Abstract
Replication-competent oncolytic adenoviruses hold considerable promise for treating malignant gliomas. The toxicity of the clinically tested E1B-55 kDa mutant virus is negligible; however, its full clinical potential is still being evaluated. The purpose of the present study is to compare the antiglioma activity in vitro and in vivo between Delta-24, an E1A mutant adenovirus, and RA55, an E1B-55 kDa mutant adenovirus. We selected human glioma cell lines that were tumorigenic in nude mice and express wild-type p53 (U-87 MG, D54 MG) or mutant p53 (U-251 MG, U-373 MG) protein. Our studies demonstrated that Delta-24 induced a more potent antiglioma effect in vitro than RA55. Moreover, Delta-24 replicated markedly more efficiently than RA55 in both wild-type and mutant-p53 scenarios. Importantly, direct intratumoral injection of Delta-24, but not RA55, significantly suppresses tumor growth in intracranial (U-87 MG, U-251 MG) or subcutaneous (D54 MG) animal models. Staining for hexon protein detected replicating adenoviruses in xenografts infected with Delta-24, but not with RA55. Collectively, these data indicate that E1A mutant adenoviruses targeting the Rb pathway are more powerful putative agents for antiglioma therapy than E1B mutant adenoviruses, and suggest that E1A mutant adenoviruses should be tested in the clinical setting for patients with malignant gliomas.
Keywords: Glioma, oncolytic adenovirus, E1A, E1B, fiber protein
Introduction
Glioblastoma is the most common and most malignant form of glioma. These tumors are locally invasive and diffusely infiltrating, thus precluding complete surgical removal. Malignant gliomas are also notoriously resistant to chemotherapy and radiotherapy [1]. For these reasons, it is imperative to design novel strategies, capitalizing on genetic anomalies of brain tumors to develop more rational and efficacious therapies. Two of the best-studied abnormalities of glioblastomas involve the regulation of Rb and p53 proteins. The Rb pathway is abnormal in most malignant gliomas [1]. However, p53 mutation is only found in a subset of malignant gliomas—secondary glioblastoma [2]. Although mutations of the p53 gene are found in only a minority of these tumors (< 50%), other players in the p53 pathway, including mdm2 and p14ARF, are abnormal in wild-type p53 tumors as well [1,2]. Replication-competent adenoviruses have been designed to take advantage of fundamental genetic defects of cancer cells to preferentially replicate in and kill cancer cells [3,4]. Although the results have not been published yet, oncolytic adenoviruses targeting p53 have recently been investigated for their suitability in the treatment of glioma in phase I clinical trials. Adenoviruses targeting Rb are second-generation agents that are currently being examined in preclinical studies [5].
The dl1520 adenovirus is the first replication-competent adenovirus developed as an anticancer tool [6]. The genome of this mutant adenovirus encompasses a 827-bp deletion in its E1B region and a point mutation that generates a stop codon, which prevents the expression of p53-binding E1B-55 kDa protein [7]. The underlying theory and characterization of dl1520 opened up a new field of gene therapy for cancer. It was initially claimed that the dl1520 adenovirus demonstrated preferential replication as well as antitumoral efficacy in some p53-deficient human tumor cells [6,8]. However, in human malignant glioma xenografts, dl1520 produced cell lysis and antitumor activity independently of p53 status [9], which could be explained if dl1520 targets the p53 pathway rather than the p53 protein [10]. Phase I and phase II clinical trials showed that dl1520 was well tolerated at the highest practical doses [11]. Unfortunately, when used as single-agent antitumor treatment, dl1520 did not induce a dramatic anticancer effect in most of the treated patients [11,12]. Apparently, efficacy rather than toxicity is the primary limitation of this virus, so that improvements in the oncolytic potency of newer constructs will be required to generate an optimal therapeutic effect.
We previously reported on the anticancer effect of Delta-24, an E1A mutant oncolytic adenovirus [13] whose adenoviral genome encompasses a deletion of 24 bp encoding for the Rb-binding region of the E1A protein (amino acids 122–129). With this genetic modification, Delta-24 lost the capability to acquire an efficient replication phenotype in quiescent normal cells, but its ability to replicate in cancer cells, whose cell cycles are dysregulated, is not impaired [13]. The differential pattern of replication in normal versus cancer cells suggests that Delta-24 selectively induces lytic activity in glioma cells while sparing postmitotic quiescent normal brain cells. Studies from our laboratory and others have demonstrated the anticancer effect of Delta-24 in vitro and in vivo [5,13–17].
This is the first study that compares the antiglioma efficacy of Delta-24 and an E1B-55 kDa mutant adenovirus RA55, which is functionally similar to dl1520. Our data demonstrate that Delta-24 induces a more potent antiglioma effect than RA55. Thus, in vitro studies of cell viability and viral replication show that Delta-24 replicates and induces cytopathic effect (CPE) in glioma cell lines with higher efficiency than RA55. Moreover, in a series of in vivo experiments, we showed that Delta-24 induces more efficacious antiglioma activity than RA55.
Materials and Methods
Cell Lines and Adenoviruses
The U-87 MG (expressing wild-type p53), U-373 MG, and U-251 MG (both expressing mutant p53) [18] human glioma cell lines were purchased from the American Type Culture Collection (Rockville, MD). D54 MG cells (expressing wild-type p53) were generously provided by Dr. Darell Bigner (Duke University, Durham, NC) [18]. The cells were cultured in DMEM/F12 supplemented with 10% FBS (Hyclone Laboratories, Inc., Logan, UT), 100 µg/ml penicillin, and 100 µg/ml streptomycin (Invitrogen, Carlsbad, CA). 293 cells were obtained from Qbiogene, Inc. (Carlbad, CA), which were maintained in DMEM supplemented with 10% FBS, 100 µg/ml penicillin, and 100 µg/ml streptomycin. The cells were cultured at 37°C in a humidified atmosphere of 5% CO2.
The E1B-55 kDa deletion in the RA55 virus was accomplished by deleting a fragment ranging from the Sau3AI restriction site to the BglII restriction site (2426–3328 bp, both included) in pXC1 (Microbix Biosystems, Inc., Ontario, Canada), resulting in pXC1-RA55. This deletion eliminates the E1B p53-binding domain [19], sparing the E1B-19K region. The pXC1-RA55 shuttle vector was cotransfected with pBHG10 (Microbix Biosystems, Inc.) into 293 cells to generate the RA55 construct through homologous recombination.
The construction of Delta-24 has been previously described [13,20]. As controls, we used the wild-type adenovirus Ad300 [20], viruses inactivated by exposure to seven cycles of 125J UV light in a GS Gene Linker UV Chamber from BioRad (Hercules, CA), and mock infections with culture medium. The viruses were grown in 293 cells and purified by CsCl gradient centrifugation, as previously described [21].
Polymerase Chain Reaction (PCR)
The E1B deletion was confirmed by PCR using sense (5′-GAATGAATGTTGTACAGGTGGCT-3′) and antisense (5′-AGGAAAACCGTACC GCTAAAATTG-3′) primers to amplify a 536-bp region of the wild-type E1B gene. Thirty-five thermal cycles were performed using the following conditions: 94°C for 30 seconds, 50°C for 30 seconds, 72°C for 1 minute. Samples were analyzed using 1% agarose (Sigma, St. Louis, MO) gel electrophoresis.
Cell Viability Assays
The cells used for crystal violet assays were seeded in six-well plates at 5 x 104 cells/well and infected 24 hours later with adenoviruses at the desired multiplicity of infection (MOI). We concluded the experiment when an MOI of 10 of one of the viruses produced more than a 75% CPE compared to mock or UV-inactivated viral treatment. The cells were then stained with 0.1% crystal violet in 20% methanol. Briefly, the medium was removed, and the cells were fixed and stained by the crystal violet solution for 3 minutes. After staining, the crystal violet solution was removed, and the cells were rinsed with water and then air-dried.
In vitro cytotoxicity was quantified using an MTT (Sigma) assay to measure cell viability. For this assay, 2 x 103 cells/well were seeded in 96-well plates and infected 24 hours later with 0, 4, 8, or 16 MOI of Delta-24 or RA55, or 16 MOI of UV-inactivated viruses, or cells were mock-infected. Eight wells were used for each condition. A total of eight wells was seeded with untreated glioma cells as a viability control, and eight wells containing only complete medium were used as a control for a nonspecific dye reduction. Medium was removed 6 days after treatment, and 100 µl/well MTT solution (2 mg/ml in DMEM/F12 medium) was added to each well. The plates were incubated for an additional 4 hours. After removing the medium, 200 µl of 10% sodium dodecyl sulfate (SDS) was added to each well. The plates were incubated for an additional 24 hours and then read on a microplate reader at a test wavelength of 570 nm.
Viral Replication Assays
Human glioma cells were seeded at 5 x 104 cells/well in six-well plates and, 24 hours later, infected with Delta-24, RA55, wild-type adenovirus Ad300, or UV-inactivated Ad300 at an MOI of 10. After 48 hours of infection, the cells were scraped into 2 ml of culture medium and lysed with three cycles of freezing (dry ice-ethanol bath) and thawing (37°C water bath). The TCID50 method was used to determine the final viral titration. Briefly, the cell lysates were clarified by centrifugation and the supernatants were serially diluted in medium to infect 293 cells in 96-well plates. The cells were analyzed for CPE 10 days after infection. Final titers were determined as plaque-forming units (pfu) according to the validation method developed by Qbiogene, Inc.
Immunoblotting
Glioma cells were collected and resuspended in PBS plus protease inhibitor cocktail (Sigma) and then lysed by adding an equal volume of 2 x SDS loading buffer. Afterward, the lysates were heated at 95°C for 10 minutes. Equal amounts of proteins from the lysates were separated by SDS polyacrylamide gel electrophoresis (PAGE) and probed with the following antibodies: adenovirus 5 E1B-55K (Oncogene, Cambridge, MA), E1B-19K (Ab-1; Oncogene), viral fiber protein (4D2; NeoMarkers, Inc., Fremont, CA), adenovirus 2 E1A (13 S-5), and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA). The secondary antibodies were horseradish peroxidase-conjugated antigoat, antirat, or antimouse IgG antibodies (Santa Cruz Biotechnology). Finally, the protein bands were visualized using an ECL Western Blotting Detection System (Amersham Pharmacia Bioechnology, Piscataway, NJ). Protein expression was quantified by densitometric analysis with the Scion Image Beta 4.02 Win computer software (available at http://www.scioncorp.com/frames/fr_scion_products.htm).
Animal Studies
For the intracranial glioma model, 5 x 105 cells of human glioma U-87 MG or U-251 MG cells were engrafted into the caudate nucleus of athymic mice, using a guide screw system as previously described [22]. Animals with U-87 MG-derived xenografted tumors were treated with virus on days 3, 5, and 8 after implantation of tumor cells. Animals with U-251 MG xenografts were treated with a single dose of virus on day 3 postimplantation of tumor cells. The animals were treated with intratumoral injections of 5 µl of one of the following: Delta-24, RA55, or UV-inactivated adenovirus. A dose of 1.5 x 108 pfu was used for each viral injection. Animals displaying general or localized symptoms of toxicity were sacrificed. The surviving animals were euthanized at 170 or 63 days after being engrafted with U-87 MG or U-251 MG human glioma cells, respectively. The removed brains were fixed in formaldehyde for 24 hours and embedded in paraffin. Hematoxylin-and eosin-stained slides were evaluated for the presence of tumor and viral nuclear inclusions.
For the subcutaneous glioma model, 107 D-54 MG cells were inoculated subcutaneously into the right flanks of 6- to 8-week-old athymic mice. When tumors reached at least 5 mm in diameter, they were injected with 109 pfu of Delta-24, RA55, or UV-inactivated adenovirus, every 5 to 7 days, a total of four times. Mice were euthanized when tumors reached the maximum acceptable size. The animal studies were carried out in the veterinary facilities of the University of Texas M. D. Anderson Cancer Center in accordance with institutional guidelines.
Immunohistochemistry
The presence of adenoviral hexon proteins in the treated xenografts was assessed by immunohistochemistry. Paraffin-embedded sections from mouse tumors were deparaffinized and rehydrated through xylene and ethanol in PBS. Endogenous peroxidase activity was quenched by incubation for 30 min in a solution of 0.3% H2O2 in methanol. Sections were probed with goat antihexon (Chemicon, Inc., Temecula, CA). Immunohistochemical staining was performed with Vector Laboratories ABC kits according to the manufacturer's instructions (Amersham Biosciences).
Statistical Analyses
For the in vitro experiments and the analysis of anticancer effect of the different constructs in the subcutaneous animal model, we performed two-tailed Student's t test. Data are represented as mean ± SD. The in vivo anticancer effect of different treatments in the intracranial animal model was assessed by plotting survival curves according to the Kaplan-Meier method, and groups were compared using the log-rank test.
Results
The E1A Mutant Delta-24 and the E1B-55 kDa Mutant RA55 Adenoviruses
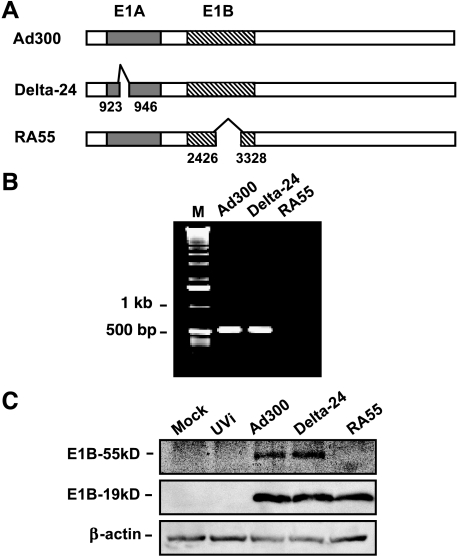
Previously, we reported on the generation and characterization of a tumor-selective adenovirus, Delta-24, which has a 24-bp deletion in the E1A region that encodes the Rb protein-binding site [13]. Immunoprecipitation analyses verified that with this deletion, Delta-24 was unable to bind the Rb protein. Viral titration experiments in 293 cells demonstrated that the Delta-24 adenovirus could replicate in and lyse cancer cells with great efficiency [13]. To construct RA55, we modified the genome of wild-type adenovirus by deleting a 903-bp region in the E1B gene (Figure 1A), which abrogated the p53-binding region of the E1B-55 kDa protein [19]. We confirmed the deletion by PCR amplification of the E1B gene using a downstream primer complementary to the sequence within the deleted region. The resulting 536-bp PCR fragment could not be amplified from the RA55 genome, although it was present when wild-type or Delta-24 genome was used as a template (Figure 1B). Sequencing the modified E1B region confirmed the deletion (data not shown). We next examined the ability of RA55 to express the wild-type E1B-55 kDa protein, but immunoblotting assays showed that RA55-infected cells did not express E1B-55 kDa protein (Figure 1C), and that the expression of E1B-19 kDa protein was not affected by the deletion (Figure 1C). Therefore, the deletion in the RA55 adenovirus results in the abolition of the wild-type E1B-55 kDa protein maintaining an intact E1B-19 kDa protein, rendering RA55 functionally similar to other E1B-55 kDa mutant adenoviruses such as d11520.
Figure 1.
The RA55 adenovirus. (A) Shown are nonscaled schematic representations of the genomes of wild-type adenovirus Ad300, Delta-24, and RA55. The nucleotides deleted in the E1A and E1B sequences are indicated. E1A, solid box; E1B, hatched box. (B) PCR amplification of E1B confirmed the deletion in the RA55 adenovirus. The antisense primer was complementary to the deleted sequence of E1B as described in Materials and Methods section. A fragment of the expected size of 536 nucleotides was amplified from the genome of Ad300 and Delta-24 adenoviruses, but no amplification was detected when RA55 was used as template. PCR products were analyzed in a 1% agarose gel. M, 1-kb ladder. (C) Immunoblot documenting the expression of E1B proteins in cells infected with RA55. Equal amounts of protein from the virus-treated cells were separated on a 10% SDS-PAGE gel. E1B-55 kDa and E1B-19 kDa were detected with specific antibodies with β-actin (42 kDa) expression as a loading control. UVi, UV-inactivated wild-type adenovirus.
Delta-24 and RA55 Induced Antiglioma Effect In Vitro
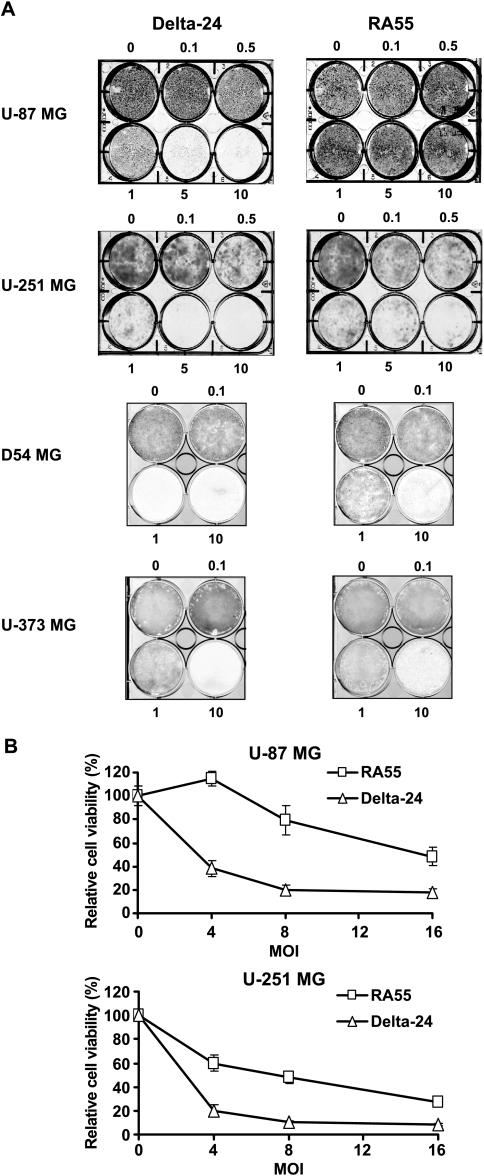
To compare the antiglioma effect of Delta-24 and RA55, we selected the U-87 MG and D54 MG glioma cell lines, which express wild-type p53 protein [18], and U-251 MG and U-373 MG glioma cell lines, which express mutant p53 protein [18]. For cell viability assays, we infected cell cultures with both viruses as single agent. Crystal violet assessment of cell death revealed that Delta-24 induced a CPE greater than 80% in U-87 MG cells at a dose of 5 MOI, 15 days after infection. At that time point, RA55-mediated anticancer effect was 10-fold less potent. Similarly, Delta-24 treatment of D54 MG cultures produced, 7 days after infection, a 10-fold increase in cell lysis compared to RA55. When the oncolytic constructs were tested on U-251 MG or U-373 MG cell lines (7 and 8 days after infection, respectively), Delta-24 produced a CPE greater than 90% at a dose of 10 MOI. In these cell lines, Delta-24 was five-fold more potent than RA55 in inducing cell lysis. Overall, the anticancer effect of Delta-24 was greater than RA55. This effect was most evident in the wild-type p53 cell lines (Figure 2A).
Figure 2.
Antiglioma effect of Delta-24 and RA55 adenovirus in vitro. (A) Crystal violet assessment of cell viability. Monolayers of human glioma cells were infected at the MOI indicated with RA55 or Delta-24. Viable cells were stained with crystal violet when 10 MOI of Delta-24 produced more than 75% of the CPE. (B) MTT colorimetric analysis of cell viability. Cells were infected at different MOI of 0–16 pfu/cell of Delta-24 or RA55, or with 16 MOI of UV-inactivated wild-type adenovirus. Cell viability was measured by MTT assay 6 days after infection. Data are mean ± SD of eight determinations, and are represented as cell viability relative to UV-inactivated adenovirus-treated cells (equal to 100%). Note that RA55 induces a more powerful antiglioma effect in U-251 MG than in U-87 MG cells.
To quantify the CPE, we performed MTT analyses of cell viability, which showed that the dose required to induce a 50% decrease in cell viability (IC50) was significantly lower in cells infected with Delta-24 adenovirus. In U-87 MG cell cultures, the IC50 of Delta-24 and RA55 was approximately 3 and 16 MOI, respectively (Figure 2B), whereas the IC50 of Delta-24 and RA55 in U-251 MG cell cultures was approximately 2 and 7 MOI, respectively (Figure 2B).
Therefore, we conclude that although both mutant adenoviruses induce cell lysis, Delta-24 displays a significantly more powerful antiglioma effect in vitro than RA55.
Delta-24 Replicated with Higher Efficiency Than RA55 in Glioma Cells
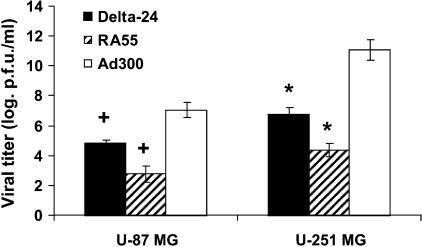
Because Delta-24 and RA55 are both replication-competent adenoviruses, we wanted to determine if they were each able to acquire a replication phenotype in human glioma cells. Using a TCID50 method to titer the production of new viral progeny, we observed that Delta-24 replicated by at least two orders of magnitude more efficiently than RA55 in both U-87 MG and U-251 MG cells (P < .001, in the two cell lines, t-test, double-sided) (Figure 3). Importantly, the RA55 titers obtained from the glioma cultures were inferior to the inoculum dose (5 x 105 pfu), showing that RA55 replicated with suboptimal efficiency. These results are consistent with observations of the poor replication of another mutant E1B-55K adenovirus, dl1520, in U-87 MG cells. Bischoff et al. [6] and Kirn [11] reported that dl1520 was unable to replicate in vitro and in vivo in U-87 MG cells. These data hold promise for the use of Delta-24 as an oncolytic agent in tumors that are resistant to the oncolytic effect of dl1520. An important caveat of these findings is that the difference between these two viruses in replication was greater in cells with wild-type p53 than in those with mutant p53. Our data corroborate previous studies showing that E1B-55 kDa mutant adenoviruses replicate with more efficiency in p53-mutant than in p53-wild-type cancer cells [6,11]. Confirming the fact that modifying the genome attenuated viral production, both mutant viruses replicated less efficiently in both glioma cultures than wild-type adenovirus (Figure 3). The differences in the ability of all three adenoviruses to replicate in the two glioma cell lines are probably explained by the differential expression of the CAR adenoviral receptor in U-87 MG (low expression) and U-251 MG (high expression) cell lines [5].
Figure 3.
Replication efficiency of Delta-24 and RA55 in human glioma cell lines. Forty-eight hours after infection with Ad300, Delta-24, or RA55 at 10 MOI, the viral titers were determined using a TCID50 procedure in 293 cells as described in Materials and Methods section. Viral titer is expressed as logarithmic scale of pfu per milliliter (mean ± SD). Both mutant viruses caused attenuated replication phenotypes compared to wild-type adenovirus. Of importance, Delta-24 replicated significantly better than RA55 in both glioma cell lines. *P < .001, t-test, double-sided; +P < .001, t-test, double-sided.
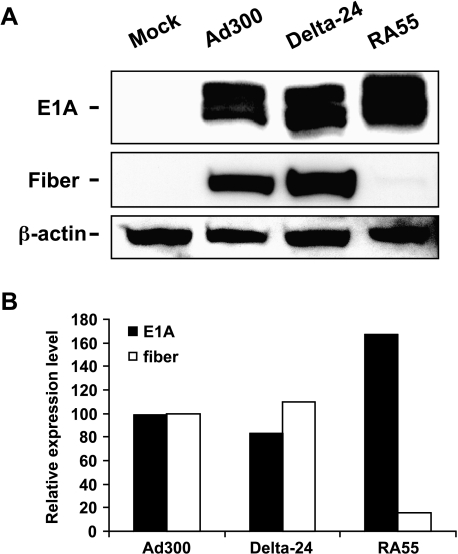
RA55-Infected Cells Showed Abnormal Expression of E1A and Fiber Proteins
Previous studies examining E1B-mediated regulation of E1A show that E1A is expressed at higher levels in the absence of E1B [23,24]. Furthermore, E1B-55 kDa reportedly promotes the exportation of viral mRNA, but inhibits the exportation of most cellular mRNA species [25]. These findings suggest that deleting E1B-55 kDa will result in a reduced level of viral replication due to the expression of a disproportionately high level of E1A (due to the lack of the E1B-mediated suppressive effect on E1A expression) and a low level of other viral proteins (due to poor nuclear mRNA export). In this work, we intended to determine if these factors impaired the replication phenotype of RA55. Examination of the expression of early and late viral genes in the cells infected with RA55, Delta-24, or wild-type adenoviruses revealed that, 24 hours after infection, the level of E1A protein was very similar in cells infected with wild-type and Delta-24 adenoviruses (Figure 4). Interestingly, cells infected with RA55 consistently had higher levels of E1A protein (Figure 4). In addition, we found high levels of adenoviral fiber protein, a structural gene expressed at the late stage of viral replication, in cells infected with either wild-type or Delta-24, whereas the expression of fiber protein was barely detectable in RA55-infected cells (Figure 4). These findings demonstrate an abnormal level of expression of adenoviral genes in the E1B-55 kDa mutant adenovirus and are consistent with the requirement for wild-type E1B protein to produce efficient adenoviral replication [25]. These data also indicate that, in addition to impairing the ability of the virus to disable p53, deleting E1B-55 kDa alters normal viral gene expression, thus possibly contributing to diminishing viral replication in infected glioma cells.
Figure 4.
Expression of adenoviral E1A and fiber proteins. (A) Cell lysates from Ad300, Delta-24, and RA55 at a dose of 50 MOI, or mock-infected U-251 MG cells were collected 24 hours after infection. Equal amount of proteins were separated on a 10% SDS-PAGE gel. E1A (35–46 kDa) and fiber protein (62 kDa) were detected with specific antibodies. β-Actin is shown as a loading control. Note that in contrast to lysates from wild-type adenovirus (Ad300) or Delta-24-infected cells, analyses of lysates from RA55-infected cells revealed a higher expression of E1A protein, whereas fiber protein was almost undetectable. (B) A quantitative representation of the expression of proteins. The amounts of E1A and fiber protein were quantified with densitometric analysis and normalized with the corresponding β-actin level. The relative protein level of E1A or fiber expressed by Ad300 is equal to 100%.
Antiglioma Efficacy of Delta-24 and RA55 In Vivo
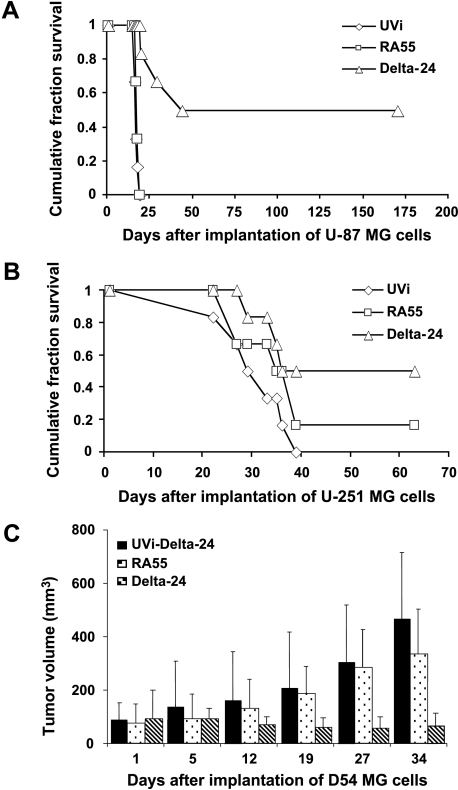
Finally, we examined the antiglioma effect of Delta-24 and RA55 adenoviruses in two intracranial glioma mouse models and one subcutaneous glioma mouse model. In the intracranial experiments, we injected U-87 MG or U-251 MG cells into the right basal ganglia of athymic mice through a guide screw implanted in the mouse skull. This screw-guided surgical model allowed repeated intratumoral injection of the viruses [22], a technique that greatly improved the reproducibility of the procedure. In the U-87 MG glioma model (with low CAR expression and containing wild-type p53 protein), the mice were injected with three doses of viruses on days 3, 5, and 8 after intracranial implantation of glioma cells. Treatment with Delta-24 largely improved survival compared to control-treated and RA55-treated groups (Figure 5A; n = 6, P < .001). Importantly, 50% of the Delta-24-treated mice, but none of the mice included in the other two groups, was tumor-free before being selectively euthanized on day 170 (Figure 5A). However, the survival of glioma-bearing mice treated with RA55 was not markedly improved compared to control animals treated with an inactivated adenovirus (Figure 5A; n = 6, P = .2).
Figure 5.
Anticancer effect in vivo. (A and B) Data are represented as a Kaplan-Meier survival curve from the day of U-87 MG (A) or U-251 MG (B) intracranial implantation, followed by intratumoral injection with RA55, Delta-24, or UV-inactivated adenovirus (UVi). The P values (determined by log-rank test) show significant differences in survival between Delta-24 (P < .05), but not RA55, and control-treated animals in those glioma animal models. (C) Representation of the growth of D54 MG-derived tumors implanted subcutaneously and treated with the indicated constructs. The largest (a) and smallest (b) diameters of each tumor were measured, and tumor volume was calculated using the formula: ab2 x 0.4 [31]. The P values (determined by two-tailed Student's t test) show significant differences in tumor volume between Delta-24-treated tumors, but not RA55, and control-treated tumors (P < .02).
We next tested the efficacy of RA55 and Delta-24 in the U-251 MG xenograft animal model. In this model, we injected U-251 MG glioma (expressing high levels of CAR and a mutant p53 protein) with a single dose of each virus on day 3 after intracranial implantation of glioma cells. We observed that Delta-24 significantly improved the survival of the glioma-bearing mice compared to the control group (Figure 5B; n = 6, P < .05). Conversely, mice treated with RA55 did not demonstrate significantly improved survival compared with the controls (Figure 5B; n = 6, P = .22). Importantly, 50% of the Delta-24-treated mice survived with no symptoms until they were selectively euthanized on day 63 postimplantation (Figure 5B). One of the mice in the group treated with RA55 survived without showing any symptoms for more than 60 days (Figure 5B).
To further evaluate the anticancer effect of the mutant E1A and E1B-55 kDa in vivo, we produced D54 MG-derived (high CAR/wild-type p53) subcutaneous tumors in nude mice. Tumors were treated with Delta-24, RA55, or UV-inactivated adenovirus (n = 6, per group) at a dose of 109 pfu, every 5 to 7 days, for a total of four doses. Delta-24 produced an inhibition in the tumor growth of > 70% within 19 days after cell implantation, and > 85% at the end of the experiment, relative to those tumors treated with UV-inactivated adenovirus (P < .02). The effect of RA55 treatment on tumor growth was not significant compared to the control-treated tumors (P > 0.2) (Figure 5C).
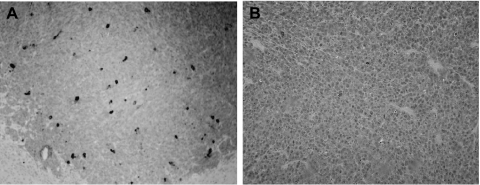
To detect adenoviral replication in vivo, we examined sections of brains where tumor tissue remained. Examination of hematoxylin and eosin-stained sections revealed a greater number of inclusion bodies, compatible with adenoviral infection and replication, in Delta-24-treated U-87 MG tumor cells than in RA55-treated cells (data not shown). In addition, immunohistochemical staining of tumor specimens showed that the expression of hexon protein, a late structural protein (and indicator of adenoviral replication), was scattered throughout the tumor in the Delta-24-treated glioma xenografts (Figure 6A) and a paucity of hexon-positive cells in RA55-treated tumors (Figure 6B). These data further confirmed the fact that Delta-24 replicated with greater efficiency than RA55 in vivo in U-87 MG glioma cells. Taken collectively, our data confirm the minimal ability of E1B-55 kDa mutants to replicate in cells with wild-type p53, and suggest that the Delta-24 adenovirus targeting the Rb pathway and expressing an intact E1B protein could be successfully used in tumors that are resistant to the anticancer effect of first-generation E1B mutant oncolytic viruses.
Figure 6.
Replication of Delta-24 and RA55 in intracranial U-87 MG human glioma xenografts. (A) Immunohistochemical analyses of hexon protein expression in a section of a U-87 MG xenograft infected with Delta-24 from a mouse that died 20 days after implantation. The adenoviral structural hexon protein is detected in cancer cells, indicating adenoviral replication in vivo (original magnification, x100). (B) Section of a U-87 MG xenograft treated with RA55 from a mouse that died 19 days after implantation stained for antihexon protein (original magnification, x100). Cells are nearly negative for the presence of viral hexon protein after immunostaining and adenoviral replication is not evident.
Discussion
In this study, we examined and compared the antiglioma activities of the E1A mutant Delta-24 adenovirus with the E1B-55 kDa mutant RA55 adenovirus in in vitro and in vivo settings. Our data showed that Delta-24 replicated more efficiently than RA55 in, and accordingly induced, a greater CPE in a panel of glioma cell lines. Delta-24 was an effective agent as antiglioma treatment in vivo in glioma cultures expressing wild-type or mutant p53. Treatment with RA55 resulted in an antiglioma effect, albeit not statistically significant, in mice bearing xenografts expressing mutant p53 protein. In human glioma xenografts expressing wild-type p53, the effect was similar to an inactive adenoviral control. Analyses of virus-treated tumor sections showed that Delta-24 replicated efficiently in glioma xenografts. In contrast, the replication of RA55 was hardly detectable, strongly suggesting that suboptimal replication of RA55 in vivo caused the reduced anticancer effect.
Our data are consistent with previous observations that E1B-55 kDa mutants such as dl1520, which are functionally similar to RA55, do not acquire a replication phenotype and do not induce an antiglioma effect in vitro or in vivo in a U-87 MG glioma cell line [6]. In addition, we observed that RA55 replicated with a higher efficiency in the mutant p53 cell line, U-251 MG. These results corroborate the tenet that E1B-55 kDa mutants target the p53 pathway and are best able to replicate in cells that express mutant p53 protein or that have an inactive p53 pathway [6].
Although the validity of the above results should, of course, be limited to the glioma cell lines tested here and to the particular mutant viruses used in the study, our results suggest that E1A mutant adenoviruses that target the Rb pathway are more potent anticancer agents than E1B-55 kDa mutants. These results are in agreement with previous reports in other cancers [26,27]. Our study is the first in vivo comparison between E1B-55 kDa and E1A mutant oncolytic adenoviruses in both intracranial and subcutaneous models of glioma. Our data suggest that tumors that are resistant to the oncolytic effect of E1B-55 kDa mutant adenoviruses might instead be sensitive to the effect of E1A mutant adenoviruses. This concept, if proven true, could have clinical relevance for patients who have been unsuccessfully treated with dl1520. Although the results of a phase I open label dose escalation trial with the E1B-attenuated adenovirus, dl1520, for patients with recurrent malignant brain tumors who are undergoing surgical resection are still pending for publication, early reports have suggested that when dl1520 is used as a single agent for treating some cancers, no dramatic antitumor effect has been produced [11]. The effect of dl1520 was, however, reported to be more beneficial when it was combined with chemotherapy [28]. Thus, further preclinical studies are needed to exploit a more effective oncolytic adenoviral therapy for gliomas. Interestingly, Delta-24 with the RGD motif in the fiber protein shows enhanced antiglioma activity [5,29]. Moreover, and of clinical relevance, it has been elegantly demonstrated that the antiglioma effect of Delta-24 RGD is further potentiated by radiotherapy [29].
Adenoviruses have evolved as a perfect adaptable genome in which the interrelation between different adenoviral proteins is critical for their efficient replication. One of the main principles used in developing an oncolytic virus is to carefully manipulate the adenoviral genome to eliminate the ability of the virus to activate normal host cells to enter into the S-phase or to block altruistic apoptosis in normal cells, while promulgating a relatively unscathed capacity for viral replication in cancer cells, thereby improving selectivity without sacrificing efficacy. This theory suggests that, to maintain therapeutic potency, oncolytic adenoviruses need to be generated by introducing only minor changes in the adenoviral genome to keep as many of the normal adenoviral protein functions as intact as possible. Accordingly, the Delta-24 genome encompasses a deletion of only 24 nucleotides in the E1A region. This small deletion is, however, sufficient to prevent binding to and inactivation of the Rb protein, but it has little effect on the functions of the E1A protein that are necessary for effective viral replication in cancer cells [13]. In contrast, the deletion in E1B-55 kDa mutant adenoviruses is larger and eliminates almost all of the functions of the E1B-55 kDa protein. Thus, the deletion in RA55 not only prevents the binding to and inactivation of p53 by E1B-55 kDa protein, but also eliminates other functions such as downmodulating E1A protein, promoting viral mRNA transport, and suppressing host cellular protein synthesis [24,25]. All of these E1B functions are key components of the adenoviral replication cycle. One of the direct consequences of the E1B-55 kDa deletion is that E1A is overexpressed. The subsequent induction of E1A-mediated apoptosis results in suboptimal adenoviral replication [30]. These observations suggest that E1B-55 kDa mutant adenoviruses whose mutations are limited to the region responsible for interacting with p53, but whose other critical functions are preserved, might be more efficacious in acquiring a replication phenotype in p53 mutant cancer cells than adenoviruses with a total abrogation of E1B-55 kDa protein. In reality, the mutation of a single amino acid is enough to generate an E1B-55 kDa mutant that is unable to bind to and inactivate p53 [19]. Further experiments are required to test the anticancer effect of these mutants in gliomas and to pursue a systematic comparison of minimally modified E1B-55 kDa mutant versus E1A mutant adenoviruses.
Taken together, our results show that Delta-24 and RA55 induce antiglioma effect in vitro and in vivo. Our data also suggest that treatment with Delta-24 will be an option for tumors that are resistant to the effect of E1B-55 kDa mutant adenoviruses, especially for cancers that express wild-type p53 protein. Finally, our data indicate that Delta-24 is a powerful antiglioma agent; thus, preclinical data that will lead to a clinical trial for testing the antiglioma effect of Delta-24 in patients with malignant glioma refractory to current forms of treatment should be generated.
Acknowledgement
We greatly appreciate the assistance of Joann Aaron (Scientific Editor, Department of Neuro-Oncology, M. D. Anderson Cancer Center) in the editing and preparation of the manuscript.
Footnotes
The work was supported by the Anthony Bullock Foundation (J.F.), National Institutes of Health (grant RO1CA90879 to J.F.), Pediatric Brain Tumor Foundation of the United States (J.F), and M.D. Anderson Cancer Center Institutional Research (grant 43721231 to H.J.).
References
- 1.Maher EA, Furnari FB, Bachoo RM, Rowitch DH, Louis DN, Cavenee RA, DePinho RA. Malignant glioma: genetics and biology of a grave matter. Genes Dev. 2001;15:1311–1333. doi: 10.1101/gad.891601. [DOI] [PubMed] [Google Scholar]
- 2.Stewart BW, Kleihues P. World Cancer Report. Lyon: IARC Press; 2003. [Google Scholar]
- 3.Chiocca EA. Oncolytic viruses. Nat Rev Cancer. 2002;2:938–950. doi: 10.1038/nrc948. [DOI] [PubMed] [Google Scholar]
- 4.Alemany R, Balague C, Curiel DT. Replicative adenoviruses for cancer therapy. Nat Biotechnol. 2000;18:723–727. doi: 10.1038/77283. [DOI] [PubMed] [Google Scholar]
- 5.Fueyo J, Alemany R, Gomez-Manzano C, Fuller GN, Khan A, Conrad CA, Liu TJ, Jiang H, Lemoine MG, Suzuki K, et al. Preclinical characterization of the antiglioma activity of a tropism-enhanced adenovirus targeted to the retinoblastoma pathway. J Natl Cancer Inst. 2003;95:652–660. doi: 10.1093/jnci/95.9.652. [DOI] [PubMed] [Google Scholar]
- 6.Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, Ng L, Nye JA, Sampson-Johannes A, Fattaey F, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 7.Barker DD, Berk J. Adenovirus proteins from both E1B reading frames are required for transformation of rodent cells by viral infection and DNA transfection. Virology. 1987;156:107–121. doi: 10.1016/0042-6822(87)90441-7. [DOI] [PubMed] [Google Scholar]
- 8.Heise C, Sampson-Johannes A, Williams A, McCormick F, Von Hoff DD, Kirn DH. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat Med. 1997;3:639–645. doi: 10.1038/nm0697-639. [DOI] [PubMed] [Google Scholar]
- 9.Geoerger B, Grill J, Opolon P, Morizet J, Aubert G, Terrier-Lacombe MJ, Bressac De-Paillerets B, Barrois M, Feunteun J, Kirn DH, et al. Oncolytic activity of the E1B-55 kDa-deleted adenovirus ONYX-015 is independent of cellular p53 status in human malignant glioma xenografts. Cancer Res. 2002;62:764–777. [PubMed] [Google Scholar]
- 10.Ries SJ, Brandts CH, Chung AS, Biederer CH, Hann BC, Lipner EM, McCormick F, Korn WM. Loss of p14ARF in tumor cells facilitates replication of the adenovirus mutant dl1520 (ONYX-015) Nat Med. 2000;6:1128–1133. doi: 10.1038/80466. [DOI] [PubMed] [Google Scholar]
- 11.Kirn D. Clinical research results with dl1520 (Onyx-015), a replication-selective adenovirus for the treatment of cancer: what have we learned? Gene Ther. 2001;8:89–98. doi: 10.1038/sj.gt.3301377. [DOI] [PubMed] [Google Scholar]
- 12.Alemany R, Gomez-Manzano C, Balague C, Yung WK, Curiel DT, Kyritsis AP, Fueyo J. Gene therapy for gliomas: molecular targets, adenoviral vectors, and oncolytic adenoviruses. Exp Cell Res. 1999;252:1–12. doi: 10.1006/excr.1999.4623. [DOI] [PubMed] [Google Scholar]
- 13.Fueyo J, Gomez-Manzano C, Alemany R, Lee PS, McDonnell TJ, Mitlianga P, Shi YX, Levin VA, Yung WK, Kyritsis AP. A mutant oncolytic adenovirus targeting the Rb pathway produces antiglioma effect in vivo. Oncogene. 2000;19:2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki K, Alemany R, Yamamoto M, Curiel DT. The presence of the adenovirus E3 region improves the oncolytic potency of conditionally replicative adenoviruses. Clin Cancer Res. 2002;8:3348–3359. [PubMed] [Google Scholar]
- 15.Cripe TP, Dunphy EJ, Holub AD, Saini A, Vasi NH, Mahlle YY, Collins MH, Snyder JD, Krasnykh V, Curiel DT, et al. Fiber knob modifications overcome low, heterogeneous expression of the coxsackievirus-adenovirus receptor that limits adenovirus gene transfer and oncolysis for human rhabdomyosarcoma cells. Cancer Res. 2001;61:2953–2960. [PubMed] [Google Scholar]
- 16.van Beusechem VW, van den Doel PB, Grill J, Pinedo HM, Gerritsen WR. Conditionally replicative adenovirus expressing p53 exhibits enhanced oncolytic potency. Cancer Res. 2002;62:6165–6171. [PubMed] [Google Scholar]
- 17.Gomez-Manzano C, Balague C, Alemany R, Lemoine MG, Mitlianga P, Jiang H, Khan A, Alonso M, Lang FF, Conrad CA, et al. A novel E1A-E1B mutant adenovirus induces glioma regression in vivo. Oncogene. 2004;23:1821–1828. doi: 10.1038/sj.onc.1207321. [DOI] [PubMed] [Google Scholar]
- 18.Gomez-Manzano C, Fueyo J, Kyritsis P, Steck PA, Roth JA, McDonnell TJ, Steck KD, Levin VA, Yung WK. Adenovirus-mediated transfer of the p53 gene produces rapid and generalized death of human glioma cells via apoptosis. Cancer Res. 1996;56:694–699. [PubMed] [Google Scholar]
- 19.Shen Y, Kitzes G, Nye JA, Fattaey A, Hermiston T. Analyses of single-amino-acid substitution mutants of adenovirus type 5 E1B-55K protein. J Virol. 2001;75:4297–4307. doi: 10.1128/JVI.75.9.4297-4307.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones N, Shenk T. Isolation of deletion and substitution mutants of adenovirus type 5. Cell. 1978;13:181–188. doi: 10.1016/0092-8674(78)90148-4. [DOI] [PubMed] [Google Scholar]
- 21.Graham FL, Prevec L. Manipulation of adenovirus vectors. Methods Mol Biol. 1991;7:109–128. doi: 10.1385/0-89603-178-0:109. [DOI] [PubMed] [Google Scholar]
- 22.Lal S, Lacroix M, Tofilon P, Fuller GN, Sawaya R, Lang FF. An implantable guide-screw system for brain tumor studies in small animals. J Neurosurg. 2000;92:326–333. doi: 10.3171/jns.2000.92.2.0326. [DOI] [PubMed] [Google Scholar]
- 23.Pilder S, Moore M, Logan J, Shenk T. The adenovirus E1B-55K transforming polypeptide modulates transport or cytoplasmic stabilization of viral and host cell mRNAs. Mol Cell Biol. 1986;6:470–476. doi: 10.1128/mcb.6.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quinlan MP. Enhanced proliferation, growth factor induction and immortalization by adenovirus E1A 12S in the absence of E1B. Oncogene. 1994;9:2639–2647. [PubMed] [Google Scholar]
- 25.Dobbelstein M, Roth J, Kimberly WT, Levine AJ, Shenk T. Nuclear export of the E1B 55-kDa and E4 34-kDa adenoviral oncoproteins mediated by a rev-like signal sequence. EMBO J. 1997;16:4276–4284. doi: 10.1093/emboj/16.14.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heise C, Hermiston T, Johnson L, Brooks G, Sampson-Johannes A, Williams A, Hawkins L, Kirn D. An adenovirus E1A mutant that demonstrates potent and selective systemic anti-tumoral efficacy. Nat Med. 2000;6:1134–1139. doi: 10.1038/80474. [DOI] [PubMed] [Google Scholar]
- 27.Howe JA, Demers GW, Johnson DE, Neugebauer SE, Perry ST, Vaillancourt MT, Faha B. Evaluation of E1-mutant adenoviruses as conditionally replicating agents for cancer therapy. Mol Ther. 2000;2:485–495. doi: 10.1006/mthe.2000.0206. [DOI] [PubMed] [Google Scholar]
- 28.Kirn D, Hermiston T, McCormick F. ONYX-015: clinical data are encouraging. Nat Med. 1998;4:1341–1342. doi: 10.1038/3902. [DOI] [PubMed] [Google Scholar]
- 29.Lamfers ML, Grill J, Dirven CM, Van Beusechem VW, Geoerger B, Van Den Berg J, Alemany R, Fueyo J, Curiel DT, Vassal G, et al. Potential of the conditionally replicative adenovirus Ad5-Delta24RGD in the treatment of malignant gliomas and its enhanced effect with radiotherapy. Cancer Res. 2002;62:5736–5742. [PubMed] [Google Scholar]
- 30.Teodoro JG, Shore GC, Branton PE. Adenovirus E1A proteins induce apoptosis by both p53-dependent and p53-independent mechanisms. Oncogene. 1995;11:467–474. [PubMed] [Google Scholar]
- 31.Attia MA, Weiss DW. Immunology of spontaneous mammary carcinomas in mice: V. Acquired tumor resistance and enhancement in strain A mice infected with mammary tumor virus. Cancer Res. 1996;26:1787–1800. [PubMed] [Google Scholar]