Abstract
Background
Delivery and tracking of endomyocardial stem cells are limited by the inability to image transplanted cells noninvasively in the beating heart. We hypothesized that mesenchymal stem cells (MSCs) could be labeled with a iron fluorophore particle (IFP) to provide MRI contrast in vivo to assess immediate and long-term localization.
Methods and Results
MSCs were isolated from swine. Short-term incubation of MSCs with IFP resulted in dose-dependent and efficient labeling. Labeled cells remained viable for multiple passages and retained in vitro proliferation and differentiation capacity. Labeled MSCs (104 to 106 cells/150 μL) were injected percutaneously into normal and freshly infarcted myocardium in swine. One, 3, and 1 animals underwent serial cardiac MRI (1.5T) for 4, 8, and 21 days, respectively. MRI contrast properties were measured both in vivo and in vitro for cells embedded in agar. Injection sites containing as few as 105 MSCs could be detected and contained intact IFP-bearing MSCs on histology.
Conclusions
IFP labeling of MSCs imparts useful MRI contrast, enabling ready detection in the beating heart on a conventional cardiac MR scanner after transplantation into normal and infarcted myocardium. The dual-labeled MSCs can be identified at locations corresponding to injection sites, both ex vivo using fluorescence microscopy and in vivo using susceptibility contrast on MRI. This technology may permit effective in vivo study of stem cell retention, engraftment, and migration.
Keywords: cells, magnetic resonance imaging, myocardial infarction, contrast media
Cellular agents such as mesenchymal stem cells (MSCs), endothelial progenitor cells, and skeletal myoblasts are under investigation as potential treatments for myocardial dysfunction.1 Once delivered to the heart, injected cells are difficult to visualize and track in vivo. Fluorescent or genetic marking2,3 identifies transplanted cells only after explantation. Nuclear scintigraphic tracking4 is limited by poor spatial resolution and radionuclide decay. In this study, we describe labeling cells with contrast agent, permitting identification under both MRI in vivo and fluorescence microscopy ex vivo.
Ultrasmall (nanometer-scale) superparamagnetic iron oxide particles have been used to track stem cells transplanted into stationary organs such as brain5,6 in small animals or to demonstrate macrophage activity within atherosclerotic plaque.7 Comparably sized magnetodendrimers, which transfect cells with MRI contrast agents, have been used for static MRI of neural cell transplants.8 To date, no approach has permitted in vivo MRI of transplanted cells in a beating heart.
Iron oxide can generate MRI contrast by disturbing the local magnetic field near excited spins, a property termed T2* relaxation. Hinds et al9 recently demonstrated efficient labeling of hematopoietic stem cells using relatively large (micron-scale) iron oxide particles by simple incubation. The particles generate significantly greater T2* effect (and more MRI contrast) than an equivalent weight of smaller (nanometer-scale) particles. The particles also contained a fluorophore, permitting both MRI and fluorescence imaging.
We hypothesized that such iron fluorescent particle (IFP) labeling of cells, specifically of mesenchymal stem cells, imparts sufficient MRI contrast that cells can be detected in vivo in a beating heart after direct injection. We show that IFP-labeled MSCs have a concentration-dependent T2* effect in vitro, can be visualized noninvasively in vivo after transplantation using a conventional scanner, can be observed serially over a period of weeks, and can then be identified ex vivo using fluorescence microscopy.
Methods
Iron Fluorescent Particle
The contrast agent consisted of iron oxide (62.4%) microparticles and a fluorescein-5-isothiocyanate analogue (Dragon Green) embedded in 0.9-μm inert polystyrene microspheres (Bangs Laboratories).
Cell Preparation, Labeling, and Characterization
MSCs were derived from bone marrow aspirates of healthy adult Yorkshire swine.10 Mononuclear cells were isolated using density gradient centrifugation (Ficoll-Paque, Amersham Biosciences), and culture was expanded based on plastic adherence. Nonadherent cells were removed every 5 days, and the adherent cells were washed and resuspended at a plating density of 1000 cells/mm2 in MSC basal media supplemented with bullet kits (MSCGM, Poeitics, BioWhittaker).
For cell-labeling procedures, early passage nonconfluent MSCs were incubated overnight with IFP (10 μL of 1% stock solution per mL medium) at 37°C in 5% CO2. Excess particles were removed after 18 hours by repeated washing. To measure intracellular IFP density, samples labeled separately were lysed with water and IFPs were counted on a hemocytometer under light and fluorescent illumination to exclude debris. Before injection, IFP-labeled cells were trypsinized and aliquotted into 150-μL injection volumes containing specified cell concentrations along with tissue-fast marking dye (Triangle Biomedical). In 2 animals (8- and 21-day survival), MSCs were stained with DAPI nuclear label (Vector Laboratory) as well as IFP before injection.
Viability was determined by Trypan Blue exclusion. Cell death was assessed by light and confocal microscopy for morphological evidence of apoptosis and chromatin abnormality. To assess the effects of the particles on cell proliferation, growth curves (MTT Roche Diagnostics) were obtained at a range of IFP concentrations (0.1 to 50 μL IFP stock per mL medium). The effect of labeling on in vitro MSC differentiation ability was tested using lineage-specific culture media.10 Adipogenic differentiation was induced with 1-methyl-3-isobutylxanthine, insulin, indomethacin, and dexamethasone. Histology specimens were stained with oil-red O, and quantitative polymerase chain reaction measured upregulation of peroxisome proliferation–activated receptor γ expression in differentiated adipocytes. Osteogenic differentiation was measured by staining for calcium deposition by the von Kossa method and for alkaline phosphatase (Fast Violet/naphthol, Sigma 85L-3R).10 We are unable to reproduce in vitro 5-azacytidine–induced cardiomyocyte differentiation11 in our laboratories.
Animal Studies
Animal protocols were approved by the National Heart, Lung, and Blood Institute Animal Care and Use Committee. Allogeneic MSCs were isolated from Yorkshire swine and delivered into Hanford mini-swine. Myocardial infarctions were created in 3- to 6-month-old Hanford mini-swine (30 to 40 kg) by transcatheter occlusion of the left anterior descending coronary artery using platinum embolization coils (VortX, Boston Scientific). MSCs were injected under x-ray guidance percutaneously using steerable guiding catheters to position a spring-actuated biocompatible 27G needle (Stilletto, provided by Boston Scientific Molecular Interventions, Natick, Mass). Two to 5 injections were performed in each animal. Five animals were progressively kept alive 4 (n=1), 8 (n=3), and 21 days (n=1) to allow serial MRI. To determine the fate of IFP in case of death or lysis of the animals’ cellular host, 1 additional healthy animal and 2 infarcted animals underwent injections of bare (acellular) IFP using high doses (0.1 to 10 μL IFP stock) per 150 μL injection, followed by MRI on days 1 and 7.
Tissue Handling and Ex Vivo MRI
Explanted hearts were snap-frozen to examine for IFP-labeled MSCs or IFP alone. Frozen sections were fixed with cold methanol, washed with PBS, and mounted with medium containing DAPI. Photographs were obtained using a Leica TCS-SP upright confocal microscope. For 3D MRI, fresh explants were retroperfused with 4% formaldehyde, and gadolinium-filled reference makers were sutured onto the epicardium for subsequent registration of MR images with pathology sections. Ex vivo MRI was performed at 1.5T with a head coil (CV/i, General Electric) using 3D fast gradient echo (voxels 0.47×0.47×1 mm, 256×256×128 matrix, repetition time 300 ms, echo time 3.3 ms, flip angle 45 degrees, bandwidth ±31 kHz, imaging time 1 hour). Hearts were then sectioned to examine for the presence of IFP-bearing cells.
Relaxometry and In Vivo MRI
MRI contrast characteristics of IFP-labeled MSCs were studied by measuring spin-lattice (T1) and spin-spin (T2 and T2*) relaxation time constants in cell suspensions and in vivo after endomyocardial injection.
IFP-labeled MSCs were suspended in 2 mL of 1% low-melting-point agarose8 at a range of densities from 102 to 107 cells/mL in 24-well plates and imaged at 1.5T (Sonata, Siemens) using the spine coil. The T1, T2, and T2* relaxation rates of in vitro preparations were measured at 37°C. T1 was measured using steady-state free precession (SSFP) inversion recovery pulse sequences and multiple inversion times (196 to 1000 ms),12 T2 by fast spin echo with multiple effective echo times (3.4 to 90 ms), and T2* by fast gradient echo (FGRE) with multiple echo times (2.6 to 60 ms). The field of view was 360 mm, which yielded voxel sizes of 1.4×1.9×6.0 mm. Voxel by voxel relaxation curves were generated and fitted using a nonlinear least-squares algorithm (Matlab v6.1, Mathworks).
For in vivo relaxation rate measurements and MRI, 1×105 IFP-labeled MSCs were suspended in 150-μL injection volumes and injected percutaneously into infarcted and normal myocardium under x-ray fluoroscopy (Multistar, Siemens). MRI was performed at multiple time points using both SSFP and FGRE pulse sequences in standard segmented, ECG-gated, and breath-held examinations. In vivo T2* relaxation rates were determined using gated FGRE with multiple echo times (3 to 20 ms). Signal to noise ratio and contrast to noise ratio (CNR) were measured for normal, infarcted, and injected myocardium according to the relation (CNR = [SImyo − SIIFP]/SDnoise), where SImyo represents the signal intensity (in arbitrary units) of normal myocardium, SIIFP represents the signal intensity of IFP-labeled cell injection sites, and SDnoise represents the standard deviation of background noise.
Results
Cell Labeling and Effect on Viability and In Vitro Differentiation
MSCs were efficiently labeled (>99% by confocal microscopy) in culture at a range of exposure times and particle concentrations, optimally 10 μL IFP stock per mL of medium for 18 to 24 hours. At higher concentrations or exposure times, there was dose-dependent attenuation of cell proliferation (data not shown). Labeled MSCs maintained viability by Trypan Blue exclusion and retained label for up to 3 months after multiple passages in culture. Cells could undergo multiple labeling cycles and be expanded up to 15 passages. MSCs bore an average of 390±360 IFPs per cell, corresponding to a mean of 0.20±0.18 ng iron oxide per cell.
IFP did not seem to alter the differentiation capacity of MSCs into multiple lineages. Appropriately stimulated IFP-labeled MSCs assumed adipocyte morphology (data not shown) and expressed similar levels of peroxisome proliferation–activated receptor γ mRNA (IFP, 393±170% versus unlabeled control, 408±173%; induced increase; P=0.86). Differentiation along osteogenic lineage also was unaffected (data not shown).
Relaxometry: Contrast Effects of IFP Labeling
Figure 1 shows T1, T2, and T2* relaxation time constants for IFP-labeled and unlabeled MSC suspension in agar at a range of cell concentrations. The cell densities corresponded to concentrations planned for 150-μL transcatheter injection mixtures. T2 and T2* values were significantly shortened compared with T1. Denser cell preparations imparted shorter T2 and especially T2* in a dose-dependent manner.
Figure 1.
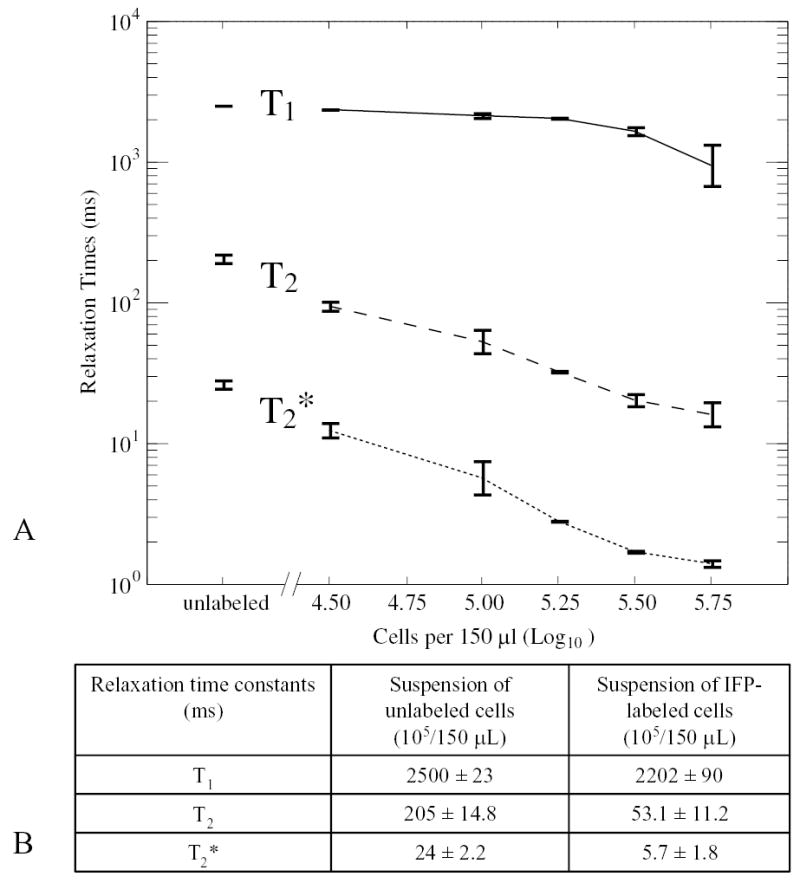
In vitro relaxometry of IFP-labeled MSCs. A, T1, T2, and T2* relaxation time constants for IFP-labeled and unlabeled MSC agar suspensions at a range of cell concentrations. Unlabeled refers to average of a range of cell concentrations from 104 to 106 MSCs without IFP. B, Comparison of relaxation time constants for labeled and unlabeled cell suspensions.
Serial In Vivo MR Imaging of IFP-Labeled MSCs
Labeled cells could be identified after injection into both normal and infarcted myocardium. A magnetic susceptibility artifact was seen as a signal void (dark region) corresponding to the injection sites (Figure 2). Injection volumes of 1×106 IFP-labeled MSCs in 150 μL (0.15 cm3) generated signal void volumes of 0.36±0.38 cm3. The CNR between normal myocardium and the signal void created by the injected IFP was 10.2±3.1 (n=6) and between infarcted myocardial and IFP signal voids was 16.5±2.3 (n=2). The T2* values for in vivo endomyocardial injections of 105/150 μL IFP-labeled MSCs are far shorter than for normal myocardium (9 versus 27 ms) and facilitate ready visualization.
Figure 2.
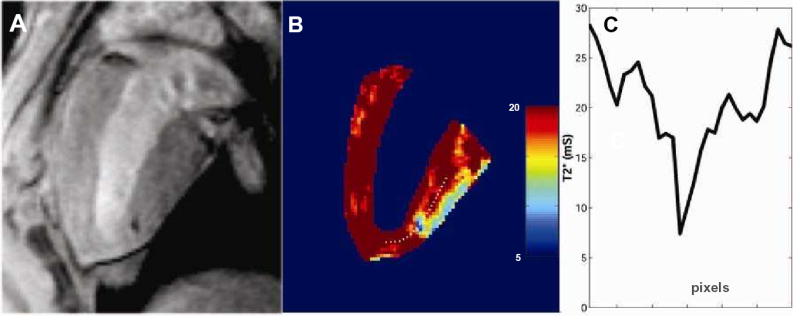
In vivo relaxometry of injected IFP-labeled MSCs. A, Typical in vivo injection of 105 IFP-labeled MSCs is shown in this still frame from a cinematic SSFP magnetic resonance image immediately after injection. This cell concentration represents the minimally detected dose in vivo and was used for all subsequent relaxometry. B, T2* parametric map of the same slice shows the lowest T2* in the region of the injection, corresponding to the location of labeled cells. The posterobasal epicardial surface also shows typical susceptibility SSFP artifact commonly attributed to adjacent diaphragmatic surfaces. The color map corresponds to T2* values indicated on the scale immediately to the right. C, A profile of T2* values along the dotted white line from the middle panel. The center of the injection has a T2* value close to the values measured in vitro (see Figure 1).
Figure 3 shows representative myocardium before and after injections of 4×106 IFP-labeled MSCs into normal and infarcted myocardium on day 1, imaged using SSFP MRI. The same slices are shown after intravenous Gd-DTPA injection and imaged using inversion recovery FGRE to show delayed hyperenhancement.
Figure 3.
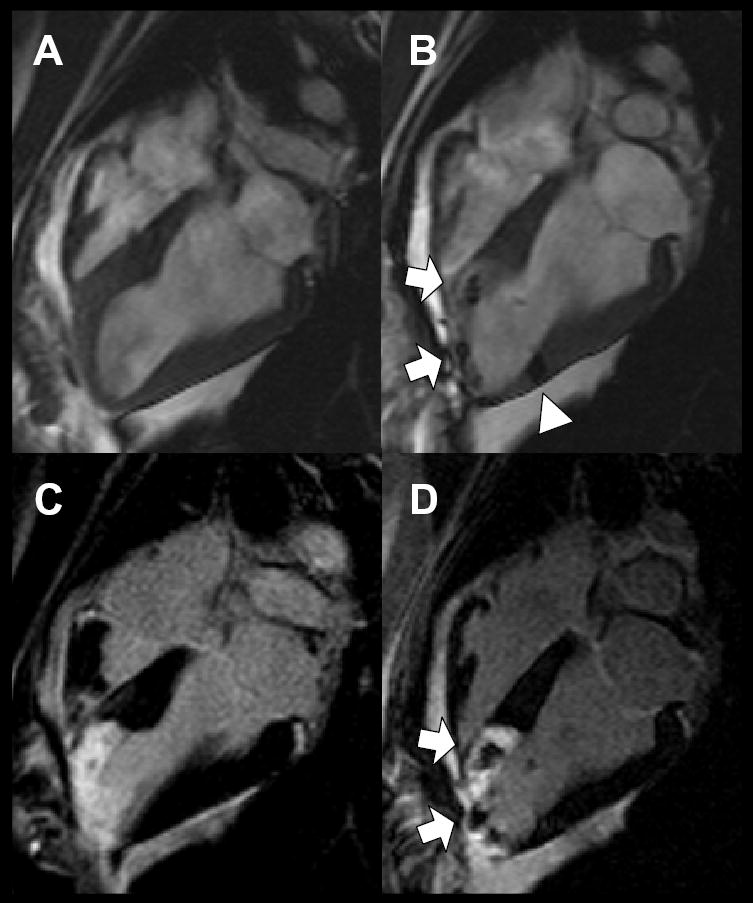
In vivo visualization immediately before and after IFP-MSC injection. Long-axis SSFP MRI view of left ventricle before (A) and after (B) transcatheter injection of 4×106 IFP-labeled MSCs into infarct at apex (arrows) and into adjoining normal myocardium (arrowhead). Delayed hyperenhancement inversion recovery FGRE MRI highlights areas of nonviable infarcted myocardium using same view as above, before (C) and after (D) injection of IFP-labeled MSCs. MSCs appear dark against hyperenhanced infarct.
Serial MRI studies were conducted in all animals. Four pigs were followed for 4 to 8 days and 1 for 21 days. Each had at least 2 follow-up MRI studies before euthanasia. A total of 18 injection sites were analyzed. Nine were into the infarct and 9 into normal myocardium. Injections of 1 to 4×106 MSCs were seen reproducibly and serially (Figure 4). The minimum detectable injection dose was 1×105 MSCs. Injections of 1 to 3.3×104 MSCs were not visualized. To investigate whether IFPs released from cells would be likely to show the same pattern after injection, up to 10 μL of IFP solution mixed in 150 μL of saline vehicle without cells was delivered in an identical manner. Only injections of 10 μL were detectable on the first day (data not shown), and there was no signal detected on subsequent MRI studies at days 4 or 7.
Figure 4.
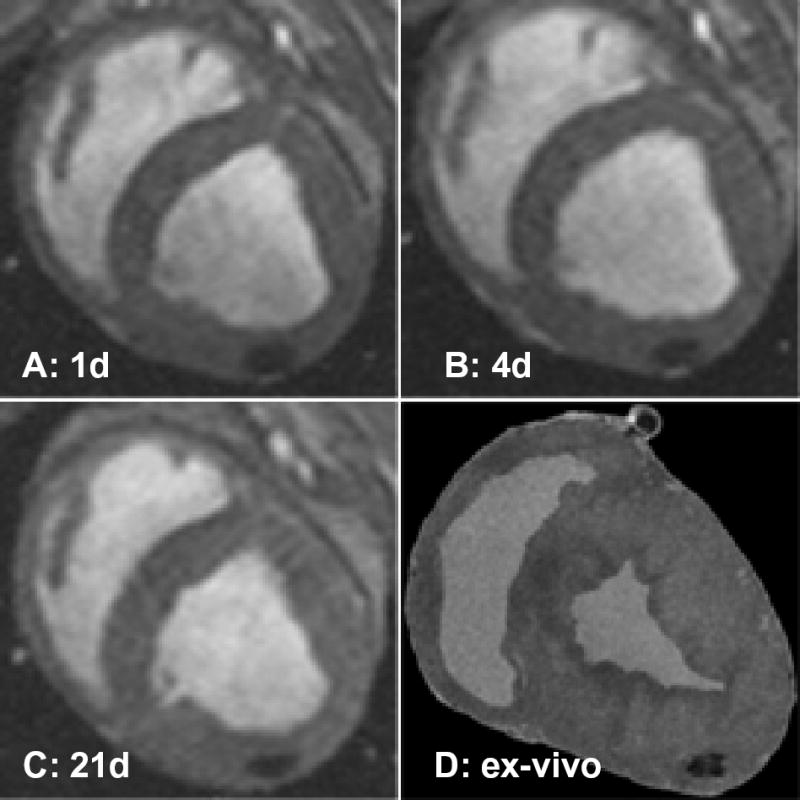
Serial in vivo and ex vivo MR imaging. Serial short-axis views of diastolic frames show a persistent susceptibility artifact (signal void) after injection of 106 IFP-labeled MSCs imaged on days 1 (A), 4 (B), and 21 (C). D, Corresponding view of explanted heart on high-resolution 3D MRI showing signal void.
Ex Vivo Correlation
To register in vivo images with histopathology sections, we used high-resolution 3D MRI (Figure 4D). Explanted hearts (n=3) had fiducial MRI markers applied over dye marks on the epicardial surface, and these were subsequently used to guide sectioning.
Figure 5 is a representative photomicrograph of a single IFP-labeled MSC under fluorescence (panel A) and differential phase (panel B) illumination. There is heavy perinuclear accumulation of IFP. Figure 6 shows correlation of labeled MSC injection sites under confocal fluorescence and differential interference microscopy. IFP-labeled MSCs are found within myocardium immediately after injection and appear rounded (Figures 6A through 6C). Other inflammatory cells are evident within needle tracts as described previously.13 When the animal shown in Figure 4 is studied after 3 weeks, IFP-labeled MSCs remain detectable, albeit with a more elongated morphology (Figures 6D through 6F). In this animal, MSCs were labeled with both IFP and DAPI before injection. Injected cells appeared viable and had preserved DAPI nuclear stain, normal chromatin pattern, and no membrane blebbing or vacuolization characteristic of apoptotic or necrotic death. Few free IFP particles appeared in the extra-cellular space.
Figure 5.
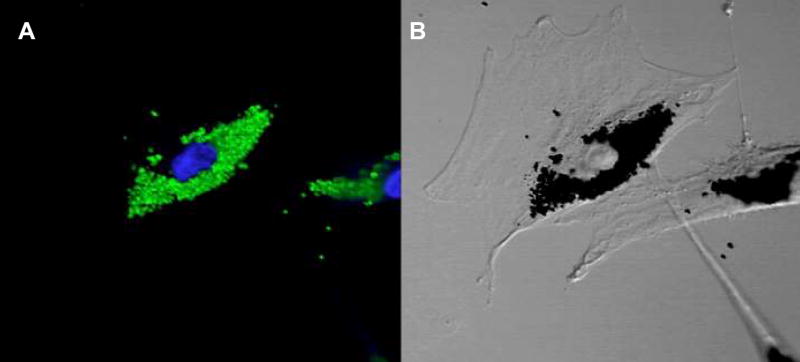
In vitro labeling. A, Confocal fluorescence micrograph of labeled MSCs (×100) showing green fluorescence of (Dragon Green) intracytoplasmic 0.9 μ IFP particles. DAPI nuclear counter-stain appears blue. B, Nomarski image (×100) shows outline of MSC and perinuclear accumulation of dense particles corresponding to the green fluorescence.
Figure 6.
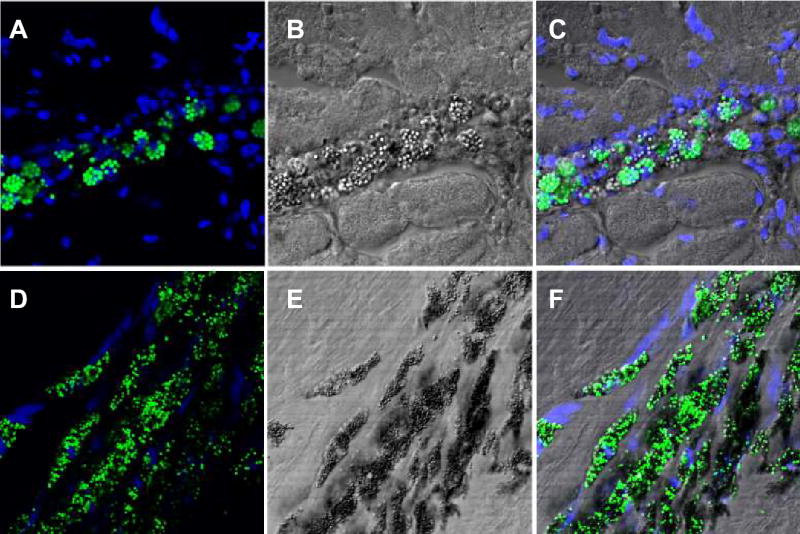
Histological confirmation of IFP-labeled cells after endomyocardial injection. A through C, Immediately after injection. A, Confocal fluorescence micrograph of round fluorescent green MSCs with DAPI nuclear counterstaining of surrounding myocardium. The cells appear intact immediately after injection with few exogenous particles in the surrounding myocardium. B, Corresponding section using differential interference microscopy. C, Overlay of images A and B. D through F, Animal shown in Figure 4, 21 days after injection. D, Endomyocardial engraftment of fluorescent green MSCs with DAPI nuclear counter-staining. MSCs appear elongated and aligned with the host myocardium. E, Corresponding section using differential interference microscopy. F, Overlay of images D and E. Cells appear intact with maintenance of DAPI nuclear staining with surrounding perinuclear IFP.
Bare IFP injection sites were all identified 7 days after injection using tissue-fast dye. Free IFPs were identified on histopathology at the highest dose (10 μL), sparse IFP at the medium dose (1 μL), and none at lower doses. Of note, 10 μL of IFP stock corresponds to the number of particles in 3.1×105 IFP-labeled MSCs.
Discussion
We demonstrate that MSCs, labeled with a large (micron-scale) iron-fluorophore intracellular contrast particle, can readily be detected in vivo within beating hearts using MRI and that their incorporation into myocardial tissue can be confirmed ex vivo using confocal fluorescence microscopy.
An ideal agent for noninvasive tracking of therapeutic cells would have several essential characteristics. It should be nontoxic without altering cell viability, growth, differentiation, or other biological activity. It should not affect surrounding tissue if released by the carrier cell. It should be durable but ideally should have some elimination pathway. It should also permit repeated, nondestructive, noninvasive detection and should be detectable with satisfactory CNR and spatial and temporal resolution at realistic doses. It should accurately reflect the behavior of the cells it labels and should indicate the location, migration, and quantity of labeled cells. Finally, an ideal agent should indicate the true disposition of labeled cells after emigration, death, or phagocytosis; non-specific interstitial deposition should not misrepresent target cell bioactivity.
The larger IFP we used to label MSCs exhibits many of the above characteristics. Porcine MSCs can be labeled with preserved in vitro viability, proliferation, and differentiation capability as well as in vivo viability after allogeneic transplantation. IFP effects on surrounding cells and the characteristics of IFP elimination are not yet understood. We have demonstrated that IFP-labeled MSCs have useful contrast characteristics, because we can distinguish labeled MSCs from unlabeled MSCs in vitro and in fresh myocardial tissue. IFP-labeled MSCs can be detected in beating myocardium with millimeter-scale spatial resolution and 40-ms temporal resolution immediately after direct injection in pigs. The contrast is satisfactory within normal or infarcted myocardium, both of which may be targeted in future MSC therapies. We have identified a minimum detectable quantity (105 cells/injection) of cells using conventional cardiac MRI on a commercial scanner. This is at least one order of magnitude lower than projected injection doses of cellular agents14 and can accommodate tracer quantities of labeled MSCs admixed with unlabeled MSCs.
Moreover, we are able to detect labeled cells serially and noninvasively when animals are kept alive for up to 3 weeks. Animals in an ongoing study reproducibly have detectable signal voids after 12 weeks (data not shown). The label precisely colocalizes with recovered cells ex vivo when histologic sections are registered with high-resolution ex vivo MRI. Bare label injections seem to exit the myocardium soon after direct injection. When we recovered myocardium 3 weeks after IFP-labeled MSC injections, we found most IFP to remain incorporated within MSCs and not free in the interstitium. However, IFPs impart contrast based on their presence, not based on whether host cells are viable. In 2 animals, injected MSCs were labeled both with DAPI and IFP. Intracellular IFPs were found within DAPI-labeled cells, suggesting retention by administered MSCs rather than ingestion by host cells. However, we cannot exclude the possibility that resident or recruited phagocytes have reincorporated free IFP after MSC death or lysis.
The IFP generated significant T2* contrast in vitro and in vivo, with T2* values significantly shorter than neighboring normal and infarcted myocardium, enabling ready detection using conventional cardiac MR to assess both immediate and long-term localization. This is consistent with the known bulk magnetic susceptibility effects of iron oxide within IFP.15,16 We observed less T1 or T2 contrast effect of IFP. This is not surprising for magnetite particles embedded in polystyrene, which cannot associate as freely with nearby water protons. Cell injections were detected as signal voids (dark regions) on MRI using both FGRE and SSFP pulse sequences. Signal voids can result from a variety of causes and are thus less specific than signal enhancement methods in MRI and can be additionally obscured by signal averaging within voxels. Nevertheless, T2* effects influence a larger volume than that occupied by the iron label itself (“blooming”), providing useful image amplification of small injections. However, whereas T2* in vitro was linearly related to the concentration of labeled cells, in vivo cellular redistribution and aggregation will probably confound quantitation of retained or expanded stem cells. Alternative contrast agents using gadolinium chelates, although attractive for their T1-shortening (signal enhancement) properties, require direct association with water protons (precluding sequestration within polymer beads) and are potentially toxic should free gadolinium be liberated during prolonged intracellular exposure. Polystyrene microspheres seem biocompatible but may be unattractive for implantation into patients, because they are not degraded and may not be excreted. Clinically approved ultrasmall iron oxide particles (Feridex, Berlex) generate significantly less MRI contrast than the IFP we describe here.9 Other biodegradable polymers such as dextran may provide a more suitable shell for magnetite labels should they be applied to human cellular therapeutics.
Although MSCs or marrow stromal cells, isolated based on density gradient centrifugation and plastic adherence, may contain both mature and progenitor cell population, there is evidence that these preparations contain many cells with multipotential capability in vitro as well as desirable effects when delivered to regions of myocardial injury.17–19 It is unlikely that our injection samples contain large numbers of other nonspecific phagocytes, such as macrophages. Most cells we inject into the myocardium have undergone multiple passages yet retain in vitro differentiation capacity. In this preliminary experience, we observed IFP-containing cells with preserved nuclear structure that have elongated and aligned with host myocardial fibers. Whether IFP-labeled MSCs indeed migrate, differentiate, and improve myocardial function after transplantation remains to be demonstrated in longer-term studies including careful controls.
This dual-fluorescence MRI contrast agent, incorporated into MSCs, imparts contrast characteristics favoring ready MRI detection and permits serial in vivo tracking of MSCs after endomyocardial delivery to the beating heart. In addition, this technology also allows accurate localization of injection sites and of retained cells within both normal and infracted myocardium. It may prove useful for real-time MRI-guided therapeutic endomyocardial injection of labeled MSCs.20 This new technique has potential to provide insight into stem cell retention, engraftment, and homing for cardiovascular cell therapy.
Acknowledgments
The authors are grateful to Joni Taylor, Diana Lancaster, Gina Orcino, Victor Wright, Bill Schenke, Ilsa Rovira, and Ted Mills for assistance, Daniel Ennis for 3D MRI, Toby Freyman and Maria Palasis of Boston Scientific for providing Stilleto catheters, and Toren Finkel and Robert Balaban for helpful comments.
References
- 1.Reinlib L, Field L. Cell transplantation as future therapy for cardiovascular disease? A workshop of the National Heart, Lung, and Blood Institute. Circulation. 2000;101:E182–E127. doi: 10.1161/01.cir.101.18.e182. [DOI] [PubMed] [Google Scholar]
- 2.Huhn RD, Tisdale JF, Agricola B, et al. Retroviral marking and transplantation of rhesus hematopoietic cells by nonmyeloablative conditioning. Hum Gene Ther. 1999;10:1783–1790. doi: 10.1089/10430349950017464. [DOI] [PubMed] [Google Scholar]
- 3.Shi PA, Hematti P, von Kalle C, et al. Genetic marking as an approach to studying in vivo hematopoiesis: progress in the non-human primate model. Oncogene. 2002;21:3274–3283. doi: 10.1038/sj.onc.1205320. [DOI] [PubMed] [Google Scholar]
- 4.Adonai N, Nguyen KN, Walsh J, et al. Ex vivo cell labeling with 64Cu-pyruvaldehyde-bis(N4-methylthiosemicarbazone) for imaging cell trafficking in mice with positron-emission tomography. Proc Natl Acad Sci U S A. 2002;99:3030–3035. doi: 10.1073/pnas.052709599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bulte JW, Zhang S, van Gelderen P, et al. Neurotransplantation of magnetically labeled oligodendrocyte progenitors: magnetic resonance tracking of cell migration and myelination. Proc Natl Acad Sci U S A. 1999;96:15256–15261. doi: 10.1073/pnas.96.26.15256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewin M, Carlesso N, Tung CH, et al. Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat Biotechnol. 2000;18:410–414. doi: 10.1038/74464. [DOI] [PubMed] [Google Scholar]
- 7.Ruehm SG, Corot C, Vogt P, et al. Ultrasmall superparamagnetic iron oxide-enhanced MR imaging of atherosclerotic plaque in hyperlipidemic rabbits. Acad Radiol. 2002;9(suppl 1):S143–S144. doi: 10.1016/s1076-6332(03)80422-1. [DOI] [PubMed] [Google Scholar]
- 8.Bulte JW, Douglas T, Witwer B, et al. Magnetodendrimers allow endosomal magnetic labeling and in vivo tracking of stem cells. Nat Biotechnol. 2001;19:1141–1147. doi: 10.1038/nbt1201-1141. [DOI] [PubMed] [Google Scholar]
- 9.Hinds KA, Hill JM, Shapiro EM, et al. Highly efficient endosomal labeling of progenitor and stem cells with large magnetic particles allows magnetic resonance imaging of single cells. Blood In press. [DOI] [PubMed]
- 10.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 11.Makino S, Fukuda K, Miyoshi S, et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheffler K, Hennig J. T(1) quantification with inversion recovery TrueFISP. Magn Reson Med. 2001;45:720–723. doi: 10.1002/mrm.1097. [DOI] [PubMed] [Google Scholar]
- 13.Kornowski R, Fuchs S, Tio FO, et al. Evaluation of the acute and chronic safety of the biosense injection catheter system in porcine hearts. Catheter Cardiovasc Interv. 1999;48:447–453. doi: 10.1002/(sici)1522-726x(199912)48:4<447::aid-ccd23>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 14.Toma C, Pittenger MF, Cahill KS, et al. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 15.Bowen C, Zhang X, Saab G, et al. Application of the static dephasing regime theory to superparamagnetic iron-oxide loaded cells. Magn Reson Med. 2002;48:52–61. doi: 10.1002/mrm.10192. [DOI] [PubMed] [Google Scholar]
- 16.Tanimoto A, Oshio K, Suematsu M, et al. Relaxation effects of clustered particles. J Magn Reson Imaging. 2001;14:72–77. doi: 10.1002/jmri.1153. [DOI] [PubMed] [Google Scholar]
- 17.Liechty KW, MacKenzie TC, Shaaban AF, et al. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000;6:1282–1286. doi: 10.1038/81395. [DOI] [PubMed] [Google Scholar]
- 18.Shake JG, Gruber PJ, Baumgartner WA, et al. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Ann Thorac Surg. 2002;73:1919–1925. doi: 10.1016/s0003-4975(02)03517-8. [DOI] [PubMed] [Google Scholar]
- 19.Wang JS, Shum-Tim D, Chedrawy E, et al. The coronary delivery of marrow stromal cells for myocardial regeneration: pathophysiologic and therapeutic implications. J Thorac Cardiovasc Surg. 2001;122:699–705. doi: 10.1067/mtc.2001.116317. [DOI] [PubMed] [Google Scholar]
- 20.Lederman RJ, Guttman MA, Peters DC, et al. Catheter-based endomyocardial injection with real-time magnetic resonance imaging. Circulation. 2002;105:1282–1284. doi: 10.1161/01.CIR.0000012425.71261.FC. [DOI] [PMC free article] [PubMed] [Google Scholar]


