Abstract
Pyruvate dehydrogenase (PDH) catalyzes the conversion of pyruvate to acetyl-coenzyme A, which enters into the Krebs cycle, providing adenosine triphosphate (ATP) to the cell. PDH activity is under the control of pyruvate dehydrogenase kinases (PDKs). Under hypoxic conditions, conversion of pyruvate to lactate occurs, a reaction catalyzed by lactate dehydrogenase 5 (LDH5). In cancer cells, however, pyruvate is transformed to lactate occurs, regardless of the presence of oxygen (aerobic glycolysis/Warburg effect). Although hypoxic intratumoral conditions account for HIF1α stabilization and induction of anaerobic metabolism, recent data suggest that high pyruvate concentrations also result in HIF1α stabilization independently of hypoxia. In the present immunohistochemical study, we provide evidence that the PDH/PDK pathway is repressed in 73% of non small cell lung carcinomas, which may be a key reason for HIF1α stabilization and “aerobic glycolysis.” However, about half of PDH-deficient carcinomas are not able to switch on the HIF pathway, and patients harboring these tumors have an excellent postoperative outcome. A small subgroup of clinically aggressive tumors maintains a coherent PDH and HIF/LDH5 expression. In contrast to cancer cells, fibroblasts in the tumor-supporting stroma exhibit an intense PDH but reduced PDK1 expression favoring maximum PDH activity. This means that stroma may use lactic acid produced by tumor cells, preventing the creation of an intolerable intratumoral acidic environment at the same time.
Keywords: PDH, PDH kinase, lung cancer, stroma, HIF
Introduction
Pyruvate is the end product of glycolysis. Under aerobic conditions, pyruvate enters the mitochondria, where it is transformed to acetyl-coenzyme A (acetyl-CoA), producing dihydronicotinamide adenine dinucleotide (NADH) and carbon dioxide. Acetyl-CoA subsequently enters into the Krebs (citric acid) cycle, providing energy (adenosine triphosphate, or ATP) to the cell. This reaction is catalyzed by the enzyme-coenzyme complex pyruvate dehydrogenase (PDH) [1]. This complex is composed of the E2 icosahedral 60-meric core (digydrolipoamide acetyltransferase) bounded to the E1 pyruvate decarboxylase and the E3 dihydrolipoamide dehydrogenase components. E3 is held on the E2 core by monomeric E3-binding proteins (E3BPs) [2–5]. PDH activity is under the control of pyruvate dehydrogenase kinases (PDKs) 1 to 4, which phosphorylate the E1 subunit of PDH and suppress the catalysis of pyruvate to acetyl-CoA [6,7]. The activity of PDK is regulated by the concentration of the metabolic products of pyruvate (NADH and acetyl-CoA).
Under hypoxic conditions or cell poisoning by inhibitors of oxidative phosphorylation (i.e., barbiturates, carbon monoxide, and cyanate), ATP production through glucose conversion to pyruvate is guaranteed by continuous nicotinamide adenine dinucleotide (NAD) production following conversion of pyruvate to lactate, a reaction catalyzed by lactate dehydrogenase 5 (LDH5). This process is called anaerobic glycolysis [8]. In cancer cells, however, pyruvate is abundantly transformed to lactate, regardless of the presence of oxygen. This phenomenon, known historically as the Warburg effect, is called aerobic glycolysis [9]. The biologic basis of this intensified glycolysis and shift of pyruvate transformation to lactate in cancer cells is thought to be related to HIF1α, a key transcription factor regulating the expression of glycolytic enzymes [10] Although hypoxic HIF activation does not explain aerobic glycolysis, oncogenes can activate this pathway. Recently, Lu et al. [11] showed that high pyruvate concentrations result in HIF1α stabilization independently of hypoxia. This could provide feedback to enhance normoxic induction.
In the present immunohistochemical study, we provide evidence that the PDH/PDK pathway is repressed in a large proportion of non small cell lung carcinomas (NSCLCs), which may contribute to HIF1α stabilization, at least in a subset of tumors. In contrast to cancer cells, the expression of PDH/PDHK enzymes was maintained in the fibroblasts of the cancer-supporting stroma. In particular, PDH predominated, suggesting that stroma may have a key role as a sump to help prevent excess acid accumulation in the tumor.
Materials and Methods
Tissue samples from 101 surgically resected NSCLCs were used for investigating the expression of PDH and PDK in cancer cells and stroma. Autopsy samples from 10 apparently normal lungs were also included. Of these 101 cases, 42 had been previously examined for hypoxic molecular parameters in relation to prognosis [12–15]. Specimens had been fixed in formalin and routinely processed to paraffin wax.
PDH/PDK Immunohistochemistry
Staining for PDH and PDK1 was performed on 3-µm paraffin sections mounted on poly-l-lysine-coated slides. The A-213226 and A21325 mouse IgG2a monoclonal antibodies (MoAb) raised against the E2/E3bp and E2 subunits of human mitochondrial PDH (Molecular Probes, Inc., Eugene, OR) were used to detect PDH immunoreactivity. These MoAbs recognize the presence of the PDH core protein E2 and the E3-binding protein essential for the binding of the E3 subunit to E2. This binding seems important for the functionality of PDH [5]. Although assessment of the E1 subunit would also be of interest, it is evident that absence of E2 or E2/E3BP expression directly reflects low PDH activity, which was the end point of the present immunohistochemical study. The concentration used was 1 µg/ml.
The C-20 goat polyclonal IgG antibody (Santa Cruz Biotechnology, Santa Cruz, CA) reactive for human PDK1 was used to stain samples for PDK at a concentration of 1 µg/ml.
A modified streptavidin technique was used for immunohistochemistry. Sections were deparaffinized and peroxidase was quenched with methanol and H2O2 3% for 15 minutes. Microwaving for antigen retrieval was used (3 x 5 minutes). The primary antibodies were applied overnight. Following washing with TBS, sections were incubated with a secondary antibody (Kwik Kit, cat. no. 404050; Thermo Shandon, Pittsburgh, PA) for 15 minutes and washed in TBS. Kwik streptavidin peroxidase reagent was applied for 15 minutes and sections were again washed in TBS. The color was developed by 15-minute incubation with DAB solution and sections were weakly counterstained with hematoxylin. Normal lung tissues were used as positive controls. Normal mouse (for A-213226) or goat (for C-20) immunoglobulin G was substituted for primary antibodies at a concentration where immunostaining of control slides gave a faint cytoplasmic staining.
Other Immunohistochemistry
For forty-two of the tissue samples analyzed for PDH reactivity, immunohistochemical data regarding hypoxia-inducible factors (HIFs) 1α and 2α (MoAbs ESEE 122 and EP190b, respectively; Oxford, UK), carbonic anhydrase-9 (CA9) catalyzing the hydration of carbon dioxide to carbonic acid (MoAb M75; Prof. J Pastorec, Bratislava, Slovak Republic), lactate dehydrogenase-5 (LDH5) (polycloncal Ab 9002; Abcam UK, Cambridge, UK), lactate dehydrogenase-1 (LDH1; polyclonal Ab 9001; Abcam UK), and angiogenesis (assessment of vascular density using the JC70 anti-CD31 MoAb) were available from previous studies [12–16]. Survival data were also available for these cases. PDH reactivity was examined in parallel tissue sections cut from the same tissue blocks used for previous immunohistochemistry studies. Extensive reports on the methods used for staining, assessment, and grouping have been previously published [12–16].
Statistical Analysis
Statistical analyses and graphs were performed using the GraphPad Prism 4 and the Instat 3.0 packages (www.graphpad.com; San Diego, CA). The chi-square t-test, the Fisher's exact t-test, or the unpaired two-tailed t-test was used for testing relationships between categorical tumor variables, as appropriate. Linear regression analysis was used to test the relationship between continuous variables. Survival curves were plotted using the method of Kaplan and Meier, and the log-rank test was used to determine statistical differences between life tables. All P values are two-sided and P < .05 was used for significance.
Results and Discussion
Normal Lung PDH/PDK1 Expression
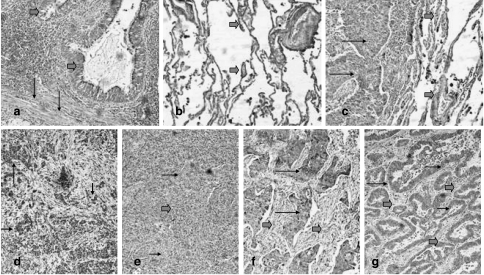
PDH and PDK1 were expressed strongly and consistently in the cytoplasm of normal bronchial and alveolar cells (Figure 1, a and b). Immunostaining using the anti-E2 and anti-E2/E3BP MoAbs showed overlapping results. Linear regression analysis of the percentage of cells strongly reacting for these Abs showed potent statistical correlation (P < .0001, r= 0.97).
Figure 1.
Expression patterns of PDH and PDK in normal lung and lung cancer. (a) Strong cytoplasmic expression of PDH in normal bronchi (thick arrows) and adjacent stroma fibroblasts (thin arrows). (b) Intense PDH expression in the alveolar tissue (thick arrows). (c) Lack of PDH expression in a squamous cell lung carcinoma (thin arrows) adjacent to PDH-positive alveolar tissue (thick arrows). (d) Focal PDH expression in cancer cells (black arrows). (e) Lack of PDH expression in squamous cell lung cancer (thin arrows) in a background of tumor-supporting stroma exhibiting a strong PDH reactivity (thick arrows). Strong expression of PDK in cancer cells (thin arrows) of a squamous cell carcinoma (f) and adenocarcinoma (g). Note the repression of PDK in the tumor-supporting stroma (thick arrows).
Cancer Cell PDH/PDK Expression
The expression of PDH and PDK1 was examined comparatively in a series of 59 (of 101) tissues from NSCLC. Both enzymes were not expressed in a large proportion of cancer cells (Figure 1, c and e) and, when expressed, the staining was weak. In the 59 cases analyzed, the percentage of cells with strong PDH expression ranged from 0% to 90% (median 10%). Lack of PDH expression was noted in 29/59 (49%) cases (negative cases), and strong expression in a minority (10–40%) of cancer cells (focal or sporadic expression; Figure 1d) was noted in 14/59 (24%) cases (classified as overall weak expression). These results are in full accordance with previously published studies by Eboli and Pasquini [17], where PDH levels are dramatically decreased in skin carcinomas compared to normal epidermis and in hepatomas compared to normal liver [18]. In our study, strong expression in more than 50% of cancer cells within tissue samples was observed in a minority of lung carcinomas (16/59; 27%) and these cases were considered as bearing high PDH reactivity.
PDK1 expression was also reduced in cancer cells (range 0–90%, median 0%). In 34/59 (58%) cases, there was lack of PDK1 expression (negative expression; Figure 1, f and g), whereas strong expression of the enzyme in 10% to 40% of cancer cells was noted in 7/59 (12%) cases (classified as overall weak expression). In 18/59 (30%) cases, there was strong PDK expression in > 50% of cancer cells, and these were considered as bearing high PDK1 reactivity.
Linear regression analysis between the percentage of cancer cells expressing PDH and PDK revealed a significant direct association (P < .0001, r= 0.49). Table 1 shows the categorical analysis of PDH and PDK expression. Low PDH expression in cancer cells was accompanied by low PDK expression in the vast majority of cases [27/29 (93%) cases]. In 10/59 (17%) cases, strong PDH/PDK expression was noted, simulating the patterns of PDH/PDK expression observed in normal lungs. In an additional 6/59 (10%) cases, PDH activity was maintained, whereas PDK was suppressed. These results show that downregulation of PDH and PDK is a common event in lung cancer, whereas a minority (27%) of cases maintain PDH expression.
Table 1.
Association of PDH Reactivity with the Expression of PDK (n = 59).
| PDK | PDH | P | ||
| Negative | Weak | High | ||
| Negative | 22 | 6 | 6 | .001 |
| Weak | 5 | 2 | 0 | |
| High | 2 | 6 | 10 | |
There was no association of PDH/PDK expression with TN stage, histology type (squamous cell cancer versus adenocarcinoma), or histologic differentiation (data not shown).
Cancer Cell PDH and HIF/LDH5 Expression
Defective PDH/PDK activity in cancer cells, as suggested by the poor PDH/PDK expression in 73% of cases, would result in an intense accumulation of pyruvate in the cells and a severe deficit of aerobic acquisition of energy. Anaerobic pathways of pyruvate metabolism to lactate could therefore take over for energy acquisition. We therefore analysed the expression of PDH in a series of 42 NSCLCs previously extensively examined for various hypoxia/metabolic parameters. We noted that lack of PDH expression was associated with HIF1α stabilization and/or LDH5 overexpression in about half of PDH-deficient tumors, (Table 2). Because of shortage of material and close association of PDH with PDK, as shown above, PDK was not analysed in this group.
Table 2.
Association of PDH Expression with HIF1α and HIF2α, LDH5, and CA9 Expression (n = 42).
| PDH | P | ||
| Low | High | ||
| HIF1α | |||
| Low | 15 | 0 | .003 |
| High | 16 | 11 | |
| HIF2α | |||
| Low | 15 | 3 | .29 |
| High | 16 | 8 | |
| LDH5 | |||
| Low | 18 | 0 | .0008 |
| High | 13 | 11 | |
| CA9 | |||
| Low | 17 | 7 | .79 |
| High | 14 | 4 | |
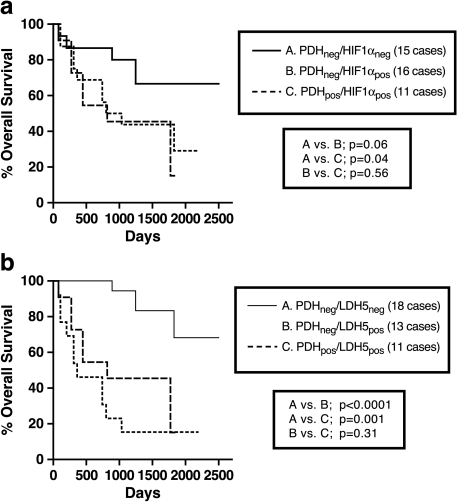
LDH5 gene is transcriptionally regulated by HIF1α [19] and, indeed, in a recent study, we noted a direct association of HIF1α and LDH5 expression in NSCLC [14]. In a recent experimental study, high pyruvate concentration induced HIF1α stabilization, independently of hypoxia [11]. It is plausible that in PDH-deficient tumors, HIF1α/LDH5 upregulation is a consequence of pyruvate accumulation. Such an effect would allow the acquisition of energy through pyruvate reduction to lactate. This, however, concerns about half of PDH-deficient tumors, as 15/31 and 18/31 of such tumors seemed not to switch on HIF1α and/or LDH5, respectively. These later category of tumors, with an apparent defective pyruvate metabolism (in both citric acid and lactate production directions), were linked with an excellent post-operative outcome in a series of 42 patients analyzed, for which survival data were available (Figure 2).
Figure 2.
Kaplan-Meier overall survival curves according to PDH expression, stratified for HIF1α (a) and LDH5 expression (b) (n = 42). Note that cases with contemporaneous defective metabolism in both aerobic (low PDH expression) and anaerobic (low HIF1α or low LDH5) directions had a particularly favorable outcome.
Of interest, a direct association of high PDH expression with HIF1α and LDH5 expression was noted, as all cases maintaining normal PDH reactivity had also high HIF1α and LDH5 expression. It would have been expected that intense PDH activity and metabolism of pyruvate to acetyl-CoA would compete for pyruvate and reduce pools that directly regulate HIF. These cases may therefore maintain HIF by hypoxia- or oncogene-mediated pathways. In any case, tumors able to generate energy by aerobic and anaerobic pathways may have an advantage in growth. However, preservation of the E2 subunit does not necessarily predict for PDH functionality as additional factors, such as PDK overexpression, may suppress PDH activity, and these cases may in fact bear an impaired aerobic metabolism.
In a previous study, we showed that, in contrast to LDH5, LDH1 is expressed consistently in all normal tissues. This expression is maintained or lost during neoplastic transformation, whereas LDH5 is expressed preferentially in tumor cells [16]. The cellular population of the tumor-supporting stroma shows consistent LDH1 reactivity and LDH5 activity in a small percentage of cases (in cases with stroma HIF1α reactivity). LDH1 is less efficient than LDH5 in catalyzing the conversion of pyruvate to lactate, and favors the conversion of pyruvate to acetyl-CoA that enters into the citric acid (Krebs) cycle. In the present study, no association of LDH1 with PDH and PDK1 was noted in cancer cells. The consistent LDH1 expression in the tumor-related stroma goes along with the strong PDH expression, further supporting the suggestion that tumoral stroma maintains strong aerobic metabolism.
PDH/HIF/LDH5 Expression and Tumor Vasculature
Analysis of VD showed no differences between PDH/HIF1α (or PDH/LDH5)-positive tumors and the remaining of tumors (data not shown). In a previous study [20], we proposed a classification of lung carcinoma vascularity according to the ability of tumors to sustain inner vasculature. High tumor angiogenic activity as assessed at the tumor invading edge was not always followed by high inner vascular density, so that highly angiogenic tumors were divided in two groups of low and high vascular survival ability (edvin scores 2 and 3, respectively; edvin: edge versus inner). Tumors with low angiogenic activity at the invading edge were classified as edvin 1. Analysis of the PDH/HIF phenotype according to the edvin score showed that tumors with simultaneously intensified PDH and HIF/LDH5 pathways were mainly of edvin 3 score, thus were tumors with high angiogenic activity and high vascular survival ability (tumors with dense vasculature throughout the tumoral mass) (Table 3). It seems, therefore, that this subgroup of tumors is less likely to be hypoxic. So concurrent PDH and HIF1/LDH5 upregulation could occur due to genetic events that switch on the entire cellular metabolism. Such tumors with intensively upregulated aerobic and anaerobic metabolic potential were linked with particularly poor prognosis (Figure 2, a and b). It should be, however, kept in mind that expression of the E2 subunit does not guarantee PDH functionality, and that HIF1 overexpression in this latter group may still be a cause of PDH inactivity.
Table 3.
Edvin Patterns of Vascularisation According to the PDH/HIF and PDH/LDH5 Expression Profile.
| PDH/HIF1α | PDH/LDH5 | |||||
| Low/Low | Low/High | High/High | Low/Low | Low/High | High/High | |
| Edvin | ||||||
| 1 | 7 | 8 | 2 | 10 | 5 | 2 |
| 2 | 6 | 4 | 2 | 6 | 4 | 2 |
| 3 | 2 | 4 | 7 | 2 | 4 | 7 |
| P = .07* and .01† | P =.06* and .01† | |||||
Edvin 1 vs. 2 vs. 3 (chi-square analysis).
Edvin 1/2 vs. 3 (Fisher's exact test).
PDH/PDK Expression in Cancer Cells Versus Tumoral Stroma
Normal lung stroma fibroblasts were positive for both PDH and PDK1. Results for the tumor stroma fibroblasts showed a major difference for PDK1. Despite the intense overall downregulation of PDH in cancer cells, fibroblasts in the tumor-supporting stroma maintained an intense PDH expression (Figure 1d). This shows that tumoral stroma maintains a high ability for aerobic pyruvate consumption. HIF and LDH5 expression in tumor fibroblasts is only exceptionally present, whereas LDH1 isoenzyme (favoring aerobic metabolism of pyruvate) is strongly expressed as previously reported [14,16].
However, PDK1 expression was impressively downregulated in stroma fibroblasts compared to normal lung (Figure 1e), suggesting that PDH activity was further facilitated by inactivation of the PDK-mediated negative regulation on PDH. Although additional studies are required to assess whether PDK2, PDK3, and PDK4 are also downregulated in the tumor-related stroma, the confirmation of PDK1 suppression is important as preliminary data show that PDK1, in contrast to PDK2 and PDK3, is not hypoxia-regulated (unpublished data). It is suggested that PDK1 suppression in the tumor-related stroma is a result of a paracrine tumor/fibroblast interaction and not a hypoxia-related effect. These observations strongly suggest that stroma fibroblasts maintain an exclusively aerobic metabolism of pyruvate, preventing lactate formation from glucose. This metabolic preference by the tumor-supporting stroma could be a consequence of the high concentration of lactic acid produced and extruded out of cancer cells, and could be very useful for tumor survival to avoid self-destruction by excessive acidosis. If tumor stroma, apart from providing a structural skeleton, also provides buffering and metabolic support to cancer cells, then drugs targeting the specific features of the cancer-supporting stroma metabolism may prove antitumorigenic and tumoricidal, which deserves further investigation.
Conclusions
It is concluded that the majority of NSCLCs has a metabolic deficit in the PDH expression regulation, the molecular basis of which remains to be elucidated. Pyruvate intracellular accumulation (due to intensively activated glycolytic pathways) [10,11] in the context of PDH downregulation, and not hypoxia, may be an important factor for HIF1α stabilization, with LDH5 overexpression and shift of the pyruvate metabolism to lactate production. Defective PDH regulation may be part of the explanation of cancer cell-specific “aerobic glycolysis” phenomenon noted by Warburg [9]. However, a large percentage of PDH-deficient carcinomas (50%) is not able to switch on the HIF pathway and these tumors are linked with an excellent postoperative outcome. A subgroup of tumors (about 20% of NSCLC) shows a coherent intense PDH and HIF/LDH5 presence, therefore sharing strong aerobic and anaerobic metabolic abilities. Such tumors are endowed with a particularly aggressive behaviour, which leads to poor postoperative outcome. The biologic significance of the fact that tumoral stroma, in contrast to cancer cells, maintains a strictly aerobic metabolism with the likelihood of maximal PDH function due to downregulation of inhibitory kinase should be further studied as a mechanism by which tumors deal with their acid load.
Abbreviations
- PDH
pyruvate dehydrogenase
- PDK
pyruvate dehydrogenase kinase
- HIF1α
hypoxia-inducible factor-1α
- ATP
adenosine triphosphate
- NAD+
nicotinamide adenine dinucleotide
- NADH
dihydronicotinamide adenine dinucleotide
- NSCLC
non small cell lung carcinoma
Footnotes
The study was financially supported by the Tumor and Angiogenesis Research Group and the Cancer Research UK.
References
- 1.Harris RA, Bowker-Kinley MM, Hyang B, Wu P. Regulation of the activity of the pyruvate dehydrogenase complex. Adv Enzyme Regul. 2002;42:2249–2259. doi: 10.1016/s0065-2571(01)00061-9. [DOI] [PubMed] [Google Scholar]
- 2.Reed LJ, Hackert ML. Structure-function relationships in dihydrolipoamide acyltransferases. J Biol Chem. 1990;265:8971–8974. [PubMed] [Google Scholar]
- 3.Sanderson SJ, Miller C, Lindsay JG. Stoichiometry, organisation and catalytic function of protein X of the pyruvate dehydrogenase complex from bovine heart. Eur J Biochem. 1996;236:68–77. doi: 10.1111/j.1432-1033.1996.00068.x. [DOI] [PubMed] [Google Scholar]
- 4.Maeng CY, Yazdi MA, Niu XD, Lee HY, Reed LJ. Expression, purification, and characterization of the dihydrolipoamide dehydrogenase-binding protein of the pyruvate dehydrogenase complex from Saccharomyces cerevisiae. Biochemistry. 1994;33:13801–13807. doi: 10.1021/bi00250a034. [DOI] [PubMed] [Google Scholar]
- 5.Harris RA, Bowker-Kinley MM, Wu P, Jeng J, Popov KM. Dihydrolipoamide dehydrogenase-binding protein of the human pyruvate dehydrogenase complex. DNA-derived amino acid sequence, expression, and reconstitution of the pyruvate dehydrogenase complex. J Biol Chem. 1997;272:19746–19751. doi: 10.1074/jbc.272.32.19746. [DOI] [PubMed] [Google Scholar]
- 6.Harris RA, Huang B, Wu P. Control of pyruvate dehydrogenase kinase gene expression. Adv Enzyme Regul. 2001;41:269–288. doi: 10.1016/s0065-2571(00)00020-0. [DOI] [PubMed] [Google Scholar]
- 7.Sugden MC, Holness MJ. Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs. Am J Physiol Endocrinol Metab. 2003;284:855–862. doi: 10.1152/ajpendo.00526.2002. [DOI] [PubMed] [Google Scholar]
- 8.Holbrook JJ, Liljas A, Steindel SJ, Rossman MG. Lactate dehydrogenase: Vol XI. In: Boyer PD, editor. The Enzymes. 3rd ed. New York: Academic Press; 1975. pp. 191–292. [Google Scholar]
- 9.Warburg O. On the origin of cancer cells. Science. 1956;123(391):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 10.Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994;269:23757–23763. [PubMed] [Google Scholar]
- 11.Lu H, Forbes RA, Verma A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J Biol Chem. 2002;277:23111–23115. doi: 10.1074/jbc.M202487200. [DOI] [PubMed] [Google Scholar]
- 12.Giatromanolaki A, Koukourakis MI, Sivridis E, Turley H, Talks K, Pezzella F, Gatter KC, Harris AL. Relation of hypoxia-inducible factor 1 alpha and 2 alpha in operable non-small cell lung cancer to angiogenic/molecular profile of tumours and survival. Br J Cancer. 2001;85:881–890. doi: 10.1054/bjoc.2001.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giatromanolaki A, Koukourakis MI, Sivridis E, Pastorek J, Wykoff CC, Gatter KC, Harris AL. Expression of hypoxia-inducible carbonic anhydrase-9 relates to angiogenic pathways and independently to poor outcome in non-small cell lung cancer. Cancer Res. 2001;61:7992–7998. [PubMed] [Google Scholar]
- 14.Koukourakis MI, Giatromanolaki A, Sivridis E, Bougioukas G, Didilis V, Gatter KC, Harris AL Tumour and Angiogenesis Research Group, author. Lactate dehydrogenase-5 (LDH-5) overexpression in non-small-cell lung cancer tissues is linked to tumour hypoxia, angiogenic factor production and poor prognosis. Br J Cancer. 2003;89:877–885. doi: 10.1038/sj.bjc.6601205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giatromanolaki A, Koukourakis M, O'Byrne K, Fox S, Whitehouse R, Talbot D, Harris AL, Gatter KC. Prognostic value of angiogenesis in operable non-small cell lung cancer. J Pathol. 1996;179:80–88. doi: 10.1002/(SICI)1096-9896(199605)179:1<80::AID-PATH547>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 16.Koukourakis MI, Giatromanolaki A, Sivridis E. Lactate dehydrogenase (LDH) isoenzymes 1 and 5 expression by neoplastic and stromal cells in non-small cell lung cancer and other epithelial malignant tumors. Tumor Biol. 2003;24:199–202. doi: 10.1159/000074430. [DOI] [PubMed] [Google Scholar]
- 17.Eboli ML, Pasquini A. Transformation linked decrease of pyruvate dehydrogenase complex in human epidermis. Cancer Lett. 1994;85:239–243. doi: 10.1016/0304-3835(94)90281-x. [DOI] [PubMed] [Google Scholar]
- 18.Eboli ML. Pyruvate dehydrogenase levels in Morris hepatomas with different growth rate. Cancer Lett. 1985;26:185–190. doi: 10.1016/0304-3835(85)90025-4. [DOI] [PubMed] [Google Scholar]
- 19.Semenza GL, Jiang BH, Leung SW, Passantino R, Concordet JP, Maire P, Giallongo A. Hypoxia response elements in the A aldolase, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem. 1996;271:32529–32537. doi: 10.1074/jbc.271.51.32529. [DOI] [PubMed] [Google Scholar]
- 20.Giatromanolaki A, Koukourakis MI, Sivridis E, O'Byrne K, Gatter KC, Harris AL. “Invading edge vs inner” (edvin) patterns of vascularization: an interplay between angiogenic and vascular survival factors defines the clinical behaviour of non-small cell lung cancer. J Pathol. 2000;192:140–149. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH693>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]