The advent of highly active antiretroviral therapy for human immunodeficiency virus-infected individuals has reduced the emergence of ganciclovir (GCV)-resistant human cytomegalovirus (HCMV) infections (3). In contrast, GCV-resistant HCMV infection in organ transplant recipients is recognized to be an increasing problem (5), whereas a recent study using a restriction fragment length polymorphism (RFLP) assay restricted to the analysis of the UL97 codons 460, 520, 594 and 595 on adult allogeneic stem cell transplant recipients did not reveal any HCMV UL97 mutations (4). In children, however, after T-cell-depleted peripheral blood stem cell transplantation (PBSCT), GCV resistance can emerge early and rapidly following short courses of GCV therapy (2). For this patient group, we successfully used an RFLP assay expanded on the UL97 codons 591, 592, and 603 (2, 6). Because this technique is a simple and yet comprehensive method for therapy surveillance, we expanded the assay.
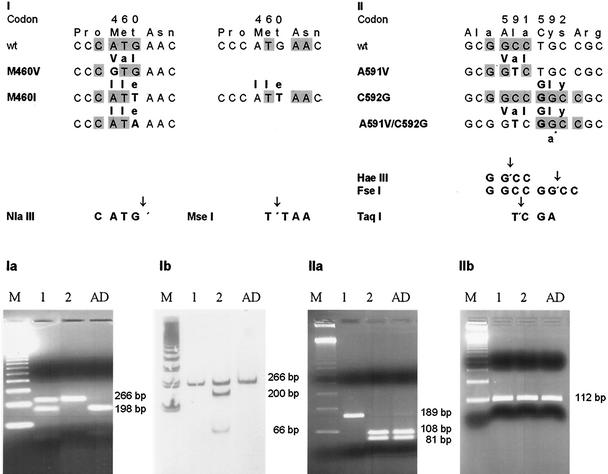
In the UL97 gene, there exist three known mutations in codon 460 conferring GCV resistance: the ATG-to-GTG (M460V) mutation, the ATG-to-ATA (M460I) mutation, and the ATG-to-ATT (M460I) mutation. Any point mutation in codon 460 will lead to the loss of an NlaIII restriction site (1). Therefore, the NlaIII digest is used to detect both M460V and M460I (1). Unfortunately, the digest is unspecific with respect to the mutation. The ATG-to-ATT (M460I) mutation generates a new MseI site. Thus, this mutation can be differentiated from the ATG-to-GTG (M460V) mutation or the ATG-to-ATA (M460V) mutation, as shown in Fig. 1, and the specific digest can be detected sensitively using a polyacrylamide gel with silver staining.
FIG. 1.
(I) Discrimination of the ATG-to-GTG/ATA versus the ATG-to-ATT mutation in UL97 codon 460. (Ia) NlaIII digest: sample 1 (tracheal secretion of a kidney transplant recipient) shows a mixture of wild-type and mutant strains (lane 1), and sample 2 (PP6, GCV-resistant laboratory strain) shows the presence of a mutant strain only (lane 2). (Ib) MseI digest with respect to NlaIII: sample 1 shows the presence of a wild-type strain (the presence of a ATG-to-GTG or -ATA mutation can be assumed) (lane 1); sample 2 shows mixture of wild-type and mutant strains (ATG to ATT must exist, and ATG to GTG or ATA can be assumed) (lane 2). Sequencing revealed a GTG and wild-type mixture for sample 1 and an ATT and GTG mixture for sample 2. (II) Pitfall of the 591/592 double mutation: use of an HaeIII digest for A591V screening can be misleading when there also exists a C592G mutation; an HaeIII digest as well as an FseI digest will result in wild-type patterns. Using the 591/592R mismatch primer (a*), a new TaqI site will be generated. (IIa) HaeIII digest: sample 1 (leukocyte, +251 days post PBSCT) shows only the mutant strain (lane 1); sample 2 (cerebrospinal fluid, + 263 days post PBSCT) shows only the wild-type strain (lane 2). (IIb) TaqI digest: exclusion of a 591V/592G double mutation. Lanes AD, AD169 GCV-sensitive laboratory strain; lanes M, 123-bp ladder.
As reported previously (2), a pediatric patient developed a GCV-resistant HCMV infection with a UL97 A591V mutation which was detected in blood on day +71 after PBSCT. The A591V mutation was the only UL97 mutation detected in this patient for 96 days. Studies performing an RFLP screening restricted on codons 460, 520, 594, and 595 (4) would have failed to detect the emergence of GCV resistance.
On day +263 after PBSCT, a HaeIII digest for screening in codon 591 was negative in a cerebrospinal fluid specimen (Fig. 1, panel IIa, sample 2). RFLP analysis of the other UL97 codons also revealed the wild-type strain. The possibility of the existence of an A591V/C592G double mutation could not be excluded (Fig. 1, panel II). For examination of this potential double mutation, a half-nested PCR on the previously published 189-bp fragment was performed using primer 595F (5′CCT CATG CGG CTG TTG GACC) (6) and, instead of primer 595R, the mismatch primer 591/592R (5′GAG CTT GCC GTT CTC CAA CGC GC GGt). Just in case both the A591V mutation and the C592G mutation exist simultaneously, a TaqI site will be generated (Fig. 1, panel II). Using this procedure, the potential double mutation can easily be detected for routine purposes.
Since there is little known about the incidence of GCV resistance in the PBSCT setting (4), the longitudinal use of an expanded and well-optimized UL97 RFLP strategy for GCV resistance screening will contribute to more reliable data on the incidence of GCV resistance.
REFERENCES
- 1.Chou, S., A. Erice, M. C. Jordan, G. M. Vercellotti, K. R. Michels, C. L. Talarico, S. C. Stanat, and K. K. Biron. 1995. Analysis of the UL97 phosphotransferase coding sequence in clinical cytomegalovirus isolates and identification of mutations conferring ganciclovir resistance. J. Infect. Dis. 171: 576-583. [DOI] [PubMed] [Google Scholar]
- 2.Eckle, T., L. Prix, G. Jahn, T. Klingebiel, R. Handgretinger, B. Selle, and K. Hamprecht. 2000. Drug-resistant human cytomegalovirus infection in children after allogeneic stem cell transplantation may have different clinical outcomes. Blood 96:3286-3289. [PubMed] [Google Scholar]
- 3.Emery, V. C. 2001. Progress in understanding cytomegalovirus drug resistance. J. Clin. Virol. 21:223-228. [DOI] [PubMed]
- 4.Gilbert, C., J. Roy, R. Belanger, R. Delage, C. Beliveau, C. Demers, and G. Boivin. 2001. Lack of emergence of cytomegalovirus UL97 mutations conferring ganciclovir (GCV) resistance following preemptive GCV therapy in allogeneic stem cell transplant recipients. Antimicrob. Agents Chemother. 45:3669-3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Limaye, A. P., G. Raghu, D. M. Koelle, J. Ferrenberg, M. L. Huang, and M. Boeckh. 2002. High incidence of ganciclovir-resistant cytomegalovirus infection among lung transplant recipients receiving preemptive therapy. J. Infect. Dis. 185: 20-27. [DOI] [PubMed] [Google Scholar]
- 6.Prix, L., K. Hamprecht, B. Holzhüter, R. Handgretinger, T. Klingebiel, and G. Jahn. 1999. Comprehensive restriction analysis of the UL97 region allows early detection of ganciclovir-resistant human cytomegalovirus in an immunocompromised child. J. Infect. Dis. 180: 491-495. [DOI] [PubMed] [Google Scholar]