Abstract
Molecular analyses have demonstrated mutations in the K-ras gene at codon 12 in the majority of pancreatic ductal adenocarcinomas (PDACs). In order to determine whether the K-ras mutation rate increases parallel to the grade of dysplasia in duct lesions, we performed a meta-analysis of the studies published between 1988 and 2003 that provide information on K-ras mutations in hyperplastic and dysplastic duct lesions in the pancreas. The described duct lesions were reclassified according to the nomenclature for pancreatic intraepithelial neoplasia (PanIN), and the molecular methods for detecting K-ras were reviewed. In PanIN lesions from pancreata of patients with PDAC, there was a stepwise increase in K-ras mutations that correlated with the grade of dysplasia of the PanIN lesion. K-ras mutations were found in 36%, 44%, and 87% of PanIN-1a, 1b, and 2–3 lesions, respectively (trend statistic P < .001). Mutation-enriched polymerase chain reaction (PCR) resulted in higher rates of K-ras mutations in PanIN than plain PCR did. The incidence of K-ras mutations in PanIN lesions associated with chronic pancreatitis (CP) or normal pancreas was low (around 10%). In CP, K-ras mutations were only found after a disease duration of 3 years. The correlation of the incidence of K-ras mutations with the grade of dysplasia in PanIN and the occurrence of these mutations in CP with a duration of more than 3 years underlines the importance of this genetic change for the development of PDAC.
Keywords: K-ras mutation, pancreatic ductal carcinoma, chronic pancreatitis, pancreatic intraepithelial neoplasia, meta-analysis
Introduction
Because of its late detection and poor response to therapy, pancreatic ductal adenocarcinoma (PDAC) ranks as one of the most lethal tumors [1]. Numerous genetic studies have been conducted to identify a suitable marker for the early recognition of PDAC [2,3]. This has resulted in the identification of a number of PDAC-associated genes [4,5]. Among these, the first gene studied in detail was the K-ras gene. It soon turned out that in the majority of PDACs [6], it shows mutations in a hot spot at codon 12, exon 1. When K-ras mutations were also found in in situ components of PDAC and PDAC-associated duct lesions [7–9], they were thought to be potential tumor precursors [10], and it was hoped that mutated K-ras might serve as a marker of the developing PDAC [11]. However, further studies revealed K-ras mutations even in the normal-appearing duct epithelium of the inflamed and noninflammed non-neoplastic pancreas [12,13]. Similar results were obtained in extrapancreatic adenocarcinomas, which were also found to have an elevated K-ras mutation rate [14]. These findings reduced the significance of K-ras as a specific tumor marker and raised the question of the role of K-ras in pancreatic carcinogenesis. Another problem was that the frequency of K-ras mutations found in duct lesions and PDACs differed from study to study, most likely because of differences in the molecular methodology [15] and the histopathologic terminology used for the analyzed duct lesions.
The aim of this study was to determine if there is a correlation between pancreatic intraepithelial neoplasia (PanIN) severity and the frequency of K-ras mutation in patients with and without PDAC by reviewing the published data on K-ras mutations in duct lesions [16] using the new PanIN classification. This recently proposed classification is based on the hypothesis that hyperplastic pancreatic duct lesions have a preneoplastic potential [10]. Hence, the duct lesions were given the name PanIN. The designation seems to be correct because three studies demonstrated an increase in loss of heterogeneity (LOH) for p16, p53, and DPC4 according to the grade of dysplasia of the PanINs [17–19]. In order to make these LOH data comparable to those of mutated K-ras in duct lesions, we applied the PanIN classification to the old histologic terms used to describe these lesions in the various studies. We also included studies on patients with chronic pancreatitis (CP) to find out whether the K-ras positivity rate in the PanINs is similar to that in the PanINs associated with PDACs.
We present a combined histopathologic and molecular meta-analysis that attempts to better define the role that both PanIN and K-ras may play in the pathogenesis of PDAC and the progression of CP to PDAC.
Materials and Methods
Data Acquisition
The study was conceived as a histopathologic meta-analysis [16]. We conducted a computer search of the Medline database for articles on K-ras mutations in pancreatic duct lesions between 1988 and 2003. We evaluated only studies published in peer-reviewed journals that presented precise data on the histopathology of the duct lesions in the form of either illustrations or detailed descriptions. Therefore, short reports, abstracts, and conference reports, which are usually included in the data acquisition of a meta-analysis, were excluded [16]. Using search terms such as dysplasia, hyperplasia, metaplasia, and intraductal tumor in conjunction with pancreas and pancreatic, we identified 15 original contributions with all the relevant information (Table 1). The way the tissue was obtained (surgery, postmortem, and micro-dissection), the associated condition (ductal adenocarcinoma, CP, and normal pancreas), and the polymerase chain reaction (PCR) technique were recorded [15]. Duct lesions reported in association with intrapancreatic mucinous tumors (IPMTs) were excluded. A quality assessment protocol [20] was generated, defining the minimum criteria necessary for a study to be included in the systematic review. These were: 1) detailed histopathologic description of the lesions; 2) histologic evidence of examples of such pancreatic lesions in printed figures; and 3) detection of K-ras mutations. The suitability of a given paper for inclusion in this study was assessed by two of the authors.
Table 1.
Frequency of K-ras Mutations in PanIN Lesions in the Pancreas of Patients with PDAC (Upper Panel) or CP, or in Normal Disease-Free Pancreas (Lower Panel).
| Study | Reference | Patients** | Lesions | Method | K-ras+/Total Foci | K-ras+ (%) |
| Lemoine et al. (1992) | [7] | PDAC | PanIN-1b | PCR | 0/9 | 0 |
| PDAC | PanIN-3 | PCR | 5/6 | 83 | ||
| Motojima et al. (1993) | [54] | PDAC | PanIN-3 | PCR | 2/2 | 100 |
| Tabata et al. (1993) | [55] | PDAC | PanIN-1a | PCR | 0/10 | 0 |
| PDAC | PanIN-2 | PCR | 5/6 | 83 | ||
| Moskaluk et al. (1997) | [8] | PDAC | PanIN-3 | PCR | 16/17 | 94 |
| PDAC | PanIN-1a | PCR | 2/7 | 29 | ||
| Sugio et al. (1997) | [56] | PDAC | PanIN-3 | PCR | 17/18 | 94 |
| PDAC | PanIN-2 | PCR | 19/23 | 83 | ||
| PDAC | PanIN-1a | PCR | 5/21 | 24 | ||
| Z'graggen et al. (1997) | [57] | PDAC | PanIN-1a | PCR | 3/11 | 27 |
| Matsubayashi et al. (1999) | [58] | PDAC | PanIN-1a | Me-PCR | 115/266 | 43 |
| Terhune et al. (1998) | [59] | PDAC | PanIN-1b | Me-PCR | 27/44 | 61 |
| PDAC | PanIN-3 | Me-PCR | 7/9 | 78 | ||
| Lüttges et al. (1999) | [9] | PDAC | PanIN-1a | PCR | 33/123 | 27 |
| PDAC | PanIN-1b | PCR | 46/112 | 41 | ||
| Mulligan et al. (1999) | [60] | PDAC | PanIN-3 | Me-PCR | 3/4 | 75 |
| Lemoine et al. (1992) | [7] | CP | PanIN-1b | PCR | 0/5 | 0 |
| Tabata et al. (1993) | [55] | CP | PanIN-2 | PCR | 0/5 | 0 |
| CP | PanIN-1a | PCR | 0/5 | 0 | ||
| Yanagisawa et al. (1993) | [23] | CP | PanIN-2 | PCR | 1/4 | 25 |
| CP | PanIN-1a | PCR | 3/3 | 60 | ||
| CP | PanIN-1b | PCR | 6/7 | 86 | ||
| Song et al. (1996) | [61] | CP | PanIN-1b | PCR | 2/12 | 17 |
| Rivera et al. (1997) | [62] | CP | PanIN-1a | PCR | 2/11 | 18 |
| Mulligan et al. (1999) | [60] | CP | PanIN-2 | Me-PCR | 2/15 | 13 |
| CP | PanIN-1a | Me-PCR | 1/17 | 6 | ||
| Lüttges et al. (2000) | [12] | CP | PanIN-2 | PCR | 0/26 | 0 |
| CP | PanIN-1a | PCR | 15/140 | 11 | ||
| CP | PanIN-1b | PCR | 4/97 | 4 | ||
| Song et al. (1996) | [61] | Normal | PanIN-1a | PCR | 1/3 | 33 |
| Tada et al. (1996) | [26] | Normal | PanIN-1a | PCR | 19/79 | 24 |
| Matsubayashi et al. (1999) | [58] | Normal | PanIN-1a | Me-PCR | 9/51 | 18 |
| Terhune et al. (1998) | [59] | Normal | PanIN-1b | Me-PCR | 8/17 | 47 |
| Lüttges et al. (1999) | [9] | Normal | PanIN-1a | PCR | 10/153 | 7 |
| Normal | PanIN-1b | PCR | 6/173 | 3 | ||
| Agoff et al. (2001) | [63] | Normal | PanIN-1a | PCR | 0/8 | 0 |
In some studies, PanINs derived from both PDAC or CP were included but separately analyzed. Therefore, these studies appear twice.
From patients with PDAC, CP, or without pancreas pathology (normal).
Evaluation of Histopathologic Data
On the basis of the PanIN classification, all studies with histopathologic reports were reevaluated and the lesions were classified [10,21] by two independent pathologists (G.K. and J.L.) (Appendix 1).
Evaluation of Molecular Data
The PCR technique employed was characterized as either plain PCR for K-ras without modification or mutation-enriched PCR (ME-PCR) technique employing special enzymes and/or PCR protocols [15]. Further, the method used to verify PCR amplificates (restriction fragment length polymorphism, direct DNA sequencing, and allele-specific oligonucleotide hybridization) was recorded.
Statistical Analysis
We performed a meta-analysis of all the available reports. The confidence interval estimates for the proportion of K-ras-positive samples for each individual study and for the overall estimates were calculated using Wilson's method [22]. For comparison of dichotomized stratified data, we used the chi-square analysis. The Mantel-Haenszel test for linear association was used to assess the significance of the trend among strata. The exact Wilcoxon-Mann-Whitney nonparametric test was used to compare the age distribution and the duration of CP with the presence or absence of K-ras mutations.
Results
We identified 15 studies dealing with K-ras mutations in pancreatic preneoplastic lesions that were histologically defined. A total of 1519 lesions was included in these studies. In all studies, it was possible to reclassify the pancreatic duct lesions according to the PanIN classification (Appendix 1).
The cases described as simple hyperplasia or mucous hypertrophy corresponded well to PanIN-1A, and those described as ductal papillary hyperplasia to PanIN-1B, as shown by the figures in the various studies (Table 1). The review of our own studies [9,17] also revealed a good correlation between the old and new terminologies. Sixty-one papillary lesions in CP with mild atypia were classified as PanIN-1B. In a study by Yanagisawa et al. [23], histologic illustrations of four lesions described as mucous papillary hyperplasia revealed a high degree of nuclear atypia but no structural abnormality. Hence, they were classified as PanIN-2 lesions. In another investigation [7], six lesions were typed as in situ carcinoma. They were accordingly classified as PanIN-3 lesions.
A total of 908 lesions was classified as PanIN-1A, 476 as PanIN-1B, 79 as PanIN-2, and 56 as PanIN-3. The overall rate for K-ras mutations in PanIN lesions was 10% in CP (95% CI, 7–14%) and 44% in PDAC (95% CI, 41–48%). There was no correlation between K-ras positivity and the source of the tissue.
The frequency of K-ras mutations in the PanIN lesions varied considerably according to the underlying disease of the patients (CP versus PDAC). Therefore, we investigated the relation between K-ras mutations and the grade of the PanIN lesions separately for each group of patients (PDAC, CP, and normal) (Tables 1 and 2).
Table 2.
PCR Data on K-ras Positivity in PanIN Lesions Associated with Various Pancreatic Disorders.
| Technique | Total | |||||
| PCR | Me-PCR | |||||
| K-ras+/Total Foci | K-ras+ % (95% CI) | K-ras+/Total Foci | K-ras+ % (95% CI) | K-ras+/Total Foci | K-ras+ % (95% CI) | |
| PDAC | ||||||
| PanIN-1A | 43/172 | 25 (19–32) | 115/266 | 43 (37–49) | 158/438 | 36 (32–41) |
| PanIN-1B | 46/121 | 38 (29–47) | 27/44 | 61 (46–75) | 73/165 | 44 (37–52) |
| PanIN-2–3 | 67/76 | 88 (78–94) | 7/9 | 78 (40–96) | 74/85 | 87 (78–93) |
| Chronic pancreatitis | ||||||
| PanIN-1A | 20/159 | 13 (8–19) | 1/17 | 6 (0–31) | 21/176 | 12 (8–18) |
| PanIN-1B | 12/121 | 10 (5–17) | - | - | 12/121 | 10 (5–17) |
| PanIN-2–3 | 1/35 | 3 (0–17) | 2/15 | 13 (2–42) | 3/50 | 6 (2–18) |
| Normal pancreas | ||||||
| PanIN-1A | 30/243 | 12 (9–17) | 9/51 | 18 (9–31) | 39/294 13 | (10–18) |
| PanIN-1B | 6/173 | 3 (1–8) | 8/17 | 47 (24–71) | 14/190 | 7 (4–12) |
In patients with PDAC, the frequency of K-ras mutations in the PanIN lesions increased with the PanIN grade: 36% in PanIN-1A lesions, 44% in PanIN-1B lesions, and 87% in PanIN-2–3 lesions (Table 2). Due to the relatively small number of lesions, PanIN-2 and PanIN-3 lesions were combined for statistical evaluation (Table 2). When data from studies using plain PCR were compared with data from studies using ME-PCR, it was found that the latter technique was associated with a higher detection rate of K-ras mutation in PanIN-IA and PanIN-IB lesions (Table 2).
Among patients with CP, the proportion of PanIN lesions harboring K-ras mutations was relatively low (10%) and independent of the PanIN grade of the lesion (Table 2). Here, the ME-PCR techniques resulted in similar rates of K-ras positivity in the PanIN-1A lesions and a higher percentage in the PanIN-2–3 lesions. The number of lesions investigated was too low in the ME-PCR group to result in any statistically significant difference.
Only low-grade PanIN (1A/1B) lesions were reported in the pancreas of patients free of pancreatic disease and, again, a low proportion (11%) of these lesions harbored K-ras mutations. There were no PanIN-3 lesions in patients with CP. The numbers of PanIN-1A and PanIN-1B lesions varied between the two PCR methods; in the ME-PCR group, the number of lesions was again low.
Using the original data reported in a previous study [12], we investigated the possible effect of age and duration of CP on the severity of PanIN lesions and on their K-ras status. Among 25 patients with CP, we found no age difference between those with PanIN-1A lesions (n = 4, median age = 47.5, range = 39–54) and those with either PanIN-1B (n = 8, median age = 54.5, range = 30–69) or PanIN-2 lesions (n = 13, median age = 50, range = 17–63; P = .59).
We also found no age difference between CP patients with K-ras-negative PanIN lesions (n = 14, median age = 46, range = 17–66) and those with K-ras-positive PanIN lesions (n = 11, median age = 53, range = 33–69; P = .42).
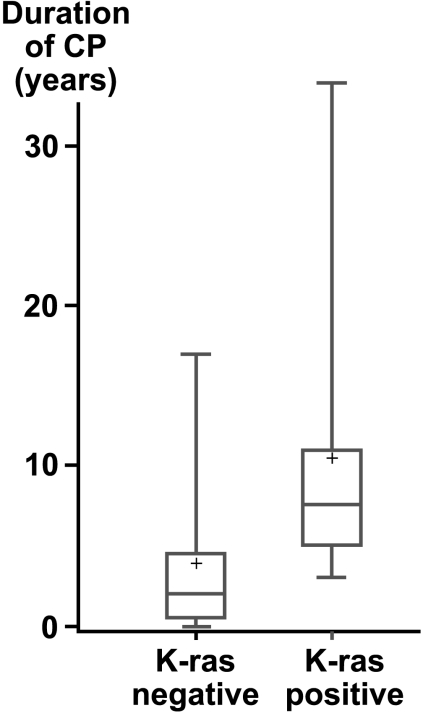
Unlike patient's age, the duration of CP was significantly associated with K-ras positivity. Of the 16 CP patients for whom information was available, the duration of CP for the eight patients with K-ras-negative PanIN lesions was shorter (median = 2 years, range = 0–17) than that of the eight CP patients with K-ras-positive PanIN lesions (median = 7.5 years, range = 3–34; P = .021) (Figure 1). No case of CP with a disease duration of less than 3 years showed K-ras mutations.
Figure 1.
Boxplot of the duration of CP with and without K-ras mutation. Median duration for K-ras-negative patients = 2 years; K-ras-positive patients = 7.5 years (P = .021).
Discussion
Mutations in the K-ras oncogene are rare in the normal disease-free pancreas [13]. Their frequency does not seem to exceed 13% [15], whereas it reaches 80% in PDAC [24]. Our meta-analysis demonstrated that the frequency of K-ras mutations in PanIN lesions corresponds to their grade of dysplasia, with PanIN-2 and PanIN-3 lesions displaying a significantly higher frequency (87%) than PanIN-1A (36%) (Table 2). The second major finding was the correlation of K-ras positivity in PanINs with the duration of CP.
We reviewed 15 studies and reclassified 1519 duct lesions. In most studies, the duct lesions could be easily reclassified, particularly when high-quality illustrations were provided. Problems arose in about 15% of the studies. They were due to uncommon phrasing of histopathologic findings and concerned mostly the distinction of PanIN-2 lesions from PanIN-3 lesions. Because of this occasional uncertainty and the small total numbers of PanIN-2 and PanIN-3 lesions reviewed, the statistical analysis was performed on a combined PanIN-2/PanIN-3 group.
In an earlier study on the sensitivity of the molecular method used for the detection of K-ras mutations and the rate of positive samples [15], we already found that so-called ME-PCR will result in higher rates of K-ras-positive samples than plain PCR. This study confirmed this result and additionally showed that the high sensitivity of the ME-PCR method was particularly evident in the low-grade PanINs. Whether the results obtained with ME-PCR reflects the K-ras mutation status better than plain PCR has been widely debated during recent years [25]. However, in order to avoid false-positive results as far as possible, the use of plain PCR techniques with subsequent DNA sequencing appears to be the method of choice.
Do K-ras mutations have a role as a diagnostic marker of PDAC? Considering the methodological ambiguities and the demonstration of K-ras positivity not only in CP but also in the disease-free pancreas [12,13,26,27], the detection of a K-ras mutation in a patient does not establish the diagnosis of PDAC. However, the results of our meta-analysis show that a K-ras mutation may be indicative of the development of a PDAC, particularly in patients with CP. The latter notion would be supported by our data that K-ras mutations were only found in CP of more than 3 years' duration. If, in addition, the patient is a smoker, the risk would further increase. Under these conditions as well as in surveillance programs for patients with hereditary pancreatitis and familial pancreatic carcinoma [28], K-ras analysis should be recommended.
This study clearly demonstrated that in PanINs from patients with PDAC, the gradual increase in K-ras positivity correlated with the grade of dysplasia. Other investigations revealed a rising incidence of LOH for p16, p53, and DPC4/SMAD4 with increasing PanIN grades [17,29]. In addition, shortened telomeres have been demonstrated in PanIN lesions of all grades [30] and were thought to predispose PanINs to accumulate progressive chromosomal abnormalities. Finally, BRCA2 and maspin, two tumor-suppressor genes known to also be involved in breast tumorigenesis, were found to be inactivated in PanIN-3 lesions [31,32]. Based on these findings [24,33–37], a progression model for genetic alteration in PanINs has been proposed. In this model, it is suggested that K-ras mutations occur early in the evolution of PanINs [38].
Recently, new experimental evidence for the tumorigenic potential of the ras oncogene was provided. Immortalization of bovine pancreatic duct cells from the adult pancreas with SV40 large-T and further transfection of mutated K-ras resulted in a malignant phenotype. Upon transplantation into nude mice, PDACs were formed [39]. In a three-step model, transfection of activated human telomerase (hTERT), SV40 large-T plus, and mutated H-ras drove human embryonic kidney cells and fibroblasts into malignancy [40]. More recently, in a study employing a conditionally transgenic mouse model for K-ras, typical PanINs were induced in animals with spontaneous progression to invasive ductal adenocarcinoma [41]. Taken together, these data demonstrate that ras does indeed play an important role in tumorigenesis and thus also in the pathogenesis of both PanINs and PDAC. How, precisely, the activated K-ras alters the cell machinery toward malignancy is not yet clear, but recent studies suggest that K-ras stimulates cell proliferation rather than inhibits apoptosis [36,42].
What may induce K-ras mutations in the pancreas? As causes of K-ras mutations, smoking and also coffee consumption have been discussed [27,43,44]. Whether the same factors may also play a role in the induction of PanINs is not known, but because PanIN-1 lesions may occur early in life [13,45], it is possible that such K-ras-positive PanIN-1 lesions may exist for a long time. Eventually, one of them is suddenly transformed into a higher-degree PanIN and invasive PDAC by accidental accumulation of additional genetic events genes such as p16, p53, and SMAD4/DPC4. Neither the time axis of the progression nor the trigger for these additional mutations is known. Recent findings may provide a clue, at least in alcoholic CP: ethanol has a dramatic synergistic effect with smoking on the formation of acetaldehyde [46]. Acetaldehyde in turn generates reactive oxygen and nitrogen species [47], resulting in reactive aldehydes, which are known to induce DNA adducts and mutations [48]. Some of the reactive aldehydes have been detected in CP already [49]. Alkylating agents may induce specific K-ras mutations, namely G-A transitions, as shown in those heavy smokers in the absence of histologic lesions [27]. Besides alkylating agents, methylating agents and organic solvents have been demonstrated to induce K-ras mutations. Analyzing patients with PDAC and occupational risks, these G-A transitions could be associated with polycyclic aromatic hydrocarbons and gasoline engine exhausts [50]. For methylating agents, the proposed mechanism lies in the reduction of O6-alkyl guanine DNA-alkyltransferase (MGMT), as shown in colorectal adenomas [51]. Reduced MGMT has also been described in PDAC [52]. The presumably long phase after the first injury (K-ras) that makes the pancreatic duct epithelium receptive to malignant transformation but unaltered—if not injured for a second time—can be deemed as a “dance on the volcano” that is harmless for most people but may be fatal for a few [53].
In summary, using the new PanIN system, we reclassified and then compared a large number of preneoplastic lesions of varying grades in published reports. In PanINs associated with PDAC, we found unambiguous evidence of a stepwise increase in K-ras mutations with increasing grade of dysplasia. We also found that the duration of CP was significantly longer in K-ras-positive than in K-ras-negative patients and that the minimum duration of CP associated with K-ras positivity was 3 years. These findings suggest that K-ras mutations play an important role in the evolution of PanINs and PDAC. However, detection of K-ras mutations as the only test cannot be recommended as a screening tool for PDAC but could be useful in combination with cytology.
Acknowledgement
The authors would like to thank K. Dege for editing the manuscript.
Appendix 1
Histopathologic description and grading of PanIN
Normal: The normal ductal and ductular epithelium is a cuboidal to low-columnar epithelium with amphophilic cytoplasm. Mucinous cytoplasm, nuclear crowding, and atypia are not seen.
Squamous (transitional) metaplasia: A process in which the normal cuboidal ductal epithelium is replaced by mature stratified squamous or pseudostratified transitional epithelium without atypia.
PanIN-1A: These are flat epithelial lesions composed of tall columnar cells with basally located nuclei and abundant supranuclear mucin. The nuclei are small and round to oval in shape. When oval, the nuclei are oriented perpendicular to the basement membrane. It is recognized that there may be considerable histologic overlap between non-neoplastic flat hyperplastic lesions and flat neoplastic lesions without atypia. Therefore, some may choose to designate these entitles with the modifier term lesion (“PanIN/L-1A”) to acknowledge that the neoplastic nature of many cases of PanIN-1A has not been unambiguously established.
PanIN-1B: These epithelial lesions have a papillary, micropapillary, or basally pseudostratified architecture but are otherwise identical to PanIN-1A.
PanIN-2: Architecturally, these mucinous epithelial lesions may be flat but are mostly papillary. Cytologically, by definition, these lesions must have some nuclear abnormalities. These abnormalities may include some loss of polarity, nuclear crowding, enlarged nuclei, pseudostratification, and hyperchromatism. These nuclear abnormalities fall short of those seen in PanIN-3. Mitoses are rare but, when present, are nonluminal (not apical) and are not atypical. True cribiform structures with luminal necrosis and marked cytologic abnormalities are generally not seen and, when present, should suggest the diagnosis of PanIN-3.
PanIN-3. Architecturally, these lesions are usually papillary or micropapillary; however, they may rarely be flat. True cribiforming, the appearance of “budding off” of small clusters of epithelial cells into the lumen, and luminal necrosis should all suggest the diagnosis of PanIN-3. Cytologically, these lesions are characterized by a loss of nuclear polarity, dystrophic goblet cells (goblet cells with nuclei oriented toward the lumen and mucinous cytoplasm oriented toward the basement membrane), mitoses that may occasionally be abnormal, nuclear irregularities, and prominent (macro)nucleoli. The lesions resemble carcinoma at the cytonuclear level, but invasion through the basement membrane is absent.
Footnotes
The work of the group was supported by the Deutsche Krebshilfe/Mildred-Scheel-Stiftung (M.L., J.L., and G.K.), the Deutsche Forschungsgemeinschaft (M.L.), the C.D. Smithers Foundation and Solvay Pharmaceuticals (A.B.L.), and the Italian Association for Cancer Research (P.M.).
References
- 1.Parkin DM, Pisari P, Ferlay J. Estimates of the world incidence of 25 major cancers in 1990. Int J Cancer. 1999;80:827–841. doi: 10.1002/(sici)1097-0215(19990315)80:6<827::aid-ijc6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 2.Urrutia R, DiMagno EP. Genetic markers: the key to early diagnosis and improved survival in pancreatic cancer? (Editorial) Gastroenterology. 1996;110:306–310. doi: 10.1053/gast.1996.v110.agast960306. [DOI] [PubMed] [Google Scholar]
- 3.Lowenfels AB, Maisonneuve P. Epidemiologic and etiologic factors of pancreatic cancer. Hematol Oncol Clin North Am. 2002;16(1):1–16. doi: 10.1016/s0889-8588(01)00003-x. (February) [DOI] [PubMed] [Google Scholar]
- 4.Grützmann R, Foerder M, Alldinger I, Staub E, Brummendorf T, Ropcke S, Li X, Kristiansen G, Jesnowski R, Sipos B, Löhr M, Lüttges J, et al. Gene expression profiles of microdissected pancreatic ductal adenocarcinoma. Virchows Arch. 2003;443(4):508–517. doi: 10.1007/s00428-003-0884-1. [DOI] [PubMed] [Google Scholar]
- 5.Buchholz M, Boeck W, Fensterer H, Muller F, Wenger C, Michl P, Adler G, Gress TM. Use of DNA arrays/microarrays in pancreatic research. Pancreatology. 2001;1(6):581–586. doi: 10.1159/000055867. [DOI] [PubMed] [Google Scholar]
- 6.Hruban RH, van Mansfeld AD, Offershaus GJ, van Weering DH, Allison DC, Goodman SN, Kensler TW, Bose KK, Camerons JL, Bos JL. k-ras oncogene activation in adenocarcinoma of the human pancreas. A study of 82 carcinomas using a combination of mutant-enriched polymerase chain reaction analysis and allele-specific oligonucleotide hybridization. Am J Pathol. 1993;143:545–554. [PMC free article] [PubMed] [Google Scholar]
- 7.Lemoine NR, Jain S, Hughes CM, Staddon SL, Maillet B, Hall PA, Klöppel G. Ki-ras oncogene activation in preinvasive pancreatic cancer. Gastroenterology. 1992;102:230–236. doi: 10.1016/0016-5085(92)91805-e. [DOI] [PubMed] [Google Scholar]
- 8.Moskaluk CA, Hruban RH, Kern SE. p16 and K-ras gene mutations in the intraductal precursors of human pancreatic adenocarcinoma. Cancer Res. 1997;57(11):2140–2143. (June 1) [PubMed] [Google Scholar]
- 9.Lüttges J, Schlehe B, Menke MAOH, Vogel I, Henne-Bruns D, Klöppel G. The k-ras mutation pattern in pancreatic ductal adenocarcinoma is usually identical to that in associated normal, hyperplastic and metaplastic duct epithelium. Cancer. 1999;85:1703–1710. [PubMed] [Google Scholar]
- 10.Hruban RH, Adsay NV, Albores-Saavedra J, Compton C, Garrett ES, Goodman SN, Kern SE, Klimstra DS, Klöppel G, Longnecker DS, Lüttges J, Offerhaus GJ. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol. 2001;25(5):579–586. doi: 10.1097/00000478-200105000-00003. (May) [DOI] [PubMed] [Google Scholar]
- 11.Maire F, Micard S, Hammel P, Voitot H, Levy P, Cugnenc PH, Ruszniewski P, Puig PL. Differential diagnosis between chronic pancreatitis and pancreatic cancer: value of the detection of KRAS2 mutations in circulating DNA. Br J Cancer. 2002;87(5):551–554. doi: 10.1038/sj.bjc.6600475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lüttges J, Diederichs A, Menke MA, Vogel I, Kremer B, Klöppel G. Ductal lesions in patients with chronic pancreatitis show k-ras mutations in a frequency similar to that in the normal pancreas and lack nuclear immunoreactivity of p53. Cancer. 2000;88(11):2495–2504. doi: 10.1002/1097-0142(20000601)88:11<2495::aid-cncr10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 13.Lüttges J, Reinecke-Lüthge A, Möllmann B, Menke MAOH, Clemens A, Klimpfinger M, Sipos B, Klöppel G. Duct changes and k-ras mutations in the disease-free pancreas: analysis of type, age relation and spatial distribution. Virchows Arch. 1999;435(1):461–468. doi: 10.1007/s004280050428. [DOI] [PubMed] [Google Scholar]
- 14.Lindforss U, Papadogiannakis N, Zetterquist H, Lindberg G, Olivecrona H. Distribution of genetic variants in preneoplastic areas of colorectal tumours. Eur J Surg Oncol. 2003;29(6):491–496. doi: 10.1016/s0748-7983(03)00075-1. [DOI] [PubMed] [Google Scholar]
- 15.Löhr M, Maisonneuve P, Lowenfels AB. K-ras mutations and benign pancreatic disease. Int J Pancreatol. 2000;27:93–103. doi: 10.1385/IJGC:27:2:093. [DOI] [PubMed] [Google Scholar]
- 16.Heatley MK. Systematic review and meta-analysis in anatomic pathology. Histopathology. 2000;36(6):481–487. doi: 10.1046/j.1365-2559.2000.00943.x. (June) [DOI] [PubMed] [Google Scholar]
- 17.Lüttges J, Galehdari H, Brocker V, Schwarte-Waldhoff I, Henne-Bruns D, Klöppel G, Schmiegel W, Hahn SA. Allelic loss is often the first hit in the biallelic inactivation of the p53 and DPC4 genes during pancreatic carcinogenesis. Am J Pathol. 2001;158(5):1677–1683. doi: 10.1016/S0002-9440(10)64123-5. (May) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosty C, Geradts J, Sato N, Wilentz RE, Roberts H, Sohn T, Cameron JL, Yeo CJ, Hruban RH, Goggins M. p16 inactivation in pancreatic intraepithelial neoplasias (PanINs) arising in patients with chronic pancreatitis. Am J Surg Pathol. 2003;27(12):1495–1501. doi: 10.1097/00000478-200312000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Wilentz RE, Iacobuzio-Donahue CA, Argani P, McCarthy DM, Parsons JL, Yeo CJ, Kern SE, Hruban RH. Loss of expression of Dpc4 in pancreatic intraepithelial neoplasia: evidence that DPC4 inactivation occurs late in neoplastic progression. Cancer Res. 2000;60(7):2002–2006. (April 1) [PubMed] [Google Scholar]
- 20.Oxman AD. Checklists for review articles. BMJ. 1994;309(6955):648–651. doi: 10.1136/bmj.309.6955.648. (September 10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lüttges J, Klöppel G. Update on the pathology and genetics of exocrine pancreatic tumors with ductal phenotype: precursor lesions and new tumor entities. Dig Dis. 2001;18:15–23. doi: 10.1159/000050649. [DOI] [PubMed] [Google Scholar]
- 22.Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Ass. 1927;22:209–212. [Google Scholar]
- 23.Yanagisawa A, Ohtake K, Ohashi K, Hori M, Kitagawa T, Sugano H, Kato Y. Frequent c-ki-ras oncogene activation in mucous cell hyperplasias of pancreas suffering from chronic inflammation. Cancer Res. 1993;53:953–956. [PubMed] [Google Scholar]
- 24.Moore PS, Beghelli S, Zamboni G, Scarpa A. Genetic abnormalities in pancreatic cancer. Mol Cancer. 2003;2(1):7. doi: 10.1186/1476-4598-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Longnecker DS, Terhune PG. What is the true rate of k-ras mutation in carcinoma of the pancreas? Pancreas. 1998;17:323–324. doi: 10.1097/00006676-199811000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Tada M, Ohashi M, Shiratori Y, Okudaira T, Komatsu Y, Kawabe T, Yoshida H, Machinami R, Kishi K, Omata M. Analysis of K-ras gene mutation in hyperplastic duct cells of the pancreas without pancreatic disease. Gastroenterology. 1996;110:227–231. doi: 10.1053/gast.1996.v110.pm8536861. [DOI] [PubMed] [Google Scholar]
- 27.Berger DH, Chang H, Wood M, Huang L, Heath CW, Lehman T, Ruggeri BA. Mutational activation of K-ras in nonneoplastic exocrine pancreatic lesions in relation to cigarette smoking. Cancer. 1999;85:326–332. doi: 10.1002/(sici)1097-0142(19990115)85:2<326::aid-cncr9>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 28.Ellis I, Lerch MM, Whitcomb DC. Genetic testing for hereditary pancreatitis: guidelines for indications, counselling, consent and privacy issues. Pancreatology. 2001;1(5):405–415. doi: 10.1159/000055840. [DOI] [PubMed] [Google Scholar]
- 29.Yamano M, Fujii H, Takagaki T, Kadowaki N, Watanabe H, Shirai T. Genetic progression and divergence in pancreatic carcinoma. Am J Pathol. 2000;156(6):2123–2133. doi: 10.1016/S0002-9440(10)65083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Heek NT, Meeker AK, Kern SE, Yeo CJ, Lillemoe KD, Cameron JL, Offerhaus GJ, Hicks JL, Wilentz RE, Goggins MG, De Marzo AM, Hruban RH, et al. Telomere shortening is nearly universal in pancreatic intraepithelial neoplasia. Am J Pathol. 2002;161(5):1541–1547. doi: 10.1016/S0002-9440(10)64432-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goggins M, Hruban RH, Kern SE. BRCA2 is inactivated late in the development of pancreatic intraepithelial neoplasia: evidence and implications. Am J Pathol. 2000;156(5):1767–1771. doi: 10.1016/S0002-9440(10)65047-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maass N, Hojo T, Ueding M, Luttges J, Kloppel G, Jonat W, Nagasaki K. Expression of the tumor suppressor gene Maspin in human pancreatic cancers. Clin Cancer Res. 2001;7(4):812–817. [PubMed] [Google Scholar]
- 33.Moore PS, Sipos B, Orlandini S, Sorio C, Real FX, Lemoine NR, Gress T, Bassi C, Kloppel G, Kalthoff H, Ungefroren H, Lohr M, et al. Genetic profile of 22 pancreatic carcinoma cell lines. Analysis of K-ras, p53, p16 and DPC4/Smad4. Virchows Arch. 2001;439(6):798–802. doi: 10.1007/s004280100474. [DOI] [PubMed] [Google Scholar]
- 34.Real FX. A catastrophic hypothesis for pancreas cancer progression. Gastroenterology. 2003;124(7):1958–1964. doi: 10.1016/s0016-5085(03)00389-5. [DOI] [PubMed] [Google Scholar]
- 35.Iacobuzio-Donahue CA, Klimstra DS, Adsay NV, Wilentz RE, Argani P, Sohn TA, Yeo CJ, Cameron JL, Kern SE, Hruban RH. Dpc-4 protein is expressed in virtually all human intraductal papillary mucinous neoplasms of the pancreas: comparison with conventional ductal adenocarcinomas. Am J Pathol. 2000;157(3):755–761. doi: 10.1016/S0002-9440(10)64589-0. (September) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lüttges J, Neumann S, Jesnowski R, Borries V, Löhr M, Klöppel G. Lack of apoptosis in PanIN-1 and PanIN-2 lesions associated with pancreatic ductal adenocarcinoma is not dependent on K-ras status. Pancreas. 2003;27(4):e57–e62. doi: 10.1097/00006676-200310000-00021. [DOI] [PubMed] [Google Scholar]
- 37.Heinmoller E, Dietmaier W, Zirngibl H, Heinmoller P, Scaringe W, Jauch KW, Hofstadter F, Ruschoff J. Molecular analysis of microdissected tumors and preneoplastic intraductal lesions in pancreatic carcinoma. Am J Pathol. 2000;157(1):83–92. doi: 10.1016/S0002-9440(10)64520-8. (July) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hruban RH, Wilentz RE, Kern SE. Genetic progression in the pancreatic ducts. Am J Pathol. 2000;156(6):1821–1825. doi: 10.1016/S0002-9440(10)65054-7. (June) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Löhr M, Müller P, Schmidt C, Zauner I, Trautmann B, Thévenod F, Capellá G, Ibrahim S, Farré A, Liebe S, Jesnowski R. Immortalized bovine pancreatic duct cells become tumorigenic after transfection with mutant k-ras. Virchows Arch. 2001;438:581–590. doi: 10.1007/s004280100397. [DOI] [PubMed] [Google Scholar]
- 40.Hahn WC, Counter CM, Lundberg AS, Beijersbergen RL, Brooks MW, Weinberg RA. Creation of human tumor cells with defined genetic elements. Nature. 1999;400:464–468. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- 41.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, Kawaguchi Y, Johann D, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4(6):437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 42.Klein WM, Hruban RH, Klein-Szanto AJ, Wilentz RE. Direct correlation between proliferative activity and dysplasia in pancreatic intraepithelial neoplasia (PanIN): additional evidence for a recently proposed model of progression. Mod Pathol. 2002;15(4):441–447. doi: 10.1038/modpathol.3880544. [DOI] [PubMed] [Google Scholar]
- 43.Löhr M, Müller P, Mora J, Brinkmann B, Ostwald C, Plath F, Farré A, Lluis F, Adam U, Hopt UT, Barten M, Nizze H, et al. p53 and ras mutations in pancreatic juice samples of patients with chronic pancreatitis. Gastrointest Endosc. 2001;53:734–743. doi: 10.1067/mge.2001.112711. [DOI] [PubMed] [Google Scholar]
- 44.Group PIs Porta M, Malats N, Guarner L, Carrato A, Rifá J, Salas A, Corominas JM, Andreu M, Real FX. Association between coffee drinking and K-ras mutations in exocrine pancreatic cancer. J Epidemiol Community Health. 1999;53:702–709. doi: 10.1136/jech.53.11.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andea A, Sarkar F, Adsay VN. Clinicopathological correlates of pancreatic intraepithelial neoplasia: a comparative analysis of 82 cases with and 152 cases without pancreatic ductal adenocarcinoma. Mod Pathol. 2003;16(10):996–1006. doi: 10.1097/01.MP.0000087422.24733.62. [DOI] [PubMed] [Google Scholar]
- 46.Salaspuro V, Salaspuro M. Synergistic effect of alcohol drinking and smoking on in vivo acetaldehyde concentration in saliva. Int J Cancer. 2004;111:480–483. doi: 10.1002/ijc.20293. (DOI 10.002/ijc.20293) [DOI] [PubMed] [Google Scholar]
- 47.Kadlubar FF, Anderson KE, Haussermann S, Lang NP, Barone GW, Thompson PA, MacLeod SL, Chou MW, Mikhailova M, Plastaras J, Marnett LJ, Nair J, et al. Comparison of DNA adduct levels associated with oxidative stress in human pancreas. Mutat Res. 1998;405(2):125–133. doi: 10.1016/s0027-5107(98)00129-8. [DOI] [PubMed] [Google Scholar]
- 48.VanderVeen LA, Hashim MF, Shyr Y, Marnett LJ. Induction of frameshift and base pair substitution mutations by the major DNA adduct of the endogenous carcinogen malondialdehyde. Proc Natl Acad Sci USA. 2003;100(24):14247–14252. doi: 10.1073/pnas.2332176100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Casini A, Galli A, Pignalosa P, Frulloni L, Grappone C, Milani S, Pederzoli P, Cavallini G, Surrenti C. Collagen type I synthesized by pancreatic periacinar stellate cells (PSC) co-localizes with lipid peroxidation-derived aldehydes in chronic alcoholic pancreatitis. J Pathol. 2000;192(1):81–89. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH675>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 50.Alguacil J, Porta M, Kauppinen T, Malats N, Kogevinas M, Carrato A. Occupational exposure to dyes, metals, polycyclic aromatic hydrocarbons and other agents and K-ras activation in human exocrine pancreatic cancer. Int J Cancer. 2003;107(4):635–641. doi: 10.1002/ijc.11431. [DOI] [PubMed] [Google Scholar]
- 51.Lees NP, Harrison KL, Hall CN, Margison GP, Povey AC. Reduced MGMT activity in human colorectal adenomas is associated with K-ras GC→AT transition mutations in a population exposed to methylating agents. Carcinogenesis. 2004;25(7):1243–1247. doi: 10.1093/carcin/bgh111. [DOI] [PubMed] [Google Scholar]
- 52.Kaina B, Christmann M. DNA repair in resistance to alkylating anticancer drugs. Int J Clin Pharmacol Ther. 2002;40(8):354–367. doi: 10.5414/cpp40354. [DOI] [PubMed] [Google Scholar]
- 53.Klöppel G, Lüttges J. The pathology of ductal-type pancreatic carcinomas and pancreatic intraepithelial neoplasia: insights for clinicians. Curr Gastroenterol Rep. 2004;6(2):111–118. doi: 10.1007/s11894-004-0037-y. [DOI] [PubMed] [Google Scholar]
- 54.Motojima K, Urano T, Nagata Y, Shiku H, Tsurifune T, Kanematsu T. Detection of point mutations in the kirsten-ras oncogene provides evidence of the multicentricity of pancreatic carcinoma. Ann Surg. 1993;217:138–143. doi: 10.1097/00000658-199302000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tabata T, Fujimori T, Maeda S, Yamamoto M, Saitoh Y. The role of ras mutation in pancreatic cancer, precancerous lesions, and chronic pancreatitis. Int J Pancreatol. 1993;14:237–244. doi: 10.1007/BF02784932. [DOI] [PubMed] [Google Scholar]
- 56.Sugio K, Molberg K, Albores-Saavedra J, Virmani AK, Kishimoto Y, Gazdar AF. K-ras mutations and allelic loss at 5q and 18q in the development of human pancreatic cancers. Int J Pancreatol. 1997;21:205–217. doi: 10.1007/BF02821606. [DOI] [PubMed] [Google Scholar]
- 57.Z'graggen K, Rivera JA, Compton CC, Pins M, Werner J, Fernández-del Castillo C, Rattner DW, Lewandrowski KB, Rustgi AK, Warshaw AL. Prevalence of activating k-ras mutations in the evolutionary stages of neoplasia in intraductal papillary mucinous tumors of the pancreas. Ann Surg. 1997;226:491–500. doi: 10.1097/00000658-199710000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matsubayashi H, Watanabe H, Yamaguchi T, Ajioka Y, Nishikura K, Iwafuchi M, Yamano M, Kijima H, Saito T. Multiple K-ras mutations in hyperplasia and carcinoma in cases of human pancreatic carcinoma. Jpn J Cancer Res. 1999;90(8):841–848. doi: 10.1111/j.1349-7006.1999.tb00825.x. (August) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Terhune PG, Phifer DM, Tosteson TD, Longnecker DS. K-ras mutation in focal proliferative lesions of human pancreas. Cancer Epidemiol Biomark Prev. 1998;7:515–521. [PubMed] [Google Scholar]
- 60.Mulligan NJ, Yang S, Andry C, Klein M, O'Brien MJ. The role of p21ras in pancreatic neoplasia and chronic pancreatitis. Hum Pathol. 1999;30(6):602–610. doi: 10.1016/s0046-8177(99)90082-5. [DOI] [PubMed] [Google Scholar]
- 61.Song MM, Nio Y, Sato Y, Tamura K, Furuse K. Clinicopathological significance of ki-ras point mutation and p21 expression in benign and malignant exocrine tumors of the human pancreas. Int J Pancreatol. 1996;20(2):85–93. doi: 10.1007/BF02825506. [DOI] [PubMed] [Google Scholar]
- 62.Rivera JA, Rall CJN, Graeme-Cook F, Fernández-del Castillo C, Shu P, Lakey N, Tepper R, Rattner DW, Warshaw AL, Rustgi AK. Analysis of k-ras oncogene mutations in chronic pancreatitis with ductal hyperplasia. Surgery. 1997;121(1):42–49. doi: 10.1016/s0039-6060(97)90181-1. [DOI] [PubMed] [Google Scholar]
- 63.Agoff SN, Crispin DA, Bronner MP, Dail DH, Hawes SE, Haggitt RC. Neoplasms of the ampulla of water with concurrent pancreatic intraductal neoplasia: a histological and molecular study. Mod Pathol. 2001;14(3):139–146. doi: 10.1038/modpathol.3880270. [DOI] [PubMed] [Google Scholar]