trans. R. Soc. Trop. Med. Hyg. 75:667-674. [DOI] [PubMed] [Google Scholar]
- 26.Miles, M. A., M. Povoa, A. Prata, R. A. Cedillos, A. A. De Souza, and V. Macedo. 1981. Do radically dissimilar Trypanosoma cruzi strains (zymodemes) cause Venezuelan and Brazilian forms of Chagas' disease? Lancet 8234:1336-1340. [DOI] [PubMed] [Google Scholar]
- 27.Miles, M. A., B. W. Apt, G. Widmer, M. M. Povoa, and C. J. Schofield. 1984. Isozyme heterogeneity and numerical taxonomy of Trypanosoma cruzi stocks from Chile. Trans. R. Soc. Trop. Med. Hyg. 78:526-535. [DOI] [PubMed] [Google Scholar]
- 28.Molina, J., O. A. Martins-Filho, Z. Brener, A. J. Romanha, D. Loebenberg, and J. A. Urbina. 2000. Activities of the triazole derivative SCH 56592 (posaconazole) against drug-resistant strain of the protozoan parasite Trypanosoma (Schizotrypanum) cruzi in immunocompetent and immunosuppressed murine hosts. Antimicrob. Agents Chemother. 44:150-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molina, J., Z. Brener, A. J. Romanha, and J. A. Urbina. 2000. In vivo activity of the bis-triazole D0870 against drug-susceptible and drug-resistant strains of the protozoan parasite Trypanosoma cruzi. J. Antimicrob. Chemother. 46:137-140. [DOI] [PubMed] [Google Scholar]
- 30.Momen, H. 1999. Taxonomy of Trypanosoma cruzi: a commentary on characterization and nomenclature. Mem. Inst. Oswaldo Cruz 94(Suppl. 1):181-184. [DOI] [PubMed] [Google Scholar]
- 31.Murta, S. M. F., R. T. Gazzinelli, Z. Brener, and A. J. Romanha. 1998. Molecular characterization of susceptible and naturally resistant strains of Trypanosoma cruzi to benznidazole and nifurtimox. Mol. Biochem. Parasitol. 93:203-214. [DOI] [PubMed] [Google Scholar]
- 32. Organización Panamericana de la Salud. 1994. Las condiciones de salud en las Américas. Publicación Científica (1) 549. Organización Panamericana de la Salud, Washington, D.C.
- 33.Revollo, S., B. Oury, J. P. Laurent, C. Barnabé, V. Quesney, V. Carrière, S. Noël, and M. Tibayrenc. 1998. Trypanosoma cruzi: impact of clonal evolution of the parasite on its biological and medical properties. Exp. Parasitol. 89:30-39. [DOI] [PubMed] [Google Scholar]
- 34.Romanha, A. J., A. A. S. Pereira, E. Chiari, and V. Kilgour. 1979. Isoenzyme patterns of cultured Trypanosoma cruzi: changes after prolonged subculture. Comp. Biochem. Physiol. 62:139-142. [DOI] [PubMed] [Google Scholar]
- 35.Souto, R. P., O. Fernandes, A. M. Macedo, D. A. Campbell, and B. Zingales. 1996. DNA markers define two major phylogenetic lineages of Trypanosoma cruzi. Mol. Biochem. Parasitol. 83:141-152. [DOI] [PubMed] [Google Scholar]
- 36.Tibayrenc, M., and F. J. Ayala. 1988. Isozyme variability in Trypanosoma cruzi, the agent of Chagas' disease: genetical, taxonomical, and epidemiological significance. Evolution 42:277-292. [DOI] [PubMed] [Google Scholar]
- 37.Tibayrenc, M., K. Neubauer, C. Barnabé, F. Guerrini, D. Skarecky, and F. J. Ayala. 1993. Genetic characterization of six parasitic protozoa: parity between random-primer DNA typing and multilocus enzyme electrophoresis. Proc. Natl. Acad. Sci. USA 90:1335-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tibayrenc, M. 1995. Population genetics of parasitic protozoa and other microorganisms, p. 47-115. In J. Baker, R. Muller, and D. E. Rollinson (ed.), Advances in parasitology. Academic Press, London, England. [DOI] [PubMed]
- 39.Toledo, M. J. O., A. L. F. Guilherme, J. C. Silva, M. V. Gasperi, A. P. Mendes, M. L. Gomes, and S. Marques de Araújo. 1997. Trypanosoma cruzi: chemotherapy with benznidazole in mice inoculated with strains from Parana state and from different endemic areas of Brazil. Rev. Inst. Med. Trop. São Paulo 39:283-290. [DOI] [PubMed] [Google Scholar]
- 40.Toledo, M. J. O., M. Lana, C. M. Carneiro, M. T. Bahia, G. L. L. Machado-Coelho, V. M. Veloso, C. Barnabé, M. Tibayrenc, and W. L. Tafuri. 2002. Impact of Trypanosoma cruzi clonal evolution on its biological properties in mice. Exp. Parasitol. 100:161-172. [DOI] [PubMed] [Google Scholar]
- 41.Urbina, J. 2001. Specific treatment of Chagas disease: current status and new developments. Curr. Opin. Infect. Dis. 14:717-718. [DOI] [PubMed] [Google Scholar]
- 42.Voller, A., D. Bidwell, and A. Bartlet. 1980. Enzyme linked immunosorbent assay, p. 359-371. In N. R. Rose and R. Friedman (ed.), Manual of clinical immunology. American Society for Microbiology, Washington, D.C.