Abstract
The variable penetration of antiretroviral drugs into sanctuary sites may contribute to the differential evolution of human immunodeficiency virus (HIV) and the emergence of drug resistance. We evaluated the penetration of indinavir, nelfinavir, and lopinavir-ritonavir (lopinavir/r) in the central nervous system, genital tract, and lymphoid tissue and assessed the correlation with residual viral replication. Plasma, cerebrospinal fluid (CSF), semen, and lymph node biopsy samples were collected from 41 HIV-infected patients on stable highly active antiretroviral therapy regimens to determine drug concentrations and HIV RNA levels. When HIV RNA was detectable, sequencing of the reverse transcriptase and protease genes was performed. Ratios of the concentration in semen/concentration in plasma were 1.9 for indinavir, 0.08 for nelfinavir, and 0.07 for lopinavir. Only indinavir was detectable in CSF, with a concentration in CSF/concentration in plasma ratio of 0.17. Differential penetration into lymphoid tissue was observed, with concentration in lymph node tissue/concentration in plasma ratios of 2.07, 0.58, and 0.21 for indinavir, nelfinavir, and lopinavir, respectively. HIV RNA levels were <50 copies/ml in all CSF samples of patients in whom HIV RNA was not detectable in plasma. HIV RNA was detectable in the semen of three patients (two patients receiving nelfinavir and one patient receiving lopinavir/r), and its detection was associated with multiple resistance mutations, while the viral load in plasma was undetectable. HIV RNA was detectable in all lymph node tissue samples. Differential drug penetration was observed among the three protease inhibitors in the sanctuary sites, but there was no correlation between drug levels and HIV RNA levels, suggesting that multiple factors are involved in the persistence of viral reservoirs. Further studies are required to clarify the role and clinical relevance of drug penetration in sanctuaries in terms of long-term efficacy and drug resistance.
Highly active antiretroviral therapy (HAART) has considerably decreased the rates of morbidity and mortality among patients infected with human immunodeficiency virus (HIV) (22). However, therapeutic failure is observed in up to half of patients after 2 to 3 years of HAART (19). The reasons for virologic failure are multiple, including adherence problems and pharmacological factors leading to the presence of subtherapeutic concentrations and, consequently, viral resistance (5, 8). The effects of HAART are usually assessed by use of blood samples, although several anatomical compartments or sanctuary sites have been described as viral reservoirs, in which viral evolution may differ from that in plasma (2, 3, 7, 10, 12, 15, 18, 24, 26). The main sanctuary sites are the central nervous system, genital tract, and lymphoid tissue. The viral loads and resistance profiles in these compartments have been described to be discordant from those in plasma (1, 4, 14, 27, 29).
Therapeutic failure may hence be caused by inefficient drug penetration in these compartments; variable protease inhibitor (PI) diffusion in sanctuary sites may contribute to sustained HIV type 1 (HIV-1) replication, resistance selection, and a subsequent failure to control the virus in plasma (6, 9, 21, 31). To date, few studies have analyzed PI concentrations in the sanctuary sites; no data are available on lopinavir-ritonavir (lopinavir/r), the most recently licensed PI, or drug concentrations in lymphoid tissue, despite its major role as a viral reservoir. In this study, we evaluated the penetration of indinavir, nelfinavir, and lopinavir/r in the plasma, cerebrospinal fluid (CSF), semen, and lymphoid tissue of HIV-infected patients and analyzed the correlation with residual viral replication in each compartment.
MATERIALS AND METHODS
Population.
Forty-one adult patients with chronic HIV-1 infection were included in this cross-sectional study. All patients had been treated for at least 6 months with a combination of two nucleoside reverse transcriptase (RT) inhibitors (NRTIs) plus one PI: indinavir (800 mg three times daily) in 16 patients, nelfinavir (1,250 mg twice daily) in 13 patients, or lopinavir/r (400 and 100 mg, respectively, twice daily) in 12 patients. All patients provided written informed consent, and the protocol was approved by the local ethics committee (Centre Hospitalier Universitaire Timone, Marseilles, France). Adherence to the HAART regimen was assessed from pill counts, and only patients with adherence rates >90% were included in the study.
Sampling schedule.
Sample collection was performed on the same day for each compartment. A plasma sample was drawn just before drug intake, about 8 h after the last indinavir dose, and 12 h after the last nelfinavir or lopinavir/r dose for the determination of trough levels. CSF and semen samples were collected through lumbar puncture and masturbation, respectively, 8 to 12 h after drug administration (trough concentration). A lymph node (LN) biopsy was then performed surgically in superficial areas 3 to 5 h after drug intake. Three additional plasma samples were drawn concomitantly with CSF, semen, and LN tissue collection to enable assessment of the ratios of the concentrations in each compartment. The collection times as they related to the time of prior drug intake were documented carefully. All samples were stored at −80°C until analysis.
Drug concentration analysis.
Quantification of indinavir, nelfinavir, lopinavir, and ritonavir concentrations was performed by a sensitive high-performance liquid chromatography method with UV detection (13, 32). Indinavir, nelfinavir, ritonavir, and lopinavir were removed from plasma by liquid-liquid extraction. Chromatographic separation was performed at 210 nm with an Inertsil ODS2, 5-μm column (4.6 by 150 mm; Precision Instrument, Marseilles, France) for indinavir, ritonavir, and lopinavir and at 220 nm with a Symmetry C18 5-μm column (4.6 by 250 mm; Waters, St. Quentin en Yvelines, France) for nelfinavir. The intra- and interassay variabilities ranged from 0.97 to 3.58% for indinavir, 11.1 to 12.3% for nelfinavir, 3.22 to 9.35% for ritonavir, and 2.9 to 13.8% for lopinavir for three quality control concentrations. The quantification limits were 20 ng/ml for indinavir and 50 ng/ml for nelfinavir, ritonavir, and lopinavir. The overall level of recovery of each PI was high, ranging from 80.8 to 93.3%. Chromatographic data were recorded and analyzed with a Millenium (version 2.0) software system (Waters).
PI concentrations in total LN tissue were measured. Blood contamination was avoided by at least two washes with cold 0.9% NaCl before homogenization. LN biopsy specimens were weighed, rinsed again with 500 μl of 0.9% NaCl, and homogenized with 1 ml of 0.9% NaCl. Cellular debris was then removed by centrifugation, and the resulting supernatant was collected and stored at −80°C until analysis. Quantification of indinavir, nelfinavir, ritonavir, and lopinavir in CSF, semen, and LN tissue samples was carried out by the same extraction and analytic method used for the plasma samples. Due to the lack of availability of blank CSF, semen, and LN biopsy samples from healthy donors, a reference curve could not be set up for each of the biologic samples. Therefore, the amounts of PIs were calculated from the same standard curve used for the plasma samples. Preliminary assays with 0.9% NaCl as an alternative matrix led to the same results. The identities of the peaks in the chromatograms for the various biologic samples were checked on a liquid chromatograph equipped with a diode array detector.
HIV-1 RNA levels.
HIV-1 RNA levels in plasma, CSF, and semen were measured by using the Amplicor Monitor kit (Roche Diagnostics, Meylan, France). When HIV-1 RNA levels were undetectable, the ultradirect procedure was used; that procedure has a lower limit of detection of 50 copies/ml (25).
CSF samples were frozen at −80°C after centrifugation to remove cells. Semen samples were processed systematically within 2 h following collection. Samples were diluted 1:1 in a 5-mg/ml bromeline solution (Sigma-Aldrich, Saint-Quentin, France) for semen fluidization and dissociation of cellular aggregates. After 10 min at 37°C, diluted specimens were layered over a two-layer Percoll (Sigma-Aldrich) gradient consisting of 95 and 47% isotonic Percoll solution, centrifuged at 300 × g for 20 min, and then collected. For semen samples with volumes greater than 1.5 ml, one-third of the volume was centrifuged at 3,600 × g for 10 min to eliminate any cellular component. The internal standard provided with the Amplicor Monitor kit was added to the lysis buffer to validate the extraction and amplification steps, and the Amplicor Monitor kit was used to quantify the HIV-1 RNA as described previously (28).
LN tissue samples were minced with a scalpel, and the cells were teased out in RPMI 1640 (Eurobio, Les Ulis, France). The LN mononuclear cells (LNMCs) were then isolated with Lymphocyte Separation Medium (Eurobio, Les Ulis, France). To quantify the viral load in the LN tissue samples, the LNMCs were counted and HIV-1 RNA was obtained from a pellet of 106 cells after treatment with RNA-B (Bioprobe Systems, Montreuil sous Bois, France). HIV-1 RNA levels were measured by using the Amplicor Monitor kit as reported previously (16); that kit has a limit of detection of 20 copies/106 cells.
HIV-1 RNA genotyping.
Sequencing of the RT and protease (PR) genes in samples with detectable HIV-1 RNA levels was done with the TruGene kit (Visible Genetics, Toronto, Ontario, Canada) according to the instructions of the manufacturer.
Statistical analysis.
HIV-1 RNA levels were log transformed. Comparison of mean values was done by the Student t test. Nonparametric tests and the Fisher exact test were used to compare drug levels and HIV RNA levels in semen and CSF. Correlation between two variables was done by the Pearson test. A P value <0.05 was considered significant.
RESULTS
Population.
Thirty-five men and six women (median age, 41 years) were included in the study. The median duration of the present HAART regimen was 32 months, and the median CD4+-cell count was 286 × 106/liter. Plasma samples were obtained from all patients. CSF and semen were collected from 40 of 41 and 34 of 41 patients, respectively. The LN biopsy failed to recover sufficient material for analysis from two patients (one patient receiving indinavir and one patient receiving nelfinavir). For other compartments, when sample material was limited, virologic tests were carried out prior to pharmacological evaluation.
PI levels. (i) Plasma.
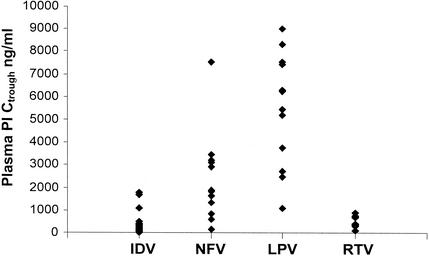
Median trough concentrations in plasma were 284 ng/ml (interquartile range [IQR], 144 to 504 ng/ml) for indinavir, 1,878 ng/ml (IQR, 1,355 to 3,211 ng/ml) for nelfinavir, 5,863 ng/ml (IQR, 3,505 to 7,453 ng/ml) for lopinavir, and 557 ng/ml (IQR, 341 to 741 ng/ml) for ritonavir. Large interindividual variability was noted (Fig. 1).
FIG. 1.
Distribution of trough concentrations (Ctrough) of indinavir (IDV), nelfinavir (NFV), lopinavir (LPV), and ritonavir (RTV) in plasma.
(ii) CSF.
The indinavir concentration was measured in the CSF of all 16 patients. The data for two patients were not used because CSF collection was done 1 h after drug intake, which corresponded to the time of the peak level in plasma. For the 14 patients whose data were analyzed, the average CSF sampling time was 7 h (range, 7 to 8 h). Indinavir concentrations in CSF were variable, with a median value of 73 ng/ml (IQR, 52 to 92 ng/ml). The corresponding value in plasma was 357 ng/ml (IQR, 155 to 914 ng/ml). The median CSF indinavir concentration/plasma indinavir concentration ratio was 0.17 (IQR, 0.10 to 0.49), and a high degree of interindividual variability was noted (Table 1). Nelfinavir, lopinavir, and ritonavir were undetectable in CSF samples from 12 patients receiving nelfinavir and all patients receiving lopinavir/r.
TABLE 1.
Ratios (medians) and IQRs of the concentrations of indinavir, nelfinavir, lopinavir, and ritonavir in CSF, semen, and LN tissue compared to those in plasma
| Sanctuary site | Concn ratio (IQR)a
|
|||
|---|---|---|---|---|
| IDV | NFV | LPV | RTV | |
| CSF | 0.17 (0.10-0.49) | 0 | 0 | 0 |
| Semen | 1.9 (1.07-3.94) | 0.08 (0.06-0.10) | 0.07 (0.01-0.45) | 0 |
| LN tissue | 2.07 (1.02-3.67) | 0.58 (0.27-0.84) | 0.21 (0.15-0.26) | 0.64 (0-1.15) |
IDV, indinavir; NFV, nelfinavir; LPV, lopinavir; RTV, ritonavir.
(iii) Semen.
Semen samples were obtained from all men receiving indinavir (n = 12), 7 of 11 men receiving nelfinavir, and 7 of 11 men receiving lopinavir/r. Semen samples were collected between 8 and 12 h postdosing according to the PI prescribed (8 h for patients receiving indinavir and 12 h for patients receiving nelfinavir or lopinavir/r).
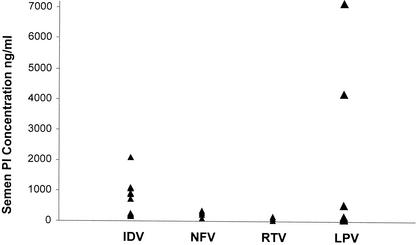
The median concentrations of indinavir, nelfinavir, and lopinavir in semen were 788 ng/ml (IQR, 213 to 1,046 ng/ml), 159 ng/ml (IQR, 94 to 268 ng/ml), and 166 ng/ml (IQR, 84 to 2,353 ng/ml), respectively. We observed a large interindividual variability in the level of PI penetration into semen (Fig. 2). Nelfinavir could be detected in only six of seven semen samples analyzed. The corresponding concentrations in plasma were similar to the trough levels in plasma. The median (IQR) concentration in semen/concentration in plasma ratios are presented in Table 1. Ritonavir was detectable in only three of seven samples at levels ranging from 50 to 149 ng/ml.
FIG. 2.
Concentrations of indinavir (IDV), nelfinavir (NFV), lopinavir (LPV), and ritonavir (RTV) in seminal plasma 8 to 12 h postdosing.
(iv) Lymphoid tissue.
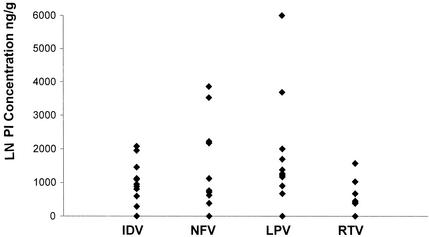
The average sampling time for LN tissue was 5 to 6 h (range, 4 to 8 h). The median concentration in LN tissue/concentration in plasma ratios for indinavir, nelfinavir, lopinavir, and ritonavir are shown in Table 1. In LN tissues, the median indinavir concentration was 1,025 ng/g (IQR, 755 to 1,108 ng/g) in 13 of 16 LN samples, and the corresponding value in plasma was 321 ng/ml (IQR, 216 to 587 ng/ml). For nelfinavir, the median concentrations were 740 ng/g (IQR, 278 to 2,175 ng/g) in 12 of 13 LN samples and 1,328 ng/ml (IQR, 1,136 to 3,040 ng/ml) in the corresponding plasma samples. The median lopinavir concentration measured in 11 of 12 LN tissue samples was 1,260 ng/g (IQR, 1,035 to 1,835 ng/g). The corresponding lopinavir level in plasma was 7,333 ng/ml (IQR, 5,400 to 9,458 ng/ml). Ritonavir, which is coformulated with lopinavir, was also detected in 7 of 11 LN tissue samples at a median concentration of 410 ng/g. Large interindividual variability was also found for PI penetration into LN tissues (Fig. 3).
FIG. 3.
Concentrations of indinavir (IDV), nelfinavir (NFV), lopinavir (LPV), and ritonavir (RTV) in lymph node tissue 6 h (range, 4 to 8) after the last drug intake.
HIV-1 RNA levels and genotyping. (i) Plasma.
Plasma HIV-1 RNA levels remained at <50 copies/ml in 28 patients for at least 6 months prior to the trial (on the basis of a monthly evaluation). HIV-1 RNA was detectable in the plasma of four patients receiving indinavir (median level, 516 copies/ml), five patients receiving nelfinavir (median level, 431 copies/ml), and four patients receiving lopinavir/r (median level, 39,090 copies/ml). The higher HIV-1 RNA levels reported in patients receiving lopinavir/r reflect the present preferential use of this drug in France for highly antiretroviral agent-experienced patients.
(ii) CSF.
HIV-1 RNA levels were <50 copies/ml in 35 of 40 CSF samples obtained, and HIV-1 RNA was detectable in 2 samples from patients receiving nelfinavir (55 and 1,330 copies/ml, respectively) and 3 samples from patients receiving lopinavir/r (median level, 350 copies/ml). For the last five patients, the detection of HIV-1 RNA in CSF was associated with a detectable viral load in plasma. In contrast, the CSF of eight other patients in whom plasma HIV-1 RNA was detectable (four receiving indinavir, three receiving nelfinavir, and one receiving lopinavir/r) had HIV-1 RNA levels <50 copies/ml. Isolates in plasma from one patient receiving nelfinavir presented more mutations (RT gene, M41L, D67N, M184V, and T215Y; PR gene, M36I and V82A) than isolates in CSF (RT gene, T215Y; PR gene, M36I); isolates from both compartments from another patient presented the same mutation patterns (RT gene, M41L; PR gene, A71V and L90 M). Isolates from the three patients receiving lopinavir/r presented several combinations of mutations on the RT and PR genes which were also detected in isolates from plasma (data not shown).
(iii) Semen.
The HIV-1 RNA load in seminal plasma was <50 copies/ml in 28 of 34 patients whose semen was analyzed, and HIV-1 RNA was detectable in the semen of 6 patients (median load, 552 copies/ml): 3 patients receiving nelfinavir and 3 patients receiving lopinavir/r. Among the six patients in whom HIV-1 RNA was detectable in seminal plasma, three had detectable plasma HIV RNA levels (one receiving nelfinavir, two receiving lopinavir/r) and three had plasma HIV-1 RNA levels <50 copies/ml (two receiving nelfinavir, one receiving lopinavir/r).
Sequencing of the HIV-1 RNA obtained from seminal plasma of the three patients with undetectable viral loads in plasma showed resistance mutations in only the PR gene in one patient treated with nelfinavir, didanosine, and stavudine (A71V) and in both the RT and the PR genes in two patients: one patient receiving nelfinavir, lamivudine, and stavudine (an M184I mutation and M36I and L63P mutations, respectively) and one patient receiving lopinavir/r, abacavir, didanosine, and stavudine (M41L and T215Y mutations and L10I, L63P, A71V, V82A, and L90M mutations, respectively). The last patient had previously failed three HAART regimens before the viral load in plasma was controlled with lopinavir/r. The resistance mutations found in the HIV-1 RNA from the seminal plasma from this patient were already present in the RNA from plasma analyzed just before the introduction of lopinavir/r (data not shown).
(iv) Lymphoid tissue.
HIV-1 RNA was detectable in LNMCs from all patients. The LNMCs of patients with plasma HIV-1 RNA loads <50 copies/ml had mean HIV-1 RNA levels of 3.56 ± 0.17 log copies/106 cells. No resistance mutations were found in LNMCs taken from nine patients on first-line therapy. Conversely, resistance mutations were found in HIV-1 RNA in LNMCs from 15 of 19 patients who had received prior suboptimal regimens (M41L in the RT gene, 6 patients; D67N in the RT gene, 1 patient; T69N in the RT gene, 3 patients; K70R in the RT gene, 11 patients; T215Y in the RT gene, 5 patients; T215F in the RT gene, 5 patients; K219Q in the RT gene, 5 patients; V82A in the PR gene, 5 patients; L90 M in the PR gene, 3 patients). Frozen plasma was available from 11 patients before initiation of the present regimen. These mutations were already present in plasma samples taken 24 to 180 weeks beforehand, when the patients had been treated with suboptimal regimens.
Correlation between PI concentrations and HIV RNA loads in the different sanctuary sites.
In the CSF, there was no statistical difference between a detectable (patients receiving indinavir) or a nondetectable (patients receiving nelfinavir and lopinavir/r) PI concentration and HIV RNA levels (P = 0.137 by the Fisher exact test).
HIV RNA was not detectable in the semen of 100, 66, and 75% of patients receiving indinavir, nelfinavir, and lopinavir/r, respectively. Among the three patients who were receiving nelfinavir and in whom HIV-1 RNA was detectable in semen, the reported nelfinavir concentrations were very low, ranging from 0 to 94 ng/ml, but no characteristic nelfinavir resistance mutations were found in isolates from the semen of these patients. Furthermore, in four other patients, the HIV-1 RNA load in seminal plasma was <50 copies/ml, despite undetectable or low nelfinavir concentrations. The nelfinavir concentrations were not statistically different between patients in whom HIV RNA was undetectable and patients in whom HIV-1 RNA was detectable (P = 0.20 by the Mann-Whitney test). Similarly, three patients who were receiving lopinavir/r and in whom HIV RNA was detectable in seminal plasma had very low lopinavir concentrations in semen, but three other patients in whom lopinavir concentrations were undetectable had seminal plasma HIV RNA loads <50 copies/ml. As for nelfinavir, no correlation was found between lopinavir concentrations and the detection of HIV-1 RNA in semen.
Among the 28 patients with undetectable viral loads in plasma, HIV-1 RNA levels in LNMCs were not statistically different between those treated with indinavir (with a concentration in LN tissue/concentration in plasma ratio >1) and those treated with nelfinavir or lopinavir/r (with a concentration in LN tissue/concentration in plasma ratio <1): 3.32 ± 0.23 versus 3.80 ± 0.25 log copies/106 cells (P = 0.17 by the Student t test). Overall, no statistically significant correlation was found between HIV-1 RNA levels in LNMCs and the concentration in LN tissue/concentration in plasma ratio for the effective PI used (r = −0.33; P = 0.1 by the Pearson test).
DISCUSSION
The effectiveness of antiretroviral therapy may depend on the ability of antiretroviral drugs to reach the sanctuary sites. Our study analyzed simultaneously viral loads and PI levels in four different compartments in a population of patients receiving long-term HAART.
Major differences were demonstrated for PI penetration into CSF. Nelfinavir and lopinavir were undetectable in the CSF of all patients, although indinavir was detectable with a concentration in CSF/concentration in plasma ratio of 0.17 and achieved concentrations in CSF that exceeded the 95% inhibitory concentration for the wild-type virus (35 to 70 ng/ml, i.e., 25 to 100 nM). Our results are concordant with other recently published data, which showed mean CSF indinavir concentrations ranging from 68 to 137 ng/ml (11, 17, 33). Protein binding variations appear to be the most likely explanation for these different levels of penetration into CSF. Indinavir is 61% bound to plasma protein, whereas nelfinavir and lopinavir/r are 98% bound. Moreover, amprenavir, which is 90% protein bound, is also present at low levels in CSF (R. Murphy, J. Currier, J. Gerber, R. D'Aquila, L. Smeaton, J. P. Sommadossi, R. Tung, and R. Gulick, 7th Conf. Retrovir. Opportunist. Infect., abstr. 314, 2000), further supporting this hypothesis. Despite these major differences in drug penetration, there was no correlation between HIV RNA levels and drug levels in CSF. These observations raise two hypotheses. First, all patients concomitantly received a combination of two NRTIs that may suffice to control HIV-1 replication in CSF. Conversely, the CSF drug concentration may not properly reflect overall activity in the brain parenchyma.
The control of viral replication in genital fluids during HAART is of particular importance, as most HIV-1 infections are transmitted through sexual contact (7). We have shown that the penetration of PIs into semen was highly variable not only between the different PIs but also between individuals. Indinavir had the highest concentration in semen/concentration in plasma ratio, as reported previously (30), and, as for CSF, reached concentrations that widely exceed the 95% inhibitory concentration for wild-type virus and that are close to those found in plasma. Indinavir would be expected to be effective in this compartment since HIV-1 RNA was not detectable in the semen of any of the patients. For the first time, we have reported on the penetration of nelfinavir into semen, even though the concentrations were very low (generally below the 50% effective concentration corrected for in vitro protein binding [630 ng/ml, i.e., 1.11 μM]) and highly variable (20). The same observations were made for lopinavir penetration into semen, with an important variability detected, despite the presence of ritonavir as a pharmacokinetic booster. To our knowledge, no data are available on lopinavir penetration alone, and we were unable to determine whether ritonavir enhances the penetration of lopinavir. Genotypic resistance in an isolate from the semen of one patient treated with lopinavir/r was related to the presence of resistant strains previously selected in plasma by failing regimens. Introduction of lopinavir/r in this case was able to control resistant HIV-1 in plasma but not in semen, where the drug concentrations may have been too low to be effective against this strain. However, neither nelfinavir nor lopinavir concentrations were correlated with HIV RNA levels in semen. Concentrations in semen should be interpreted with caution, since the percentage of protein binding of the drugs in this compartment is unknown. Regarding CSF, the presence of high NRTI concentrations in semen also makes it difficult to draw conclusions about the efficacies of PIs for the suppression of viral replication in semen (23).
In lymphoid tissue, residual HIV-1 RNA was detected in all LN tissue samples of patients who had previously received suboptimal regimens, even when HIV-1 RNA was undetectable in plasma for 2 to 3 years. All three PIs were detectable in LN tissue samples, but with wide differences in the concentration in LN tissue/concentration in plasma ratio, suggesting a better penetration for indinavir. Despite these differences in drug penetration, similar levels of HIV-1 RNA in LN tissue samples were found in patients with undetectable viral loads in plasma, and no correlation between drug penetration and levels of residual viral RNA was found. This suggests that persisting HIV-1 RNA in LN tissue is related to multiple factors other than PI diffusion, e.g., the existence of a population of T cells producing viral RNA without new cellular infection cycles (10).
The present study has some limitations. Indeed, use of a ratio value to describe PI penetration into CSF, semen, or LN tissue is not accurate because these ratios tend to change over time due to different time-concentration profiles in the nonblood compartments. However, it was not feasible to perform several lumbar punctures, lymph node biopsies, or semen collections to calculate ratios of areas under the curve. Therefore, our results did not allow complete evaluation of PI penetration during a dosing interval and the pharmacokinetic properties of the respective PIs in the different compartments.
In conclusion, major differences between different PIs in terms of penetration into nonblood compartments were demonstrated, but we were unable to establish a correlation between the pharmacological data and the HIV RNA levels. These discrepancies suggest that PI penetration is not the only factor which contributes to viral suppression in the different viral reservoirs. Indeed, protein binding may lead to differences in drug penetration into the CSF and the genital tract among the different PIs. Mostly, however, PIs are substrates of P glycoprotein, which is present in the blood-brain barrier and the blood-testis barrier and which actively pumps out PIs from these compartments. Furthermore, other cellular mechanisms and genetic factors are likely involved in the persistence of residual viral replication and viral reservoirs. These major differences require further study in order to clarify the importance of the role and the clinical relevance of drug penetration into these sanctuary sites in terms of long-term efficacy and drug resistance.
Acknowledgments
We thank Segolene Durand (INSERM U379 ORS PACA) for contributions to the statistical analysis.
This study was supported in part by grants from Roche, Merck Sharp & Dohme, and Abbott.
REFERENCES
- 1.Byrn, R. A., and A. A. Kiessling. 1998. Analysis of human immunodeficiency virus in semen: indication of a genetically distinct virus reservoir. J. Reprod. Immunol. 41:161-176. [DOI] [PubMed] [Google Scholar]
- 2.Chun, T. W., L. Carruth, D. Finzi, X. Shen, J. A. DiGiuseppe, H. Taylor, M. Hermankova, K. Chadwick, J. Margolick, T. C. Quinn, Y. H. Kuo, R. Brookmeyer, M. A. Zeiger, P. Barditch-Crovo, and R. F. Siliciano. 1997. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 387:183-188. [DOI] [PubMed] [Google Scholar]
- 3.Chun, T. W., L. Stuyver, and S. B. Mizell. 1997. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc. Natl. Acad. Sci. USA 94:13193-13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DiStefano, M., J. R. Fiore, L. Monno, A. Lepera, G. Pastore, and G. Angarano. 1999. Detection of multiple drug-resistance-associated pol mutations in cervicovaginal secretions from women. AIDS 13:992-994. [DOI] [PubMed] [Google Scholar]
- 5.Durant, J., P. Clevenbergh, R. Garraffo, P. Halfon, S. Icard, P. Del Giudice, N. Montagne, J. M. Schapiro, and P. Dellamonica. 2000. Importance of protease inhibitor plasma levels in HIV-infected patients treated with genotypic-guided therapy: pharmacologic data from the Viradapt Study. AIDS 14:1333-1339. [DOI] [PubMed] [Google Scholar]
- 6.Enting, R. H., M. Hoetelmans, J. M. A. Lange, D. M. Burger, J. H. Beijnen, and P. Portegies. 1998. Antiretroviral drugs and the central nervous system. AIDS 12:1941-1955. [DOI] [PubMed] [Google Scholar]
- 7.Eron, J. J., P. L. Vernazza, D. M. Johnston, F. Seillier-Moiseiwitsch, T. M. Alcorn, S. A. Fiscus, and M. S. Cohen. 1998. Resistance of HIV-1 to antiretroviral agents in blood and seminal plasma: implications for transmission. AIDS 12:F181-F189. [DOI] [PubMed] [Google Scholar]
- 8.Gallant, J. 2000. Strategies for long-term success in the treatment of HIV infection. JAMA 283:1329-1334. [DOI] [PubMed] [Google Scholar]
- 9.Groothuis, D., and R. Levy. 1997. The entry of antiviral and antiretroviral drugs into the central nervous system. J. Neurovirol. 3:387-400. [DOI] [PubMed] [Google Scholar]
- 10.Günthard, H., D. Havlir, S. Fiscus, Z. Zhang, J. Eron, J. Mellors, R. Gulick, S. Frost, A. Leigh Brown, W. Schleif, F. Valentine, L. Jonas, A. Meibohm, C. Ignacio, R. Isaacs, R. Gamagami, E. Emini, A. Haase, D. Richman, and J. Wong. 2001. Residual human immunodeficiency virus (HIV) type 1 RNA and DNA in lymph nodes and HIV RNA in genital secretions and in cerebrospinal fluid after suppression of viremia for 2 years. J. Infect. Dis. 183:1318-1327. [DOI] [PubMed] [Google Scholar]
- 11.Haas, D. W., J. Stone, L. A. Clough, B. Johnson, P. Spearman, V. L. Harris, J. Nicotera, R. H. Johnson, S. Raffanti, L. Zhong, P. Bergqwist, S. Chamberlin, V. Hoagland, and W. D. Ju. 2000. Steady-state pharmacokinetics of indinavir in cerebrospinal fluid and plasma among adults with human immunodeficiency virus type I infection. Clin. Pharmacol. Ther. 68:367-374. [DOI] [PubMed] [Google Scholar]
- 12.Hoetelmans, R. 1998. Sanctuary sites in HIV-1 infection. Antivir. Ther. 3:S13-S17. [PubMed] [Google Scholar]
- 13.Jayewardene, A. L., F. Zhu, F. T. Aweeka, and J. G. Gambertoglio. 1998. Simple high-performance liquid chromatographic determination of the protease inhibitor indinavir in human plasma. J. Chromatogr. B 707:203-211. [DOI] [PubMed] [Google Scholar]
- 14.Kiessling, A., L. Fitzgerald, D. Zhang, H. Chhay, D. Brettler, R. Eyre, J. Steinberg, K. McGowan, and R. Byrn. 1998. Human immunodeficiency virus in semen arises from a genetically distinct virus reservoir. AIDS Res. Hum. Retrovir. 14(Suppl. 1):S33-S41. [PubMed] [Google Scholar]
- 15.Lafeuillade, A., L. Chollet, G. Hittinger, N. Profizi, O. Costes, and C. Poggi. 1997. Residual human immunodeficiency virus type 1 RNA in lymphoid tissue of patients with sustained plasma RNA of <200 copies/mL. J. Infect. Dis. 177:235-238. [DOI] [PubMed] [Google Scholar]
- 16.Lafeuillade, A., C. Poggi, A. Tamalet, and N. Profizi. 1997. Human immunodeficiency virus type 1 dynamics in different lymphoid tissue compartments. J. Infect. Dis. 176:804-806. [DOI] [PubMed] [Google Scholar]
- 17.Letendre, S. L., E. V. Capparelli, R. J. Ellis, J. A. McCutchan, and the HIV Neurobehavioral Research Center Group. 2000. Indinavir population pharmacokinetics in plasma and cerebrospinal fluid. Antimicrob. Agents Chemother. 44:2173-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayer, K. H., S. Boswell, R. Goldstein, W. Lo, C. Xu, L. Tucker, M. P. DePasquale, R. D'Aquila, and D. Andreson. 1999. Persistence of human immunodeficiency virus in semen after adding indinavir to combination antiretroviral therapy. Clin. Infect. Dis. 28:1252-1259. [DOI] [PubMed] [Google Scholar]
- 19.Mocroft, A., H. Devereux, S. Kinloch-de-Loes, M. Wilson, D., S. M. Youle, M. Tyrer, C. Loveday, A. Phillips, M. Johnson, et al. 2000. Immunological, virological and clinical response to highly active antiretroviral therapy treatment regimens in a complete clinic population. AIDS 14:1545-1552. [DOI] [PubMed] [Google Scholar]
- 20.Molla, A., S. Vasavanonda, G. Kumar, H. L. Sham, M. Johnson, B. Grabowski, J. F. Denissen, W. Kohlbrenner, J. J. Plattner, J. M. Leonard, D. W. Norbeck, and D. J. Kempf. 1998. Human serum attenuates the activity of protease inhibitors toward wild-type and mutant human deficiency virus. Virology 250:255-262. [DOI] [PubMed] [Google Scholar]
- 21.Moyle, G. J., M. Sadler, and N. Buss. 1999. Plasma and cerebrospinal fluid saquinavir concentrations in patients receiving combination antiretroviral therapy. Clin. Infect. Dis. 28:403-404. [DOI] [PubMed] [Google Scholar]
- 22.Palella, F. J. J., K. M. Delaney, A. C. Moorman, M. O. Loveless, J. Fuhrer, G. A. Satten, D. J. Aschman, and S. D. Holmberg. 1998. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N. Engl. J. Med. 338:853-860. [DOI] [PubMed] [Google Scholar]
- 23.Pereira, A., A. Kashuba, S. Fiscus, J. Hall, R. Tidwell, L. Troiani, J. Dunn, J. J. Eron, and M. Cohen. 1999. Nucleoside analogues achieve high concentrations in seminal plasma: relationship between drug concentration and virus burden. J. Infect. Dis. 180:2039-2043. [DOI] [PubMed] [Google Scholar]
- 24.Pomerantz, R. J. 1999. Residual HIV-1 disease in the era of highly active antiretroviral therapy. N. Engl. J. Med. 340:1672-1674. [DOI] [PubMed] [Google Scholar]
- 25.Schockmel, G., S. Yerly, and L. Perrin. 1997. Detection of low HIV-1 RNA levels in plasma. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 17:179-183. [DOI] [PubMed] [Google Scholar]
- 26.Schrager, L. K., and M. P. D'Souza. 1998. Cellular and anatomical reservoirs of HIV-1 in patients receiving potent antiretroviral combination therapy. JAMA 280:67-71. [DOI] [PubMed] [Google Scholar]
- 27.Si-Mohamed, A., M. Kazatchkine, I. Heard, C. Goujon, T. Prazuck, G. Aymard, G. Cessot, Y. Kuo, M. Bernard, B. Diquet, J. Malkin, L. Gutmann, and L. Belec. 2000. Selection of drug-resistant variants in the female genital tract of human immunodeficiency virus type 1-infected women receiving antiretroviral therapy. J. Infect. Dis. 182:112-122. [DOI] [PubMed] [Google Scholar]
- 28.Tachet, A., E. Dulioust, D. Salmon, M. De Almeida, S. Rivalland, L. Finkielsztejn, I. Heard, P. Jouannet, D. Sicard, and C. Rouzioux. 1999. Detection and quantification of HIV-1 in semen: identification of a subpopulation of men at high potential risk of viral sexual transmission. AIDS 13:823-831. [DOI] [PubMed] [Google Scholar]
- 29.Tang, Y. W., J. T. Huong, R. M. Lloyd, P. Spearman, and D. W. Haas. 2000. Comparison of human immunodeficiency virus type I RNA sequence heterogeneity in cerebrospinal fluid and plasma. J. Clin. Microbiol. 38:4637-4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor, S., D. Back, S. Drake, J. Workman, H. Reynolds, S. Gibbons, D. White, and D. Pillay. 2001. Antiretroviral drug concentrations in semen of HIV-infected men: differential penetration of indinavir, ritonavir and sa-quinavir. J. Antimicrob. Chemother. 48:351-354. [DOI] [PubMed] [Google Scholar]
- 31.Taylor, S., D. J. Back, J. Workman, S. M. Drake, D. J. White, B. Choudhury, P. A. Cane, G. M. Beards, K. Halifax, and D. Pillay. 1999. Poor penetration of the male genital tract by HIV-1 protease inhibitors. AIDS 13:859-872. [DOI] [PubMed] [Google Scholar]
- 32.Wu, E. Y., J. M. Wilkinson, D. G. Naret, V. L. Daniels, L. J. Williams, D. A. Khalil, and B. V. Shetty. 1997. High performance liquid chromatographic method for the determination of nelfinavir, a novel HIV-1 protease inhibitor, in human plasma. J. Chromatogr. B 695:373-380. [DOI] [PubMed] [Google Scholar]
- 33.Zhou, X. J., D. V. Havlir, D. D. Richman, E. P. Acosta, M. Hirsch, A. C. Collier, P. Tebas, J. P. Sommadossi, and the AIDS Clinical Trials Group Study 343. 2000. Plasma population pharmacokinetics and penetration into cerebrospinal fluid of indinavir in combination with zidovudine and lamivudine in HIV-1-infected patients. AIDS 14:2869-2876. [DOI] [PubMed] [Google Scholar]